Generation and Characterization of Human Mesenchymal Stem Cell-Derived Smooth Muscle Cells
Abstract
:1. Introduction
2. Results
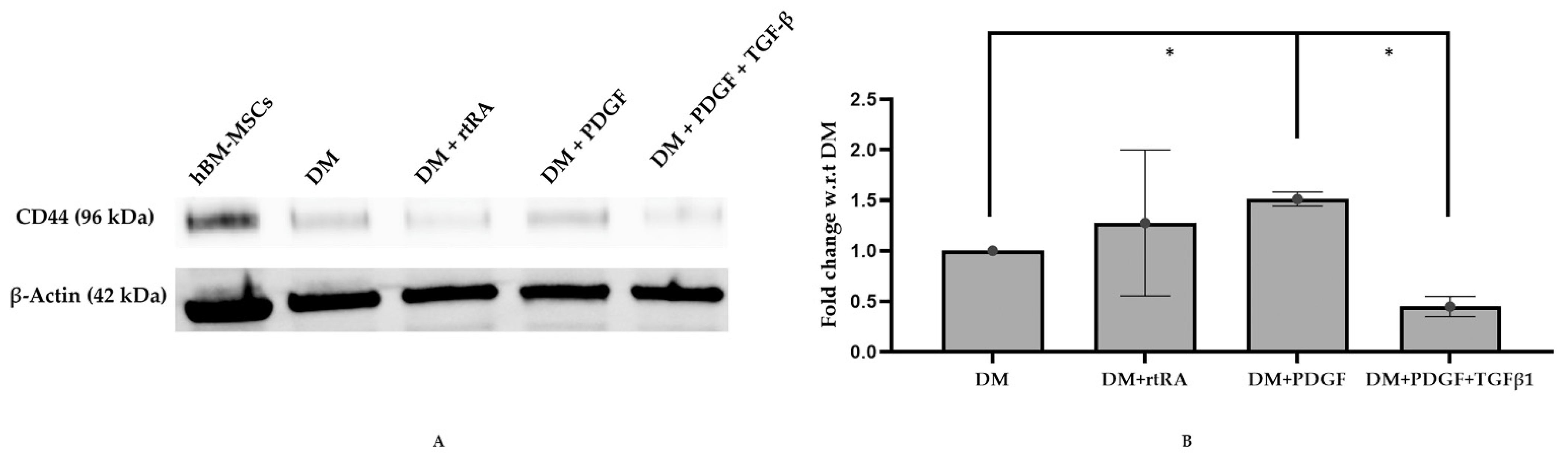
2.1. MSCs Cultured in PDGF and TGF β1 Supplemented Differentiation Medium Indicates SMC Formation
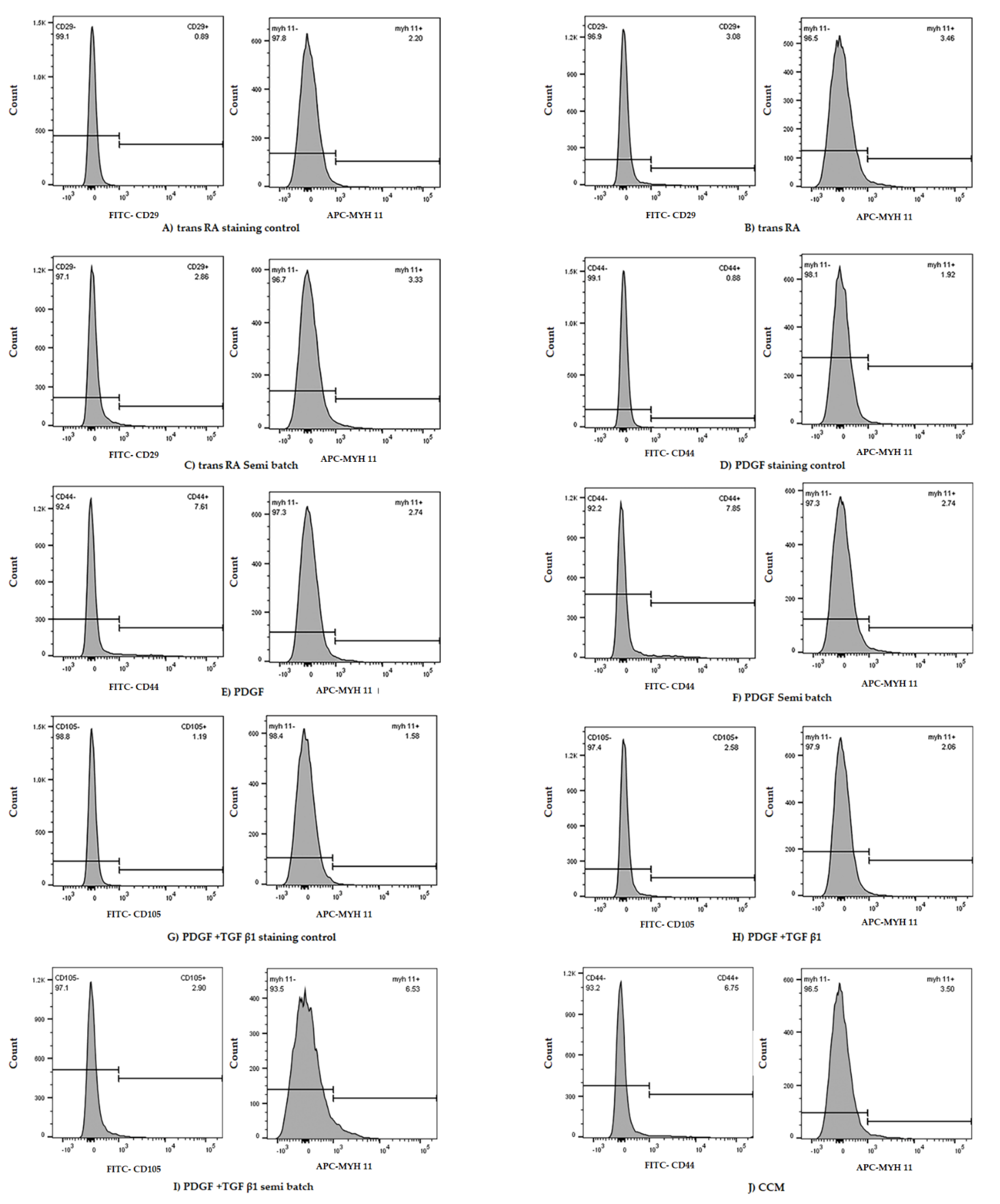
2.2. Treatment of MSCs with DM Containing PT Resulted in Minimal Increase in MYH II Expression
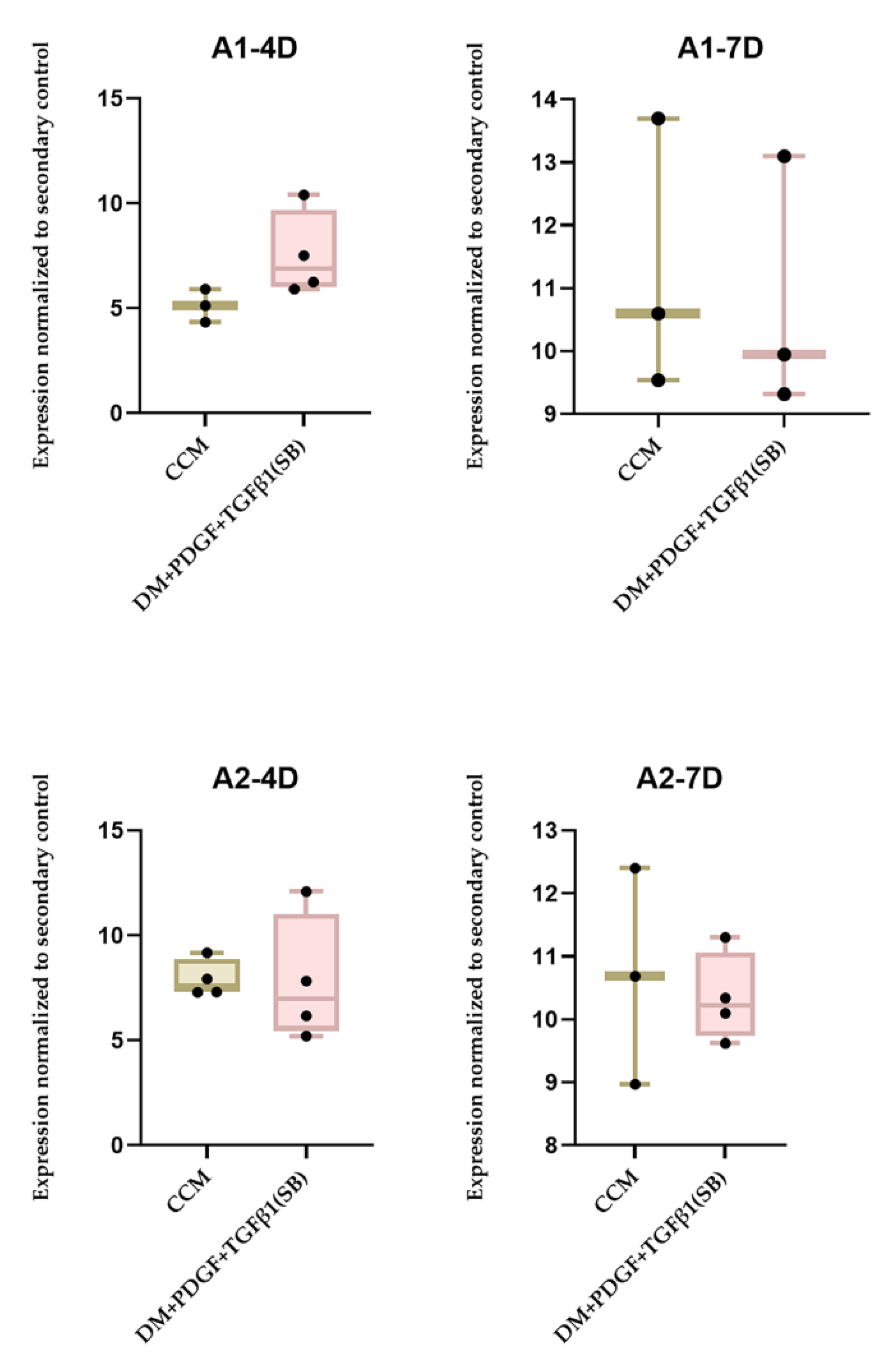
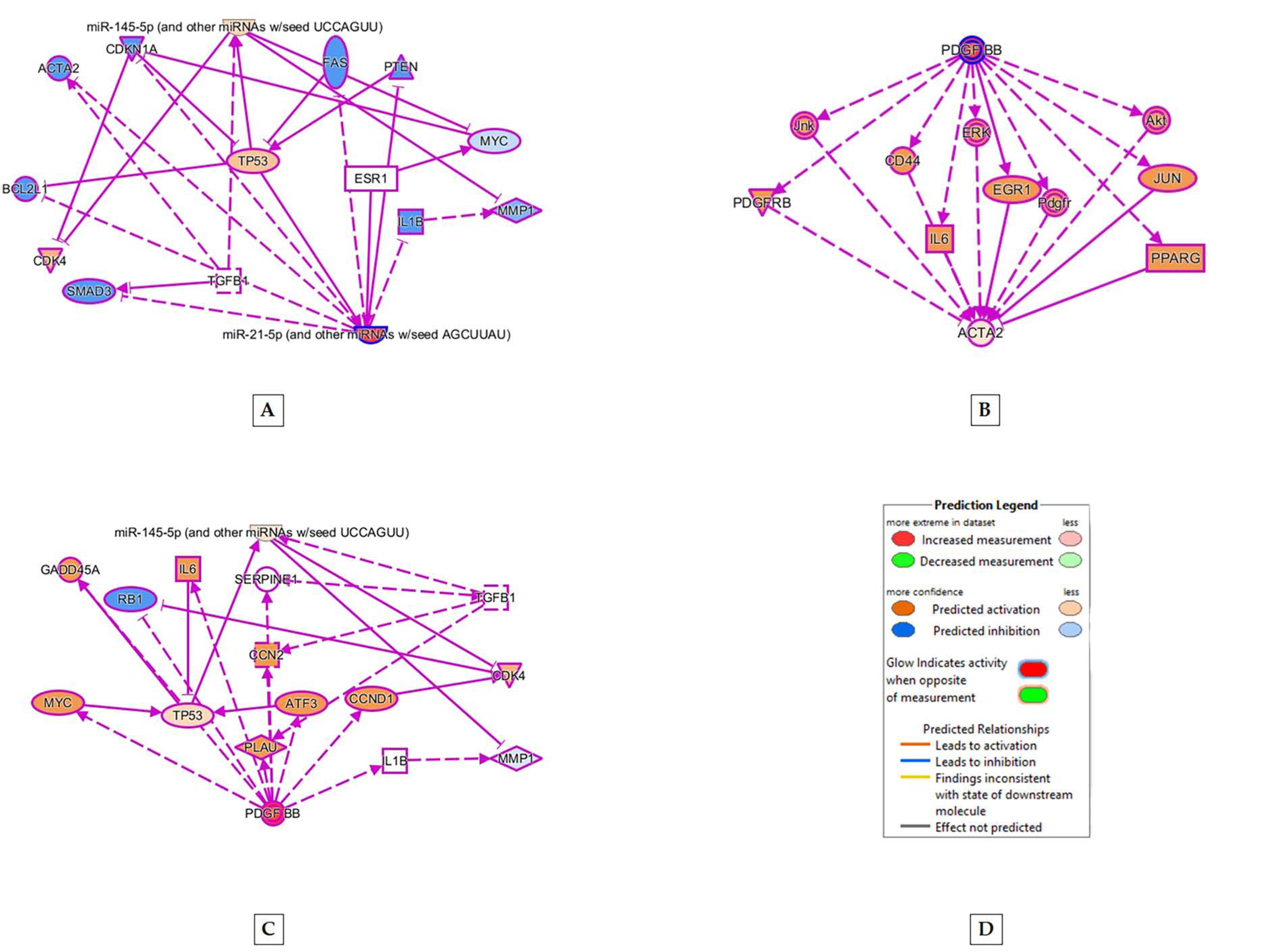
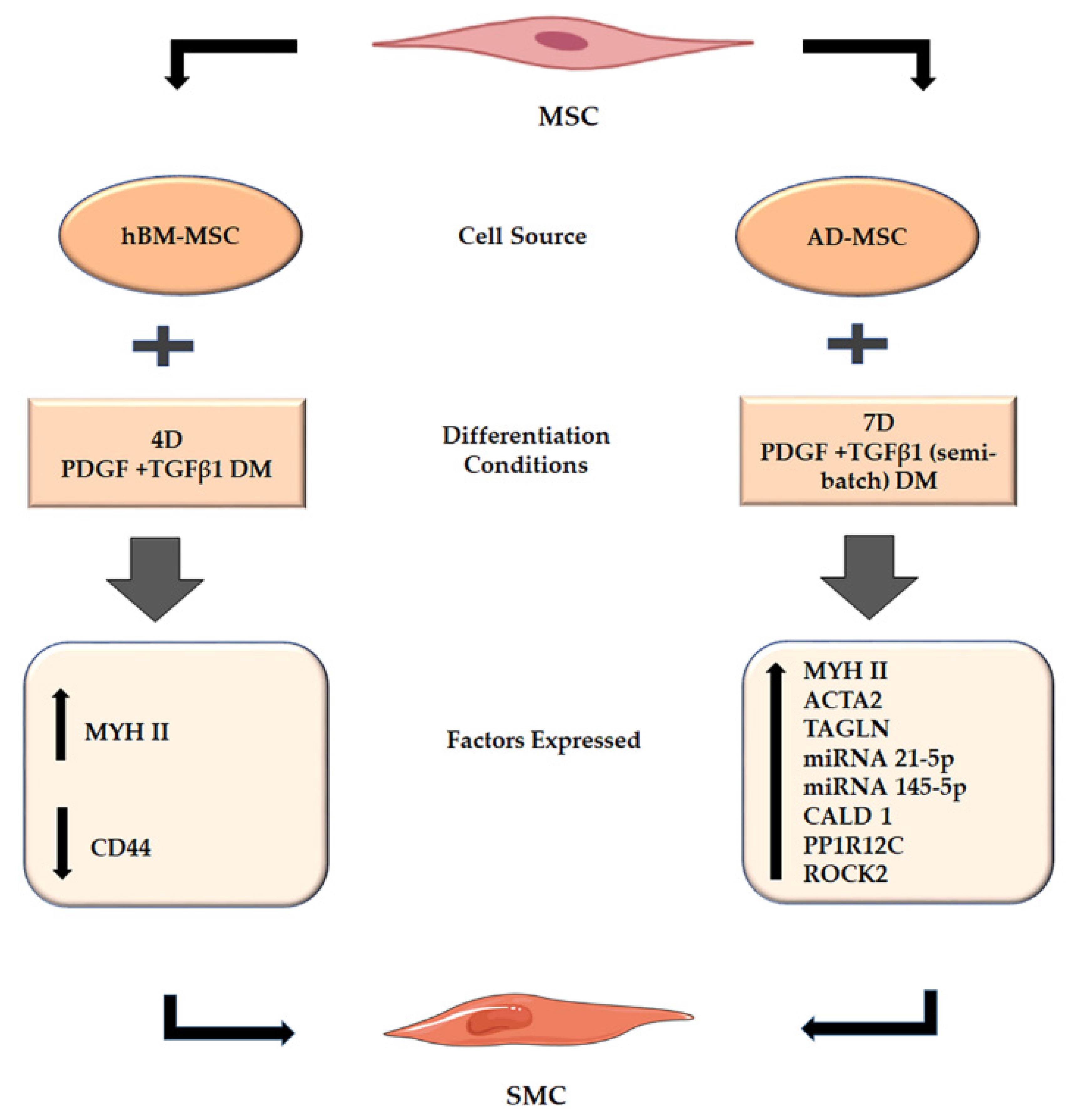
2.3. Origin of Stem Cells and PT Treatment Both Play a Role in MSC Differentiation to SMC
3. Discussion
4. Materials and Methods
4.1. Procurement of Mesenchymal Stem Cells
4.2. Directed Differentiation of MSCs into SMCs
4.3. Western Blot Assessment of Cell Differentiation
4.4. BCA Protein Assay
4.5. Western Blotting
4.5.1. Sample Preparation
4.5.2. Gel Electrophoresis
4.5.3. Transfer
4.5.4. Blotting
4.5.5. Imaging
4.5.6. Blot Stripping
4.5.7. Protein Normalization
4.6. Intracellular and Extracellular Marker Staining of Cells for Flow Cytometry
4.7. Flow-Cytometry Assessment of Cell Differentiation
4.8. RNA Seq Analysis of Cell Differentiation
4.8.1. Stem Cell Differentiation and Pellet formation
4.8.2. RNA Isolation
4.8.3. RNA Quality Determination
4.8.4. Ingenuity Pathway Analysis (IPA)
Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stratton, M.S.; Farina, F.M.; Elia, L. Epigenetics and vascular diseases. J. Mol. Cell Cardiol. 2019, 133, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Selle, J.G.; Robicsek, F.; Daugherty, H.K.; Cook, J.W. Thoracoabdominal aortic aneurysms. A review and current status. Ann. Surg. 1979, 189, 158–164. [Google Scholar] [CrossRef]
