Cudratricusxanthone A Inhibits Lipid Accumulation and Expression of Inducible Nitric Oxide Synthase in 3T3-L1 Preadipocytes
Abstract
:1. Introduction
2. Results
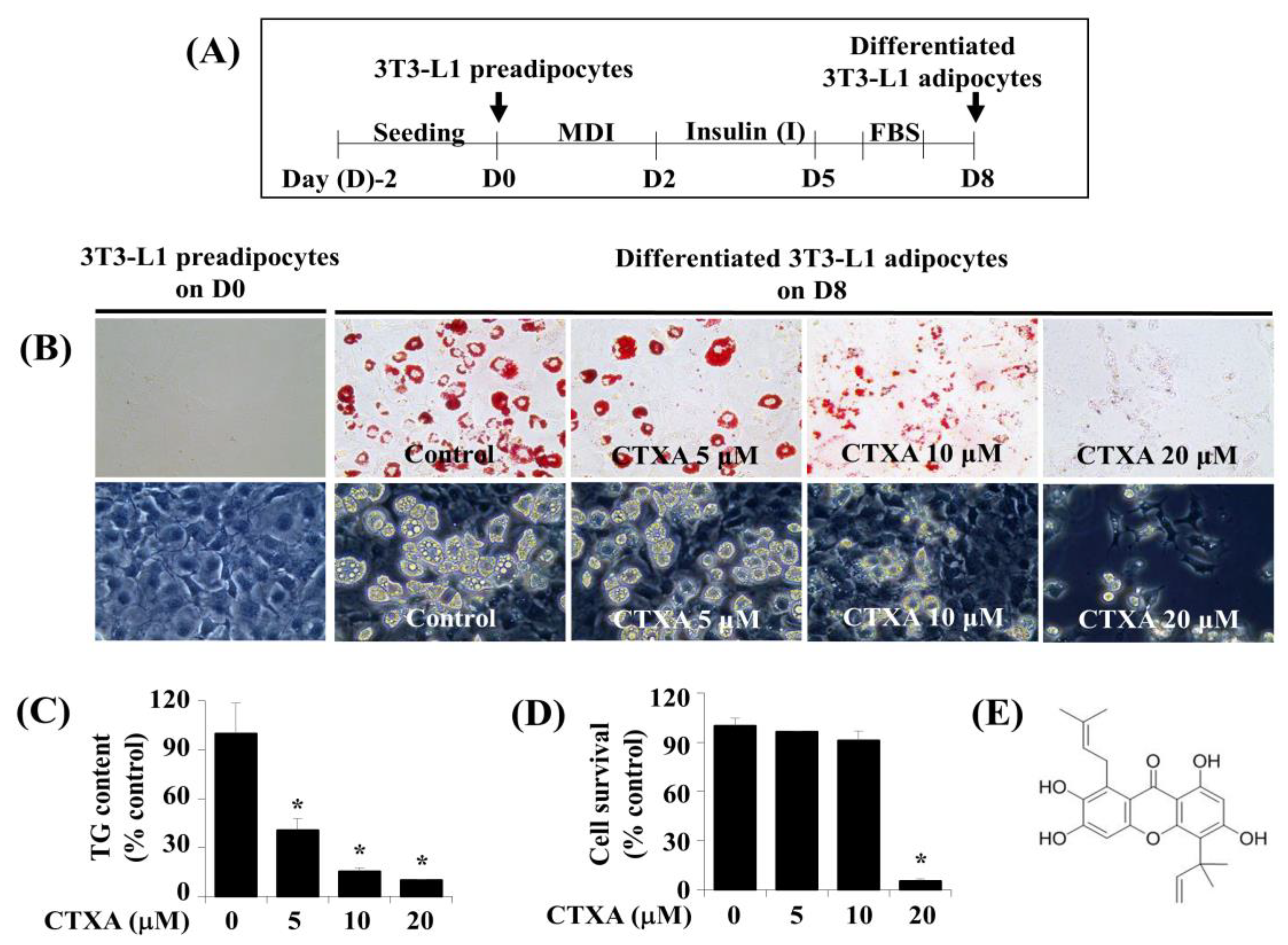
2.1. CTXA Induces a Concentration-Dependent Inhibition of Lipid Accumulation during 3T3-L1 Preadipocyte Differentiation
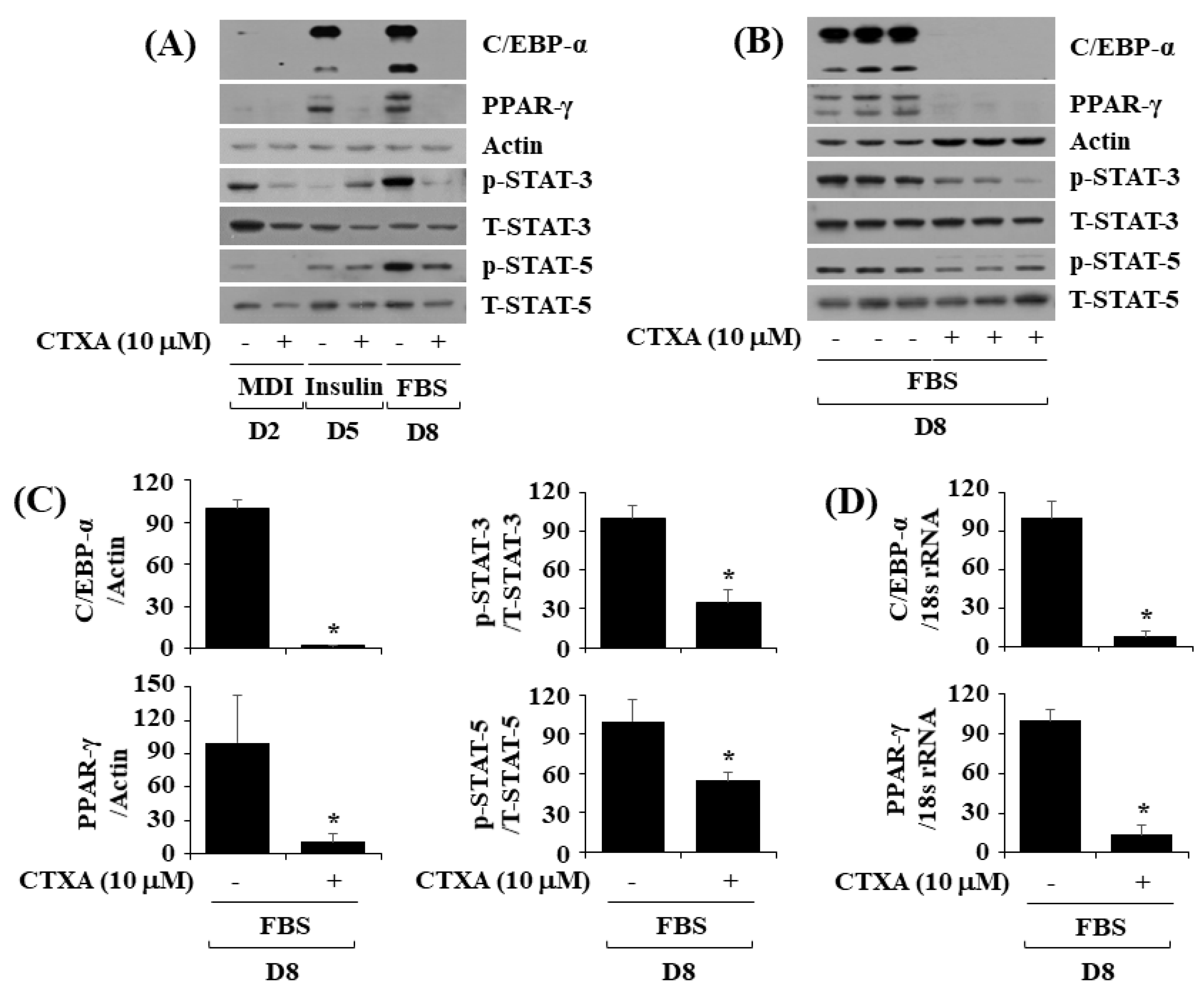
2.2. CTXA at 10 µM Reduces Protein Expression Level of C/EBP-α and PPAR-γ as Well as Phosphorylation Level of STAT-3 and STAT-5 during 3T3-L1 Preadipocyte Differentiation
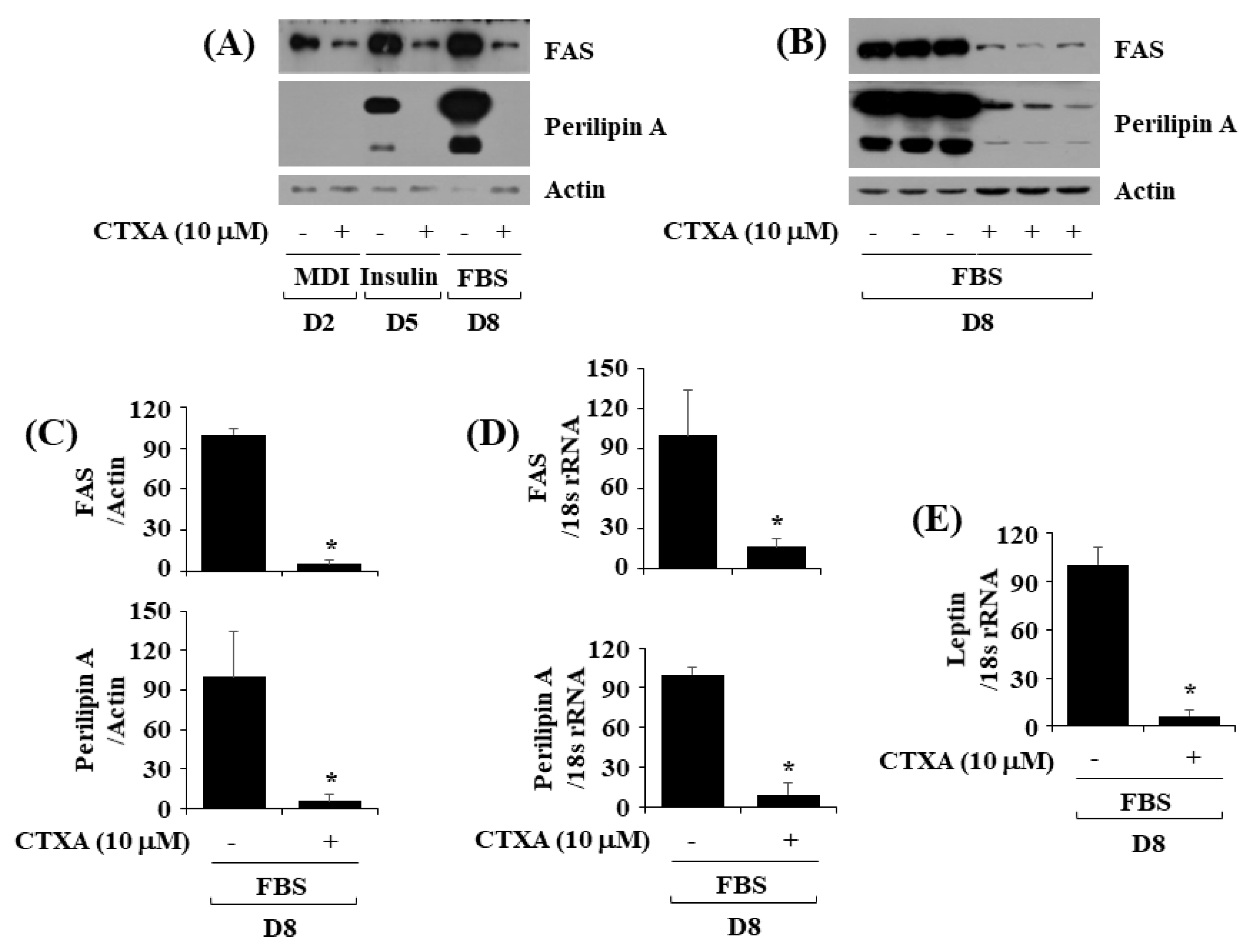
2.3. CTXA at 10 µM Downregulates Expression Level of FAS and Perilipin A during 3T3-L1 Preadipocyte Differentiation
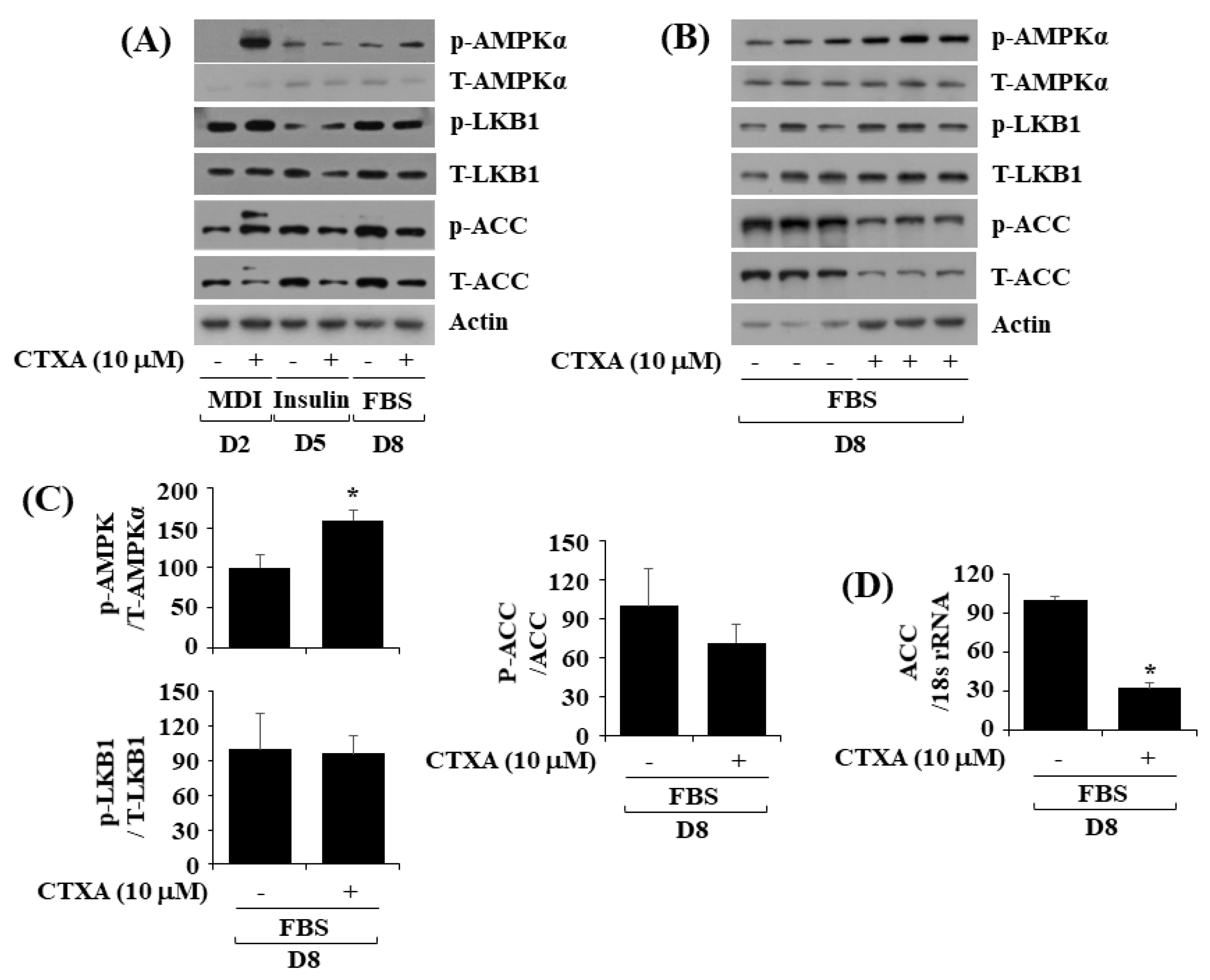
2.4. CTXA at 10 µM Alters Phosphorylation and Expression Level of AMPKα, LKB1, and ACC during 3T3-L1 Preadipocyte Differentiation
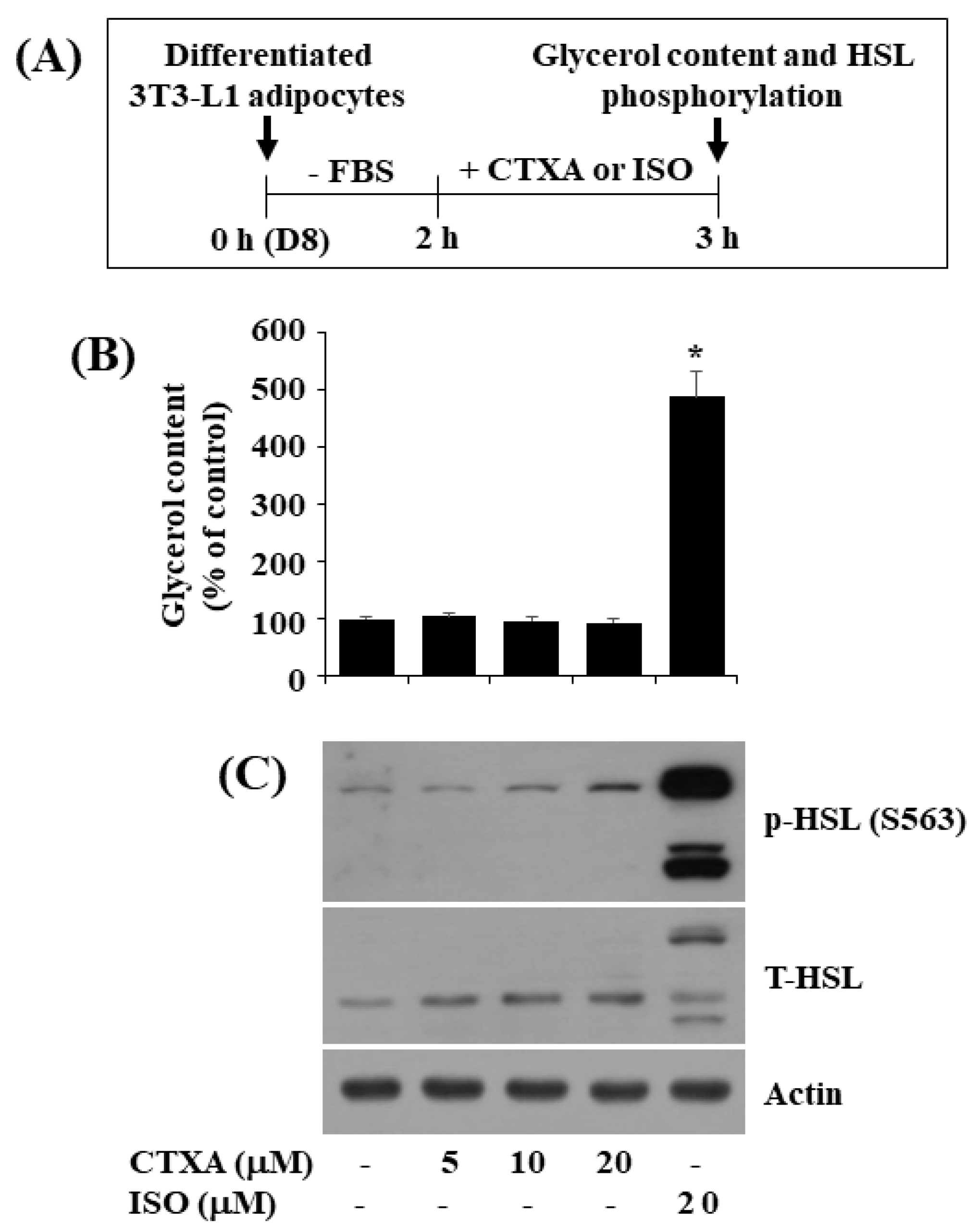
2.5. CTXA at 10 µM Does Not Stimulate Glycerol Release and Phosphorylation of HSL in Mature 3T3-L1 Adipocytes
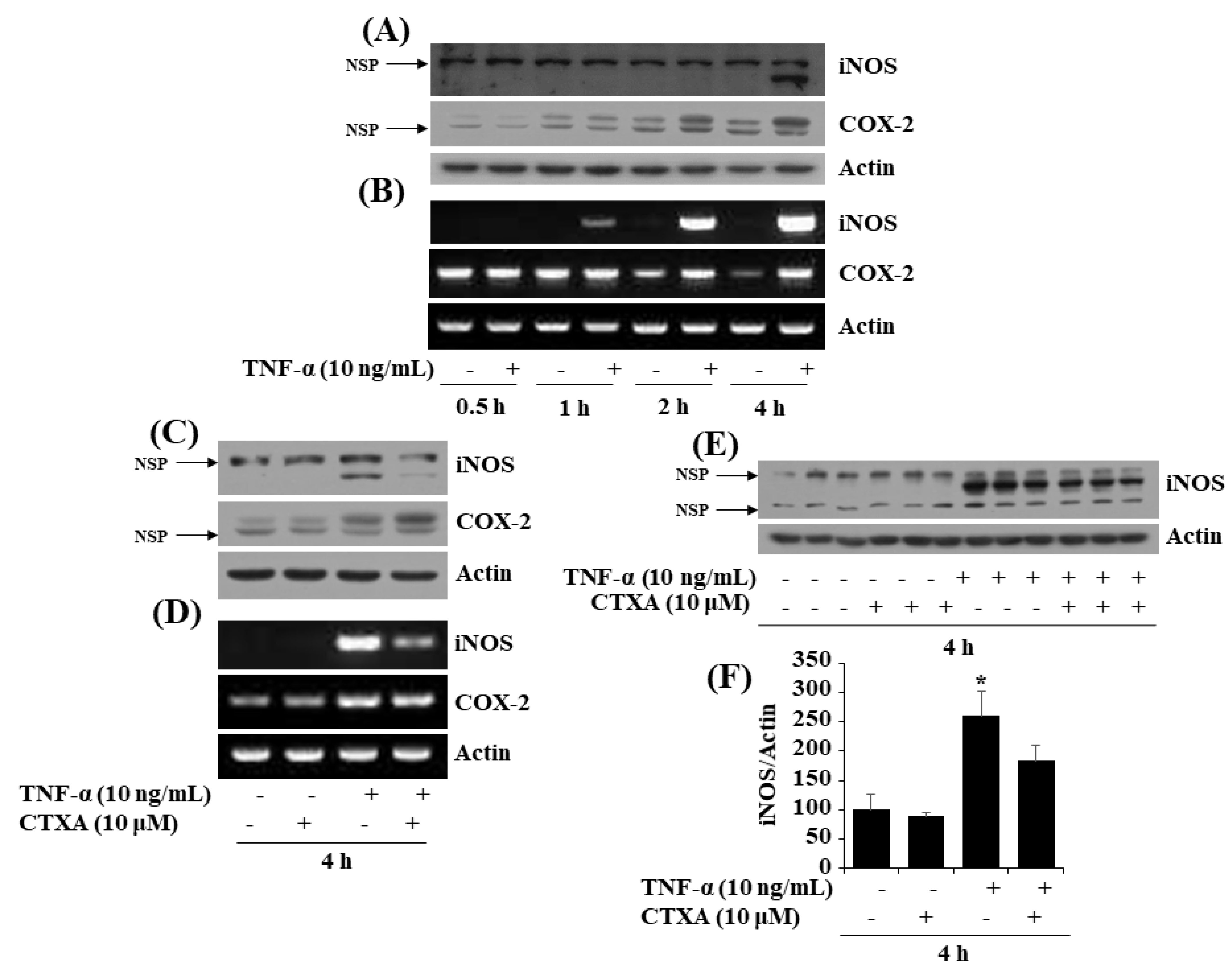
2.6. CTXA at 10 µM Inhibits TNF-α-Induced Expression of iNOS, but Not COX-2, in 3T3-L1 Preadipocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Differentiation of 3T3-L1 Preadipocytes
