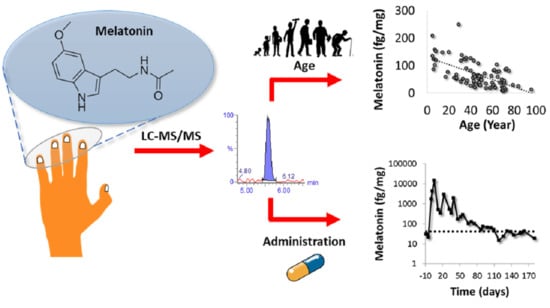
Nail Melatonin Content: A Suitable Non-Invasive Marker of Melatonin Production
Abstract
:1. Introduction
2. Results
2.1. Method Development
2.2. Method Validation
2.2.1. Validation Using Melatonin-Free Nails
2.2.2. Validation Using Real Nail Matrix
2.3. Method Application
2.3.1. Establishment of Endogenous Levels of Fingernail Melatonin
2.3.2. Potential of Toenails for Melatonin Determination
2.3.3. Effect of Exogenous Melatonin Administration in the Fingernail’s Melatonin Levels
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Instrumentation
4.3. Sample Treatment
4.4. Preparation of Melatonin-Free Nails
4.5. Method Validation
4.5.1. Method Validation Using Melatonin-Free Nails
4.5.2. Method Validation Using Real Nail Matrix
4.6. Method Application
4.7. Samples
4.7.1. Method Validation
4.7.2. Method Application
Endogenous Melatonin Levels in Fingernails
Comparison of Melatonin Levels between Fingernails and Toenails
Effect of Melatonin Administration in Melatonin Fingernails’ Levels
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLMO | Dim light melatonin onset |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| LOD | Limit of detection |
| CV | Coefficient of variation |
| CDER | Center for Drug Evaluation and Research |
| SRM | Selected reaction monitoring |
| QC | Melatonin-free nails spiked at different levels |
| S/N | Signal to noise ratio |
References
- Kennaway, D.J. Measuring melatonin by immunoassay. J. Pineal Res. 2020, 69, e12657. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C. Melatonin: A potent endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Barrenetxe, J.; Delagrange, P.; Martínez, J.A. Physiological and metabolic functions of melatonin. J. Physiol. Biochem. 2004, 60, 61–72. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olcese, J. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlomagno, G.; Minini, M.; Tilotta, M. Vittorio Vittorio Unfer for The Experts Group on Inositol in Basic and Clinical Research From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skene, D.; Vivien-Roels, B.; Sparks, D.; Hunsaker, J.; Pévet, P.; Ravid, D.; Swaab, D. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: Effect of age and Alzheimer’s disease. Brain Res. 1990, 528, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K.; Okamoto, N.; Ohara, K.; Morita, Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996, 717, 154–159. [Google Scholar] [CrossRef]
- Aoki, M.; Yokota, Y.; Hayashi, T.; Kuze, B.; Murai, M.; Mizuta, K.; Ito, Y. Disorder of the saliva melatonin circadian rhythm in patients with Meniere’s disease. Acta Neurol. Scand. 2006, 113, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Shen, G.; Yin, S.; Xu, W.; Hu, B. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Adv. Ther. 2009, 26, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Grin, W.; Grünberger, W. A significant correlation between melatonin deficiency and endometrial cancer. Gynecol. Obstet. Investig. 1998, 45, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Stumpf, I.; Bazwinsky, I.; Litvak, L.; Dralle, H.; Mühlbauer, E. Melatonin and type 2 diabetes ? a possible link? J. Pineal Res. 2007, 42, 350–358. [Google Scholar] [CrossRef]
- Hardeland, R. Neurobiology, Pathophysiology, and Treatment of Melatonin Deficiency and Dysfunction. Sci. World J. 2012, 2012, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Puy, H.; Deybach, J.-C.; Baudry, P.; Callebert, J.; Touitou, Y.; Nordmann, Y. Decreased nocturnal plasma melatonin levels in patients with recurrent acute intermittent porphyria attacks. Life Sci. 1993, 53, 621–627. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Reiter, R.J. Melatonin therapy in fibromyalgia. J. Pineal Res. 2005, 40, 98–99. [Google Scholar] [CrossRef]
- Monteleone, P.; Martiadis, V.; Maj, M. Circadian rhythms and treatment implications in depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 1569–1574. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef]
- Armstrong, S.; Redman, J. Melatonin: A chronobiotic with anti-aging properties? Med. Hypotheses 1991, 34, 300–309. [Google Scholar] [CrossRef]