- McPhee, J.; Eslami, M.H.; Arous, E.J.; Messina, L.M.; Schanzer, A. Endovascular treatment of ruptured abdominal aortic aneurysms in the United States (2001–2006): A significant survival benefit over open repair is independently associated with increased institutional volume. J. Vasc. Surg. 2009, 49, 817–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Robson, P.; Hew, Y.; Keeley, F.W. Decreased elastin synthesis in normal development and in long-term aortic organ and cell cultures is related to rapid and selective destabilization of mRNA for elastin. Circ. Res. 1995, 77, 1107–1113. [Google Scholar] [CrossRef]
- Bashur, C.A.; Rao, R.R.; Ramamurthi, A. Perspectives on stem cell-based elastic matrix regenerative therapies for abdominal aortic aneurysms. Stem Cells Transl. Med. 2013, 2, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Yamawaki-Ogata, A.; Ueda, Y.; Wagner, W.R.; Narita, Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J. Vasc Surg 2011, 54, 1743–1752. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wen, Y.; Li, Y.H.; Wei, Y.; Green, M.; Wani, P.; Zhang, P.; Pera, R.R.; Chen, B. Smooth Muscle Precursor Cells Derived from Human Pluripotent Stem Cells for Treatment of Stress Urinary Incontinence. Stem Cells Dev. 2016, 25, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, G.; Gadepalli, V.S.; Stoilov, I.; Mecham, R.P.; Rao, R.R.; Ramamurthi, A. Pro-elastogenic