4.3. Oil Red O Staining
4.4. Cell Count Analysis
4.5. Quantification of Cellular TG Content
4.6. Preparation of Whole-Cell Lysates
4.7. Western Blot Analysis
4.8. Quantitative Real-Time RT-PCR
4.9. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Measurement of Glycerol Content
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [PubMed]
- Sudhakaran, M.; Doseff, A.I. The Targeted Impact of Flavones on Obesity-Induced Inflammation and the Potential Synergistic Role in Cancer and the Gut Microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell. Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell. Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Dokmeci Emre, S. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell. Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Burrell, J.A.; Boudreau, A.; Stephens, J.M. Latest advances in STAT signaling and function in adipocytes. Clin. Sci. (Lond.) 2020, 134, 629–639. [Google Scholar]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Klöting, N.; Fasshauer, M.; Schön, M.R.; Körner, A.; Stumvoll, M.; Blüher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordonier, E.L.; Jarecke, S.K.; Hollinger, F.E.; Zempleni, J. Inhibition of acetyl-CoA carboxylases by soraphen A prevents lipid accumulation and adipocyte differentiation in 3T3-L1 cells. Eur. J. Pharmacol. 2016, 780, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, P.A.; Di Gregorio, G.; Lu, T.; Rassouli, N.; Ranganathan, G. Perilipin expression in human adipose tissue is elevated with obesity. J. Clin. Endocrinol. Metab. 2004, 89, 1352–1358. [Google Scholar] [CrossRef] [Green Version]
- Lage, R.; Diéguez, C.; Vidal-Puig, A.; López, M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008, 14, 539–549. [Google Scholar] [CrossRef]
- Martini, C.N.; Plaza, M.V.; Vila Mdel, C. PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol. Cell. Endocrinol. 2009, 298, 42–47. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–462232. [Google Scholar] [PubMed] [Green Version]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998, 273, 32111–321120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar]
- Chirumbolo, S.; Franceschetti, G.; Zoico, E.; Bambace, C.; Cominacini, L.; Zamboni, M. LPS response pattern of inflammatory adipokines in an in vitro 3T3-L1 murine adipocyte model. Inflamm. Res. 2014, 63, 495–507. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose Tissue in Obesity-Related Inflammation and Insulin Resistance: Cells, Cytokines, and Chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [PubMed] [Green Version]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar]
- Kim, H.L.; Ha, A.W.; Kim, W.K. Effect of saccharin on inflammation in 3T3-L1 adipocytes and the related mechanism. Nutr. Res. Pract. 2020, 14, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Han, H.J.; Hong, S.S.; Hwang, J.H.; Hwang, B.Y.; Yoo, H.S.; Jin, Y.R.; Lee, J.J.; Yu, J.Y.; Lee, K.H.; et al. Cudratricusxanthone A isolated from the root bark of Cudrania tricuspidata inhibits the proliferation of vascular smooth muscle cells through the suppression of PDGF-receptor beta tyrosine kinase. Biol. Pharm. Bull. 2007, 30, 805–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, G.S.; Lee, D.S.; Kim, Y.C. Cudratricusxanthone A from Cudrania tricuspidata suppresses pro-inflammatory mediators through expression of anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Int. Immunopharmacol. 2009, 9, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Lee, D.S.; Jeomg, G.S. Cudraticusxanthone A isolated from the roots of Cudrania tricuspidata inhibits metastasis and induces apoptosis in breast cancer cells. J. Ethnopharmacol. 2016, 194, 57–62. [Google Scholar] [CrossRef]