- Karasek, M.; Reiter, R.J. Melatonin and aging. Neuro Endocrinol. Lett. 2002, 23, 14–16. [Google Scholar]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef] [PubMed]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, E.A.; Di Mascio, P.; Harumi, T.; Spence, D.W.; Moscovitch, A.; Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Measurement of melatonin in body fluids: Standards, protocols and procedures. Child’s Nerv. Syst. 2011, 27, 879–891. [Google Scholar] [CrossRef] [Green Version]
- Kennaway, D.J. A critical review of melatonin assays: Past and present. J. Pineal Res. 2019, 67, e12572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockley, S.W. Journal of Pineal Research guideline for authors: Measuring melatonin in humans. J. Pineal Res. 2020, 69, e12664. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Cook, M.R.; Kavet, R.; Sastre, A.; Smith, D.K. Prediction of nocturnal plasma melatonin from morning urinary measures. J. Pineal Res. 1998, 24, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Saksvik-Lehouillier, I.; Harrison, S.L.; Marshall, L.M.; Tranah, G.J.; Ensrud, K.; Ancoli-Israel, S.; Clemons, A.; Redline, S.; Stone, K.L.; Schernhammer, E.S.; et al. Association of Urinary 6-Sulfatoxymelatonin (aMT6s) Levels and Objective and Subjective Sleep Measures in Older Men: The MrOS Sleep Study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2015, 70, 1569–1577. [Google Scholar] [CrossRef]
- Stebelova, K.; Roska, J.; Zeman, M. Impact of Dim Light at Night on Urinary 6-Sulphatoxymelatonin Concentrations and Sleep in Healthy Humans. Int. J. Mol. Sci. 2020, 21, 7736. [Google Scholar] [CrossRef]
- Palmeri, A.; Pichini, S.; Pacifici, R.; Zuccaro, P.; Lopez, A. Drugs in Nails. Clin. Pharmacokinet. 2000, 38, 95–110. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, K.; Xu, P.; Xue, J.; Xu, X.; Liu, L. Associations of perceived stress with the present and subsequent cortisol levels in fingernails among medical students: A prospective pilot study. Psychol. Res. Behav. Manag. 2018, 11, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, I.; Negrusz, A.; Jones, J.; Lewis, U. Detection of Drugs in Nails: Three Year Experience. J. Anal. Toxicol. 2015, 39, 624–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitku, J.; Skodova, T.; Sosvorova, L.; Hampl, R.; Hill, M.; Heracek, J.; Bicikova, M.; Stárka, L. Development and validation of LC–MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 2015, 140, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Kellermann, G. Simultaneous determination of three estrogens in human saliva without derivatization or liquid-liquid extraction for routine testing via miniaturized solid phase extraction with LC-MS/MS detection. Talanta 2018, 178, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.J.; Wyndham, E.L.; Burrows, G.D.; Norman, T.R. The effect of gender on the melatonin suppression by light: A dose response relationship. J. Neural Transm. 2000, 107, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Shechter, A.; Boudreau, P.; Begum, E.A.; Ying-Kin, N.M.K.N. Diurnal and circadian variation of sleep and alertness in men vs. naturally cycling women. Proc. Natl. Acad. Sci. USA 2016, 113, 10980–10985. [Google Scholar] [CrossRef] [Green Version]
- Mahlberg, R.; Tilmann, A.; Salewski, L.; Kunz, D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology 2006, 31, 634–641. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Lushington, K.; Dawson, D.; Lack, L.; Heuvel, C.V.D.; Rogers, N. Urinary 6-sulfatoxymelatonin excretion and aging: New results and a critical review of the literature. J. Pineal Res. 1999, 27, 210–220. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human Melatonin Production Decreases With Age. J. Pineal Res. 1986, 3, 379–388. [Google Scholar] [CrossRef]
- Wetterberg, L.; Bergiannaki, J.D.; Paparrigopoulos, T.; Von Knorring, L.; Eberhard, G.; Bratlid, T.; Yuwiler, A. Normative melatonin excretion: A multinational study. Psychoneuroendocrinology 1999, 24, 209–226. [Google Scholar] [CrossRef]