effects of bone marrow mesenchymal stem cell-derived smooth muscle cells on cultured aneurysmal smooth muscle cells. J. Tissue Eng. Regen Med. 2017, 11, 679–693. [Google Scholar] [CrossRef]
- Ghorbel, M.T.; Jia, H.; Swim, M.M.; Iacobazzi, D.; Albertario, A.; Zebele, C.; Holopherne-Doran, D.; Hollander, A.; Madeddu, P.; Caputo, M. Reconstruction of the pulmonary artery by a novel biodegradable conduit engineered with perinatal stem cell-derived vascular smooth muscle cells enables physiological vascular growth in a large animal model of congenital heart disease. Biomaterials 2019, 217, 119284. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Pacelli, L.; Krampera, M. Immunological properties of embryonic and adult stem cells. World J. Stem Cells 2010, 2, 50–60. [Google Scholar] [CrossRef]
- Sommer, C.A.; Mostoslavsky, G. The evolving field of induced pluripotency: Recent progress and future challenges. J. Cell Physiol. 2013, 228, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.; Harmouch, C.; Lataillade, J.J.; Laurent-Maquin, D.; Labrude, P.; Menu, P.; Kerdjoudj, H. Stem cells: A promising source for vascular regenerative medicine. Stem Cells Dev. 2014, 23, 2931–2949. [Google Scholar] [CrossRef]
- Housman, T.S.; Lawrence, N.; Mellen, B.G.; George, M.N.; Filippo, J.S.; Cerveny, K.A.; DeMarco, M.; Feldman, S.R.; Fleischer, A.B. The safety of liposuction: Results of a national survey. Dermatol. Surg. 2002, 28, 971–978. [Google Scholar] [CrossRef]
