The Spatiotemporal Coupling: Regional Energy Failure and Aberrant Proteins in Neurodegenerative Diseases
Abstract
:1. Introduction
2. The Region-Specific Metabolic Coupling
3. An Integrated Model of Synaptic Plasticity
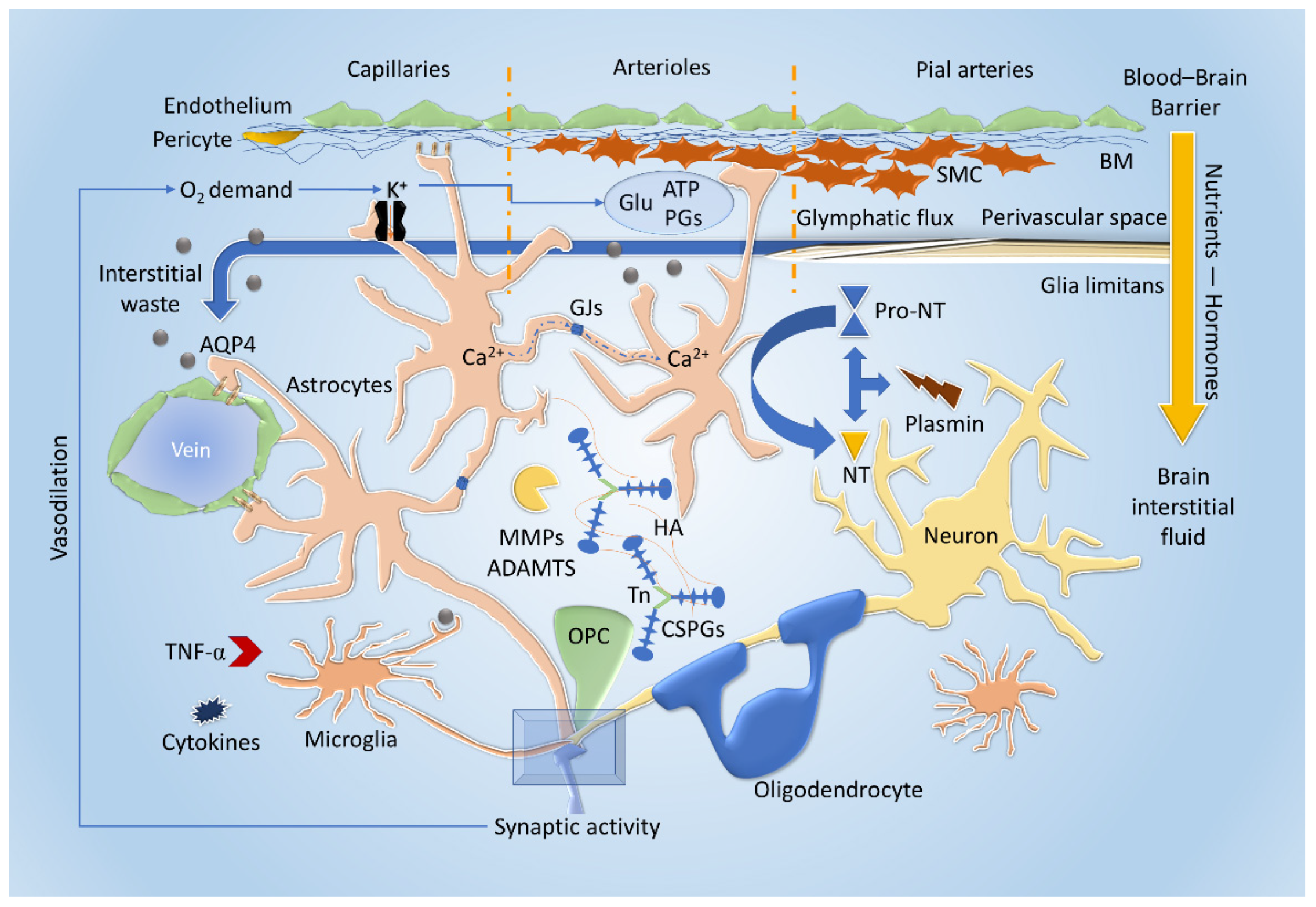
3.1. Cellular and Extracellular Interactions
3.2. The Emergent Roles of the Extracellular Matrix and the Neurovascular Unit
4. The Burdensome Variable Energy Key Issue
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, I.R.; Harel, D. Explaining a Complex Living System: Dynamics, Multi-Scaling and Emergence. J. R. Soc. Interface 2007, 4, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.; Papa, M. Matrix Metalloproteinases, Neural Extracellular Matrix, and Central Nervous System Pathology. Prog. Mol. Biol. Transl. Sci. 2017, 148, 167–202. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Virtuoso, A.; Alberghina, L.; Papa, M. Neurons, Glia, Extracellular Matrix and Neurovascular Unit: A Systems Biology Approach to the Complexity of Synaptic Plasticity in Health and Disease. Int. J. Mol. Sci. 2020, 21, 1539. [Google Scholar] [CrossRef] [Green Version]
- Grothe, M.J.; Sepulcre, J.; Gonzalez-Escamilla, G.; Jelistratova, I.; Schöll, M.; Hansson, O.; Teipel, S.J. Alzheimer’s Disease Neuroimaging Initiative Molecular Properties Underlying Regional Vulnerability to Alzheimer’s Disease Pathology. Brain 2018, 141, 2755–2771. [Google Scholar] [CrossRef] [PubMed]
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A.V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-Synuclein Oligomers Interact with ATP Synthase and Open the Permeability Transition Pore in Parkinson’s Disease. Nat. Commun. 2018, 9, 2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragnolini, A.B.; Lampitella, G.; Virtuoso, A.; Viscovo, I.; Panetsos, F.; Papa, M.; Cirillo, G. Regional Brain Susceptibility to Neurodegeneration: What Is the Role of Glial Cells? Neural Regen. Res. 2020, 15, 838–842. [Google Scholar] [CrossRef]
- Vlassenko, A.G.; Raichle, M.E. Brain Aerobic Glycolysis Functions and Alzheimer’s Disease. Clin. Transl. Imaging 2015, 3, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Qiang, W.; Yau, W.-M.; Lu, J.-X.; Collinge, J.; Tycko, R. Structural Variation in Amyloid-β Fibrils from Alzheimer’s Disease Clinical Subtypes. Nature 2017, 541, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Bodis-Wollner, I. Visual Electrophysiology in Parkinson’s Disease: PERG, VEP and Visual P300. Clin. Electroencephalogr. 1997, 28, 143–147. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Gori, S.; Mazzucchi, S.; Dini, E.; Cafalli, M.; Siciliano, G.; Papa, M.; Baldacci, F. Supersaturation of VEP in Migraine without Aura Patients Treated with Topiramate: An Anatomo-Functional Biomarker of the Disease. J. Clin. Med. 2021, 10, 769. [Google Scholar] [CrossRef]
- Yener, G.G.; Başar, E. Biomarkers in Alzheimer’s Disease with a Special Emphasis on Event-Related Oscillatory Responses. Suppl. Clin. Neurophysiol. 2013, 62, 237–273. [Google Scholar] [CrossRef]
- Chen, J.J. Functional MRI of Brain Physiology in Aging and Neurodegenerative Diseases. Neuroimage 2019, 187, 209–225. [Google Scholar] [CrossRef]
- Ovsepian, S.V.; O’Leary, V.B. Neuronal Activity and Amyloid Plaque Pathology: An Update. J. Alzheimer’s Dis. 2016, 49, 13–19. [Google Scholar] [CrossRef]