- Choi, E.H.; Kim, E.N.; Jeong, G.S. Inhibitory effect of Cudratricusxanthone A on osteoclast differentiation and function. Phytomedicine 2018, 43, 86–91. [Google Scholar]
- Yoon, C.S.; Kim, D.C.; Quang, T.H.; Seo, J.; Kang, D.G.; Lee, H.S.; Oh, H.; Kim, Y.C. A Prenylated Xanthone, Cudratricusxanthone A, Isolated from Cudrania tricuspidata Inhibits Lipopolysaccharide-Induced Neuroinflammation through Inhibition of NF-κB and p38 MAPK Pathways in BV2 Microglia. Molecules 2016, 21, 1240. [Google Scholar] [CrossRef]
- Cohen, P.; Spiegelman, B.M. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.; Witkowski, A.; Joshi, A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003, 42, 289–317. [Google Scholar] [CrossRef]
- Itabe, H.; Yamaguchi, T.N.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakhill, J.S.; Scott, J.W.; Kemp, B.E. Structure and function of AMP-activated protein kinase. Acta. Physiol. (Oxf.) 2009, 196, 3–14. [Google Scholar] [CrossRef]
- Winder, W.W.; Hardue, D.G. AMP-activated protein kinase, a metabolic master switch: Possible roles in type 2 diabetes. Am. J. Physiol. 1999, 277, E1–E10. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanism of adipogenesis: The anti-adipogenic role of AMP activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar]
- Wu, L.; Whang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase(AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Park, Y.K.; Obiang-Obounou, B.W.; Lee, K.B.; Choi, J.S.; Jang, B.C. AZD1208, a pan-Pim kinase inhibitor, inhibits adipogenesis and induces lipolysis in 3T3-L1 adipocytes. J. Cell. Mol. Med. 2018, 22, 2488–2497. [Google Scholar] [CrossRef] [Green Version]
- Lapid, K.; Graff, J.M. Form(ul)ation of adipocytes by lipids. Adipocyte 2017, 6, 176–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [PubMed]
- Lehrke, M.; Lazar, M.A. The many faces of PPAR gamma. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londos, C.; Brasaemle, D.L.; Schultz, C.J.; Segrest, J.P.; Kimmel, A.R. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell. Dev. Biol. 1999, 10, 51–58. [Google Scholar] [CrossRef]
- Wolins, N.E.; Brasaemle, D.L.; Bickel, P.E. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006, 580, 5484–5491. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar]