- Su, Z.Y.; Li, Y.; Zhao, X.L.; Zhang, M. All-trans retinoic acid promotes smooth muscle cell differentiation of rabbit bone marrow-derived mesenchymal stem cells. J. Zhejiang Univ. Sci. B 2010, 11, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.C.; Cui, L.; Li, G.; Yin, S.; Gao, Y.J.; Cao, Y.L. PDGF-BB initiates vascular smooth muscle-like phenotype differentiation of human bone marrow mesenchymal stem cells In Vitro. Chin. J. Plast. Surg. 2007, 23, 335–339. [Google Scholar]
- Tian, H.; Bharadwaj, S.; Liu, Y.; Ma, H.; Ma, P.X.; Atala, A.; Zhang, Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials 2010, 31, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, G.; Stoilov, I.; Broekelmann, T.; Mecham, R.; Ramamurthi, A. Phenotype-based selection of bone marrow mesenchymal stem cell-derived smooth muscle cells for elastic matrix regenerative repair in abdominal aortic aneurysms. J. Tissue Eng. Regen Med. 2018, 12, e60–e70. [Google Scholar] [CrossRef]
- Stegemann, J.P.; Hong, H.; Nerem, R.M. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J. Appl. Physiol. 2005, 98, 2321–2327. [Google Scholar] [CrossRef] [Green Version]
- Fadini, G.P.; de Kreutzenberg, S.; Albiero, M.; Coracina, A.; Pagnin, E.; Baesso, I.; Cignarella, A.; Bolego, C.; Plebani, M.; Nardelli, G.B.; et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: The role of female estrogens. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 997–1004. [Google Scholar] [CrossRef] [Green Version]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schafer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.F.; Wen, S.H.; Chen, R.L.; Lu, C.J.; Zheng, Y.J.; Yang, S.H.; Chu, S.C.; Kao, R.H.; Chen, S.H. Factors associated with peripheral blood stem cell yield in volunteer donors mobilized with granulocyte colony-stimulating factors: The impact of donor characteristics and procedural settings. Biol. Blood Marrow. Transplant. 2008, 14, 1305–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, M.E.; Conboy, I.M. Loss of stem cell regenerative capacity within aged niches. Aging Cell 2007, 6, 371–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamama, K.; Sen, C.K.; Wells, A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008, 17, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Broekelman, T.; Mecham, R.P.; Ramamurthi, A. Maintaining Elastogenicity of Mesenchymal Stem Cell-Derived Smooth Muscle Cells in Two-Dimensional Culture. Tissue Eng. Part. A 2018, 24, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Krawiec, J.T.; Weinbaum, J.S.; St Croix, C.M.; Phillippi, J.A.; Watkins, S.C.; Rubin, J.P.; Vorp, D.A. A cautionary tale for autologous vascular tissue engineering: Impact of human demographics on the ability of adipose-derived mesenchymal stem cells to recruit and differentiate into smooth muscle cells. Tissue Eng. Part. A 2015, 21, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, T.; Wang, S.; Wei, J.; Fan, J.; Li, J.; Han, Q.; Liao, L.; Shao, C.; Zhao, R.C. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013, 10, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, E.M.; Dimmeler, S. MicroRNAs and stem cells: Control of pluripotency, reprogramming, and lineage commitment. Circ. Res. 2012, 110, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Davis-Dusenbery, B.N.; Wu, C.; Hata, A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2370–2377. [Google Scholar] [CrossRef] [Green Version]
- Davis-Dusenbery, B.N.; Chan, M.C.; Reno, K.E.; Weisman, A.S.; Layne, M.D.; Lagna, G.; Hata, A. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J. Biol. Chem. 2011, 286, 28097–28110. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef]
- Parmacek, M.S. Myocardin-related transcription factors: Critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 2007, 100, 633–644. [Google Scholar] [CrossRef]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alimperti, S.; You, H.; George, T.; Agarwal, S.K.; Andreadis, S.T. Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function In Vivo. J. Cell Sci. 2014, 127, 2627–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prussin, C.; Metcalfe, D.D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J. Immunol. Methods 1995, 188, 117–128. [Google Scholar] [CrossRef]
- Jung, T.; Schauer, U.; Heusser, C.; Neumann, C.; Rieger, C. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 1993, 159, 197–207. [Google Scholar] [CrossRef]
- Ross, J.J.; Hong, Z.; Willenbring, B.; Zeng, L.; Isenberg, B.; Lee, E.H.; Reyes, M.; Keirstead, S.A.; Weir, E.K.; Tranquillo, R.T.; et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J. Clin. Investig. 2006, 116, 3139–3149. [Google Scholar] [CrossRef]