- Martorana, F.; Foti, M.; Virtuoso, A.; Gaglio, D.; Aprea, F.; Latronico, T.; Rossano, R.; Riccio, P.; Papa, M.; Alberghina, L.; et al. Differential Modulation of NF-ΚB in Neurons and Astrocytes Underlies Neuroprotection and Antigliosis Activity of Natural Antioxidant Molecules. Oxidative Med. Cell. Longev. 2019, 2019, e8056904. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Gathagan, R.J.; Covell, D.J.; Medellin, C.; Stieber, A.; Robinson, J.L.; Zhang, B.; Pitkin, R.M.; Olufemi, M.F.; Luk, K.C.; et al. Cellular Milieu Imparts Distinct Pathological α-Synuclein Strains in α-Synucleinopathies. Nature 2018, 557, 558–563. [Google Scholar] [CrossRef]
- Braak, H.; Sastre, M.; Del Tredici, K. Development of α-Synuclein Immunoreactive Astrocytes in the Forebrain Parallels Stages of Intraneuronal Pathology in Sporadic Parkinson’s Disease. Acta Neuropathol. 2007, 114, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Siddoway, B.; Kaeser, G.E.; Segota, I.; Rivera, R.; Romanow, W.J.; Liu, C.S.; Park, C.; Kennedy, G.; Long, T.; et al. Somatic APP Gene Recombination in Alzheimer’s Disease and Normal Neurons. Nature 2018, 563, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Alegre-Abarrategui, J.; Brimblecombe, K.R.; Roberts, R.F.; Velentza-Almpani, E.; Tilley, B.S.; Bengoa-Vergniory, N.; Proukakis, C. Selective Vulnerability in α-Synucleinopathies. Acta Neuropathol. 2019, 138, 681–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguet, F.; Brown, A.A.; Castel, S.E.; Davis, J.R.; He, Y.; Jo, B.; Mohammadi, P.; Park, Y.; Parsana, P.; Segrè, A.V.; et al. Genetic Effects on Gene Expression across Human Tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef]
- Cykowski, M.D.; Coon, E.A.; Powell, S.Z.; Jenkins, S.M.; Benarroch, E.E.; Low, P.A.; Schmeichel, A.M.; Parisi, J.E. Expanding the Spectrum of Neuronal Pathology in Multiple System Atrophy. Brain 2015, 138, 2293–2309. [Google Scholar] [CrossRef]
- Post, M.R.; Lieberman, O.J.; Mosharov, E.V. Can Interactions Between α-Synuclein, Dopamine and Calcium Explain Selective Neurodegeneration in Parkinson’s Disease? Front. Neurosci. 2018, 12, 161. [Google Scholar] [CrossRef]
- Surmeier, D.J. Determinants of Dopaminergic Neuron Loss in Parkinson’s Disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, G.; Cirillo, M.; Panetsos, F.; Virtuoso, A.; Papa, M. Selective Vulnerability of Basal Ganglia: Insights into the Mechanisms of Bilateral Striatal Necrosis. J. Neuropathol. Exp. Neurol. 2019, 78, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Van de Haar, H.J.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.M.; Burgmans, S.; Verhey, F.R.J.; Backes, W.H. Neurovascular Unit Impairment in Early Alzheimer’s Disease Measured with Magnetic Resonance Imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef]
- Van de Haar, H.J.; Burgmans, S.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Hofman, P.A.M.; Verhey, F.R.J.; Backes, W.H. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology 2016, 281, 527–535. [Google Scholar] [CrossRef]