- Lee, H.; Kang, R.; Bae, S.; Yoon, Y. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar]
- Poudel, B.; Lim, S.W.; Ki, H.H.; Nepali, S.; Lee, Y.M.; Kim, D.K. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int. J. Mol. Med. 2014, 34, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Gormand, A.; Henriksson, E.; Strom, K.; Jensen, T.E.; Sakamoto, K.; Goransson, O. Regulation of AMP-activated protein kinase by LKB1 and CaMKK in adipocytes. J. Cell. Biochem. 2011, 112, 1364–1375. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hardie, D.G. AMPK: A cellular energy sensor primarily regulated by AMP. Biochem. Soc. Trans. 2014, 42, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [PubMed] [Green Version]
- Anthonsen, M.W.; Rönnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [PubMed] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.W.; Tan, X.; Sun, J.Y.; Gu, C.M.; Liu, C.; Guo, X. Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS ONE 2019, 14, e0217090. [Google Scholar]
- Lee, W.; Lee, Y.; Jeong, G.S.; Ku, S.K.; Bae, J.S. Cudratricusxanthone A attenuates renal injury in septic mice. Food Chem. Toxicol. 2017, 106, 404–410. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, G.S.; Kim, K.M.; Lee, W.; Bae, J.S. Cudratricusxanthone A attenuates sepsis-induced liver injury via SIRT1 signaling. J. Cell. Physiol. 2018, 233, 5441–5446. [Google Scholar] [CrossRef]
- Liu, W.; Li, D.; Cao, H.; Li, H.; Wang, Y. Expansion and inflammation of white adipose tissue-focusing on adipocyte progenitors. Biol. Chem. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-S.; Jeong, G.-S.; Jang, B.-C. Cudratricusxanthone A Inhibits Lipid Accumulation and Expression of Inducible Nitric Oxide Synthase in 3T3-L1 Preadipocytes. Int. J. Mol. Sci. 2021, 22, 505. https://doi.org/10.3390/ijms22020505
Kwon H-S, Jeong G-S, Jang B-C. Cudratricusxanthone A Inhibits Lipid Accumulation and Expression of Inducible Nitric Oxide Synthase in 3T3-L1 Preadipocytes. International Journal of Molecular Sciences. 2021; 22(2):505. https://doi.org/10.3390/ijms22020505
Chicago/Turabian StyleKwon, Hyo-Shin, Gil-Saeng Jeong, and Byeong-Churl Jang. 2021. "Cudratricusxanthone A Inhibits Lipid Accumulation and Expression of Inducible Nitric Oxide Synthase in 3T3-L1 Preadipocytes" International Journal of Molecular Sciences 22, no. 2: 505. https://doi.org/10.3390/ijms22020505