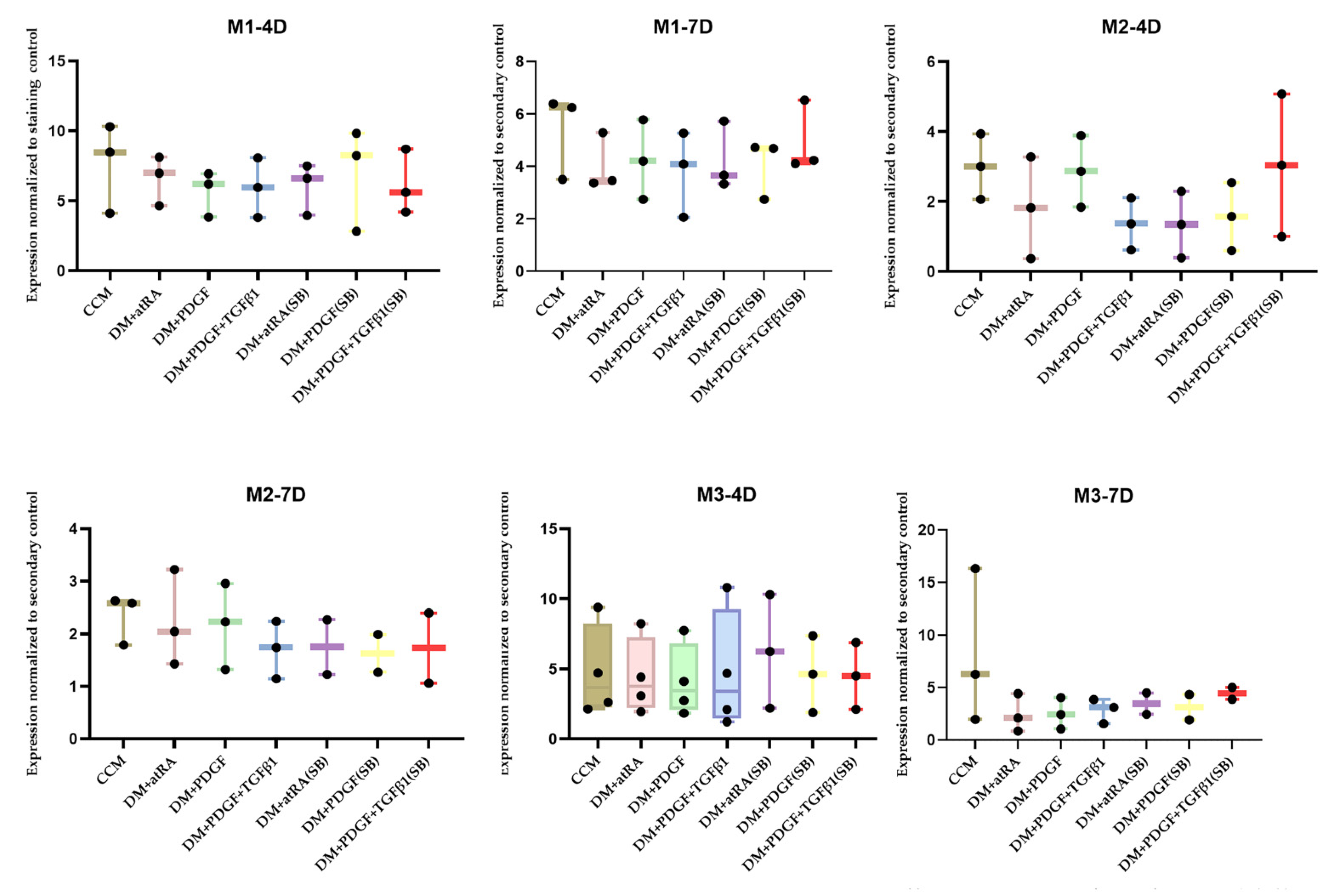
| Number of Trials | Cell Line | Time Period (Days) | Treatments with MYH11 Expression Greater than Cell Culture Medium Control |
|---|---|---|---|
| 3 | M1–30 Y Female Hispanic | 4 | PDGF + TGF β1 semi batch (1) |
| 3 | M1–30 Y Female Hispanic | 7 | PDGF + TGF β1 semi batch (1) |
| 3 | M2–18 Y Female African | 4 | PDGF + TGF β1 semi batch (1) |
| 3 | M2–18 Y Female African | 7 | N/A (3) |
| 3 | M3–21 Y Male Asian | 4 | PDGF + TGF β1 (1) |
| 3 | M3–21 Y Male Asian | 7 | N/A (3) |
| Number of Trials | Cell Lines (Adipose Tissue) | Time Period (Days) | Treatment with MYH II Expression Greater than CCM Control |
|---|---|---|---|
| 3 | A1–24 Y Female Caucasian | 4 | A1-PT (2) |
| 3 | A1–24 Y Female Caucasian | 7 | A1-PT (1) |
| 3 | A2–29 Y Female Native American | 4 | A2-PT (1) |
| 3 | A2–29 Y Female Native American | 7 | A2-PT (1) |
| Gene Name | Significance | Expression Log Ratio | Result | |
|---|---|---|---|---|
| A2PT vs. A2CC | actin alpha 2 (ACTA2) | Expressed in smooth muscle and aids in vascular contraction | 3.758 | Increased expression in A2PT |
| miR-17-5p | Mesenchymal stem cell differentiation | 2.130 | ||
| miR-145-5p | Vascular smooth muscle cell differentiation | 3.682 | ||
| A1PT vs. A1CC | transgelin (TAGLN) | Expressed in vascular and smooth muscles and is an early marker of smooth muscle differentiation. | 3.272 | Increased expression in A1PT |