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 Leads to Blood-Brain Barrier Dysfunction Predicting Cognitive Decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hafezi-Moghadam, A.; Thomas, K.L.; Wagner, D.D. ApoE Deficiency Leads to a Progressive Age-Dependent Blood-Brain Barrier Leakage. Am. J. Physiol. Cell Physiol. 2007, 292, C1256–C1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lontay, B.; Kiss, A.; Virág, L.; Tar, K. How Do Post-Translational Modifications Influence the Pathomechanistic Landscape of Huntington’s Disease? A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 4282. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, N.M.; Bordelon, Y.; Brickman, A.M.; Angulo, S.; Khan, U.; Muraskin, J.; Griffith, E.Y.; Wasserman, P.; Menalled, L.; Vonsattel, J.P.; et al. Regional Vulnerability in Huntington’s Disease: FMRI-Guided Molecular Analysis in Patients and a Mouse Model of Disease. Neurobiol. Dis. 2013, 52, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mink, J.W.; Blumenschine, R.J.; Adams, D.B. Ratio of Central Nervous System to Body Metabolism in Vertebrates: Its Constancy and Functional Basis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1981, 241, R203–R212. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain Energy Rescue: An Emerging Therapeutic Concept for Neurodegenerative Disorders of Ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic Energy Use and Supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieraszko, A. Changes in the Hippocampal Slices Energy Metabolism Following Stimulation and Long-Term Potentiation of Schaffer Collaterals-Pyramidal Cell Synapses Tested with the 2-Deoxyglucose Technique. Brain Res. 1982, 237, 449–457. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellerin, L.; Magistretti, P.J. Glutamate Uptake into Astrocytes Stimulates Aerobic Glycolysis: A Mechanism Coupling Neuronal Activity to Glucose Utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calì, C.; Tauffenberger, A.; Magistretti, P. The Strategic Location of Glycogen and Lactate: From Body Energy Reserve to Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, A.M.; Cirillo, G.; Lavitrano, M.L.; Alberghina, L.; Papa, M. Targeting Reactive Astrogliosis by Novel Biotechnological Strategies. Biotechnol. Adv. 2012, 30, 261–271. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Eroglu, C. The Interplay between Neurons and Glia in Synapse Development and Plasticity. Curr. Opin. Neurobiol. 2017, 42, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic Structural Plasticity and Neuropsychiatric Disease. Nat. Rev. Neurosci. 2018, 19, 215–234. [Google Scholar] [CrossRef]
- Zuo, Y.; Lin, A.; Chang, P.; Gan, W.-B. Development of Long-Term Dendritic Spine Stability in Diverse Regions of Cerebral Cortex. Neuron 2005, 46, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Caroni, P.; Donato, F.; Muller, D. Structural Plasticity upon Learning: Regulation and Functions. Nat. Rev. Neurosci. 2012, 13, 478–490. [Google Scholar] [CrossRef] [Green Version]
- Zenke, F.; Gerstner, W.; Ganguli, S. The Temporal Paradox of Hebbian Learning and Homeostatic Plasticity. Curr. Opin. Neurobiol. 2017, 43, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Turrigiano, G. Homeostatic Synaptic Plasticity: Local and Global Mechanisms for Stabilizing Neuronal Function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef] [Green Version]
- Herring, B.E.; Nicoll, R.A. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu. Rev. Physiol. 2016, 78, 351–365. [Google Scholar] [CrossRef]