- Skene, D. Melatonin rhythmicity: Effect of age and Alzheimer’s disease. Exp. Gerontol. 2003, 38, 199–206. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Swaab, D.F. The human pineal gland and melatonin in aging and Alzheimer’s disease. J. Pineal Res. 2005, 38, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Konturek, S.J. Melatonin and aging: Prospects for human treatment. J. Physiol. Pharmacol. 2011, 62, 13–19. [Google Scholar] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative Stress in Aging-Matters of the Heart and Mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [Green Version]
- Shekhidem, H.A.; Sharvit, L.; Leman, E.; Manov, I.; Roichman, A.; Holtze, S.; Huffman, D.M.; Cohen, H.Y.; Hildebrandt, T.; Shams, I.; et al. Telomeres and Longevity: A Cause or an Effect? Int. J. Mol. Sci. 2019, 20, 3233. [Google Scholar] [CrossRef] [Green Version]
- Webb, M.; Sideris, D.P. Intimate Relations—Mitochondria and Ageing. Int. J. Mol. Sci. 2020, 21, 7580. [Google Scholar] [CrossRef]
- Ellington, E. Chronic Nail Biting in Youth. J. Psychosoc. Nurs. Ment. Health Serv. 2017, 55, 23–26. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, C.; Zhang, J.; Wang, Z. A UHPLC-MS/MS method for profiling multifunctional steroids in human hair. Anal. Bioanal. Chem. 2017, 409, 4751–4769. [Google Scholar] [CrossRef]
- Palliyil, B.B.; Li, C.; Owaisat, S.; Lebo, D.B. Lateral Drug Diffusion in Human Nails. Ageing Int. 2014, 15, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Saner, M.V.; Kulkarni, A.D.; Pardeshi, C.V. Insights into drug delivery across the nail plate barrier. J. Drug Target. 2014, 22, 769–789. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res. 2012, 56, 17252. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on bioanalytical method validation. EMA Guidel. 2012, 44, 1–23. [Google Scholar]
- Olesti, E.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Ramaekers, J.G.; de la Torre, R.; Pozo, O.J. Quantification of endogenous neurotransmitters and related compounds by liquid chromatography coupled to tandem mass spectrometry. Talanta 2019, 192, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, A.; Miranda, J.; Feixas, G.; Betegon, A.A.; Crispi, F.; Gratacós, E.; Pozo, O.J. Determination of the steroid profile in alternative matrices by liquid chromatography tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2020, 197, 105520. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, A.; Gomez-Gomez, A.; Fabregat, A.; Segura, J.; Yang, S.; Xing, Y.; Moutian, W.; Marcos, J.; Joglar, J.; Ventura, R.; et al. Evaluation of markers out of the steroid profile for the screening of testosterone misuse. Part I: Transdermal administration. Drug Test. Anal. 2017, 10, 821–831. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Gomez-Gomez, A.; Fabregat, A.; Segura, J.; Yang, S.; Xing, Y.; Moutian, W.; Marcos, J.; Joglar, J.; Ventura, R.; et al. Evaluation of markers out of the steroid profile for the screening of testosterone misuse. Part II: Intramuscular administration. Drug Test. Anal. 2017, 10, 849–859. [Google Scholar] [CrossRef]




| Melatonin-Free Matrix (CDER Regulations) | ||||||
|---|---|---|---|---|---|---|
| Linearity | Weight. | Range (fg/mg) | ||||
| 1/x | 6.5–830 | |||||
| LLOQ | LQC | MQC | HQC | LOD | ||
| Level | 6.5 | 13 | 0.104 | 830 | 3.5 | |
| Acc. (%) | 113 | 115 | 104 | 103 | - | |
| CV (%) | 8 | 6 | 4 | 4 | - | |
| Real nail matrix (Standard additions) | ||||||
| Within-run (n = 6) | Total (n = 12) | M.E. (n = 6) | Endog Range | |||
| Acc. (%) | 100 | 104 | Rec. (%) | 105 | Conc. | 30–110 |
| CV (%) | 10 | 13 | CV (%) | 7 | (fg/mg) | |
| Specificity | ||||||
| Standard | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| Q/q ratio | 0.22 | 0.23 | 0.21 | 0.24 | 0.23 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Gomez, A.; Montero-San-Martin, B.; Haro, N.; Pozo, O.J. Nail Melatonin Content: A Suitable Non-Invasive Marker of Melatonin Production. Int. J. Mol. Sci. 2021, 22, 921. https://doi.org/10.3390/ijms22020921
Gomez-Gomez A, Montero-San-Martin B, Haro N, Pozo OJ. Nail Melatonin Content: A Suitable Non-Invasive Marker of Melatonin Production. International Journal of Molecular Sciences. 2021; 22(2):921. https://doi.org/10.3390/ijms22020921
Chicago/Turabian StyleGomez-Gomez, Alex, Blanca Montero-San-Martin, Noemí Haro, and Oscar J. Pozo. 2021. "Nail Melatonin Content: A Suitable Non-Invasive Marker of Melatonin Production" International Journal of Molecular Sciences 22, no. 2: 921. https://doi.org/10.3390/ijms22020921