| A2PT vs. A1PT | actin alpha 2 (ACTA2) | Expressed in smooth muscles and aids in vascular contraction | 3.044 | Increased Expression in A2PT Decreased Expression in A2PT |
| miR-21-5p | Adipose mesenchymal stem cell differentiation | −3.523 |
| Gene Name | Significance | Result | |
|---|---|---|---|
| A2PT vs. A2CC | CALD1 | Regulates smooth muscle contraction | Increased expression in A2PT |
| ROCK2 | Regulates formation of actin stress fibers | ||
| PPP1R12C | Regulates the assembly of the actin cytoskeleton | ||
| A1PT vs. A1CC | CALD1 | Regulates smooth muscle contraction | Increased expression in A1PT |
| A2PT vs. A1PT | CALD1 | Regulates smooth muscle contraction | Increased expression in A2PT |
| PP1R12C | Regulates the assembly of the actin cytoskeleton |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaraman, S.; Hedrick, J.; Ismail, S.; Slavin, C.; Rao, R.R. Generation and Characterization of Human Mesenchymal Stem Cell-Derived Smooth Muscle Cells. Int. J. Mol. Sci. 2021, 22, 10335. https://doi.org/10.3390/ijms221910335
Sivaraman S, Hedrick J, Ismail S, Slavin C, Rao RR. Generation and Characterization of Human Mesenchymal Stem Cell-Derived Smooth Muscle Cells. International Journal of Molecular Sciences. 2021; 22(19):10335. https://doi.org/10.3390/ijms221910335
Chicago/Turabian StyleSivaraman, Srikanth, Jackson Hedrick, Samia Ismail, Chris Slavin, and Raj R. Rao. 2021. "Generation and Characterization of Human Mesenchymal Stem Cell-Derived Smooth Muscle Cells" International Journal of Molecular Sciences 22, no. 19: 10335. https://doi.org/10.3390/ijms221910335