- Grinman, E.; Nakahata, Y.; Avchalumov, Y.; Espadas, I.; Swarnkar, S.; Yasuda, R.; Puthanveettil, S.V. Activity-Regulated Synaptic Targeting of LncRNA ADEPTR Mediates Structural Plasticity by Localizing Sptn1 and AnkB in Dendrites. Sci. Adv. 2021, 7, eabf0605. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; Aylor, K.W.; Barrett, E.J. Unravelling the Regulation of Insulin Transport across the Brain Endothelial Cell. Diabetologia 2017, 60, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Gordon, G.R.J.; Choi, H.B.; Rungta, R.L.; Ellis-Davies, G.C.R.; MacVicar, B.A. Brain Metabolism Dictates the Polarity of Astrocyte Control over Arterioles. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Blanco, V.M.; Stern, J.E.; Filosa, J.A. Tone-Dependent Vascular Responses to Astrocyte-Derived Signals. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H2855–H2863. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Park, L.; Anrather, J.; Zhou, P.; Iadecola, C. Cerebrovascular Nitrosative Stress Mediates Neurovascular and Endothelial Dysfunction Induced by Angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and Neuronal Control of Brain Blood Flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A. Binaural Blood Flow Control by Astrocytes: Listening to Synapses and the Vasculature. J. Physiol. 2017, 595, 1885–1902. [Google Scholar] [CrossRef]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V.; Meredith, A.L.; Wilkerson, M.K.; Aldrich, R.W.; Nelson, M.T. Local Potassium Signaling Couples Neuronal Activity to Vasodilation in the Brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- McCaslin, A.F.H.; Chen, B.R.; Radosevich, A.J.; Cauli, B.; Hillman, E.M.C. In Vivo 3D Morphology of Astrocyte—Vasculature Interactions in the Somatosensory Cortex: Implications for Neurovascular Coupling. J. Cereb. Blood Flow Metab. 2011, 31, 795–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The Perivascular Astroglial Sheath Provides a Complete Covering of the Brain Microvessels: An Electron Microscopic 3D Reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Meijer, R.I.; Gray, S.M.; Aylor, K.W.; Barrett, E.J. Pathways for Insulin Access to the Brain: The Role of the Microvascular Endothelial Cell. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H1132–H1138. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Sakaguchi, M.; Lockhart, S.M.; Cai, W.; Li, M.E.; Homan, E.P.; Rask-Madsen, C.; Kahn, C.R. Endothelial Insulin Receptors Differentially Control Insulin Signaling Kinetics in Peripheral Tissues and Brain of Mice. Proc. Natl. Acad. Sci. USA 2017, 114, E8478–E8487. [Google Scholar] [CrossRef] [Green Version]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi-Montalcini, R. The Nerve Growth Factor 35 Years Later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef]
- Alberghina, L.; Colangelo, A.M. The Modular Systems Biology Approach to Investigate the Control of Apoptosis in Alzheimer’s Disease Neurodegeneration. BMC Neurosci. 2006, 7, S2. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B. Autophagy in Mammalian Development and Differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.A.D.; Rabin, S.J.; Colangelo, A.M.; Brooker, G.; Mocchetti, I. TrkA Mediates the Nerve Growth Factor-Induced Intracellular Calcium Accumulation (∗). J. Biol. Chem. 1996, 271, 6092–6098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, P.M.; Zucker, R.S.; Bentley, D. Induction of Filopodia by Direct Local Elevation of Intracellular Calcium Ion Concentration. J. Cell Biol. 1999, 145, 1265–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New Roles for the Synaptic Stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 2018, 19, 318. [Google Scholar] [CrossRef] [Green Version]
- Weinhard, L.; di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia Remodel Synapses by Presynaptic Trogocytosis and Spine Head Filopodia Induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef] [Green Version]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2016, 8, a020453. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor Skill Learning Requires Active Central Myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Asi, Y.T.; Simpson, J.E.; Heath, P.R.; Wharton, S.B.; Lees, A.J.; Revesz, T.; Houlden, H.; Holton, J.L. Alpha-Synuclein MRNA Expression in Oligodendrocytes in MSA. Glia 2014, 62, 964–970. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.; Virtuoso, A.; Maggio, N.; Papa, M. Neuro-Coagulopathy: Blood Coagulation Factors in Central Nervous System Diseases. Int. J. Mol. Sci. 2017, 18, 2128. [Google Scholar] [CrossRef] [PubMed]
- Liauw, J.; Hoang, S.; Choi, M.; Eroglu, C.; Choi, M.; Sun, G.; Percy, M.; Wildman-Tobriner, B.; Bliss, T.; Guzman, R.G.; et al. Thrombospondins 1 and 2 Are Necessary for Synaptic Plasticity and Functional Recovery after Stroke. J. Cereb. Blood Flow Metab. 2008, 28, 1722–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, S.; Kitagawa, H. Formation and Remodeling of the Brain Extracellular Matrix in Neural Plasticity: Roles of Chondroitin Sulfate and Hyaluronan. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.; Gottschling, C.; Aguado, A.; Rauch, U.; Wetzel, C.H.; Hatt, H.; Faissner, A. Primary Hippocampal Neurons, Which Lack Four Crucial Extracellular Matrix Molecules, Display Abnormalities of Synaptic Structure and Function and Severe Deficits in Perineuronal Net Formation. J. Neurosci. 2013, 33, 7742–7755. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System. Front. Cell. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirillo, G.; Colangelo, A.M.; Bianco, M.R.; Cavaliere, C.; Zaccaro, L.; Sarmientos, P.; Alberghina, L.; Papa, M. BB14, a Nerve Growth Factor (NGF)-like Peptide Shown to Be Effective in Reducing Reactive Astrogliosis and Restoring Synaptic Homeostasis in a Rat Model of Peripheral Nerve Injury. Biotechnol. Adv. 2012, 30, 223–232. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central Nervous System Pericytes in Health and Disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Faissner, A.; Roll, L.; Theocharidis, U. Tenascin-C in the Matrisome of Neural Stem and Progenitor Cells. Mol. Cell. Neurosci. 2017, 81, 22–31. [Google Scholar] [CrossRef]
- Andrews, M.R.; Soleman, S.; Cheah, M.; Tumbarello, D.A.; Mason, M.R.J.; Moloney, E.; Verhaagen, J.; Bensadoun, J.-C.; Schneider, B.; Aebischer, P.; et al. Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent. eNeuro 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Zenaro, E.; Pietronigro, E.; Bianca, V.D.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils Promote Alzheimer’s Disease—like Pathology and Cognitive Decline via LFA-1 Integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, C.; Baldacci, F.; Mazzucchi, S.; Lombardo, I.; Curto, L.; Ulivi, M.; Chico, L.; Papa, M.; Siciliano, G.; Gori, S. CGRP Inhibitors and Oxidative Stress Biomarkers in Resistant Migraine: A Real-Life Study with Erenumab, Fremanezumab, and Galcanezumab. J. Clin. Med. 2021, 10, 4586. [Google Scholar] [CrossRef] [PubMed]
- Dini, E.; Mazzucchi, S.; De Luca, C.; Cafalli, M.; Chico, L.; Lo Gerfo, A.; Siciliano, G.; Bonuccelli, U.; Baldacci, F.; Gori, S. Plasma Levels of Oxidative Stress Markers, before and after BoNT/A Treatment, in Chronic Migraine. Toxins 2019, 11, 608. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, A.A.; Lee, D.Y.; Datta, R.; Hauser, M.; Budworth, H.; Holt, A.; Mihalik, S.; Goldschmidt, P.; Frankel, K.; Trego, K.; et al. Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice. Cell Metab. 2019, 29, 1258–1273.e11. [Google Scholar] [CrossRef]
- Papa, M.; Sellitti, S.; Sadile, A.G. Remodeling of Neural Networks in the Anterior Forebrain of an Animal Model of Hyperactivity and Attention Deficits as Monitored by Molecular Imaging Probes. Neurosci. Biobehav. Rev. 2000, 24, 149–156. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Bush, T.G.; Puvanachandra, N.; Horner, C.H.; Polito, A.; Ostenfeld, T.; Svendsen, C.N.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Leukocyte Infiltration, Neuronal Degeneration, and Neurite Outgrowth after Ablation of Scar-Forming, Reactive Astrocytes in Adult Transgenic Mice. Neuron 1999, 23, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional Ablation of Stat3 or Socs3 Discloses a Dual Role for Reactive Astrocytes after Spinal Cord Injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Hamby, M.E.; Coppola, G.; Ao, Y.; Geschwind, D.H.; Khakh, B.S.; Sofroniew, M.V. Inflammatory Mediators Alter the Astrocyte Transcriptome and Calcium Signaling Elicited by Multiple G-Protein-Coupled Receptors. J. Neurosci. 2012, 32, 14489–14510. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [Green Version]
- Motori, E.; Puyal, J.; Toni, N.; Ghanem, A.; Angeloni, C.; Malaguti, M.; Cantelli-Forti, G.; Berninger, B.; Conzelmann, K.-K.; Götz, M.; et al. Inflammation-Induced Alteration of Astrocyte Mitochondrial Dynamics Requires Autophagy for Mitochondrial Network Maintenance. Cell Metab. 2013, 18, 844–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Klionsky, D.J. Mitochondria Removal by Autophagy. Autophagy 2011, 7, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, S.M.; Singh, K.; Ritchie, T.M.; Aguiar, J.A.; Fan, I.Y.; Portillo, A.L.; Rojas, E.A.; Vahedi, F.; El-Sayes, A.; Xing, S.; et al. Metabolic Flexibility Determines Human NK Cell Functional Fate in the Tumor Microenvironment. Cell Metab. 2021, 33, 1205–1220.e5. [Google Scholar] [CrossRef] [PubMed]
- Virtuoso, A.; Giovannoni, R.; De Luca, C.; Gargano, F.; Cerasuolo, M.; Maggio, N.; Lavitrano, M.; Papa, M. The Glioblastoma Microenvironment: Morphology, Metabolism, and Molecular Signature of Glial Dynamics to Discover Metabolic Rewiring Sequence. Int. J. Mol. Sci. 2021, 22, 3301. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtuoso, A.; Colangelo, A.M.; Maggio, N.; Fennig, U.; Weinberg, N.; Papa, M.; De Luca, C. The Spatiotemporal Coupling: Regional Energy Failure and Aberrant Proteins in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 11304. https://doi.org/10.3390/ijms222111304
Virtuoso A, Colangelo AM, Maggio N, Fennig U, Weinberg N, Papa M, De Luca C. The Spatiotemporal Coupling: Regional Energy Failure and Aberrant Proteins in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2021; 22(21):11304. https://doi.org/10.3390/ijms222111304
Chicago/Turabian StyleVirtuoso, Assunta, Anna Maria Colangelo, Nicola Maggio, Uri Fennig, Nitai Weinberg, Michele Papa, and Ciro De Luca. 2021. "The Spatiotemporal Coupling: Regional Energy Failure and Aberrant Proteins in Neurodegenerative Diseases" International Journal of Molecular Sciences 22, no. 21: 11304. https://doi.org/10.3390/ijms222111304





