Tenofovir Modulates Semaphorin 4D Signaling and Regulates Bone Homeostasis, Which Can Be Counteracted by Dipyridamole and Adenosine A2A Receptor
Abstract
:1. Introduction
2. Results
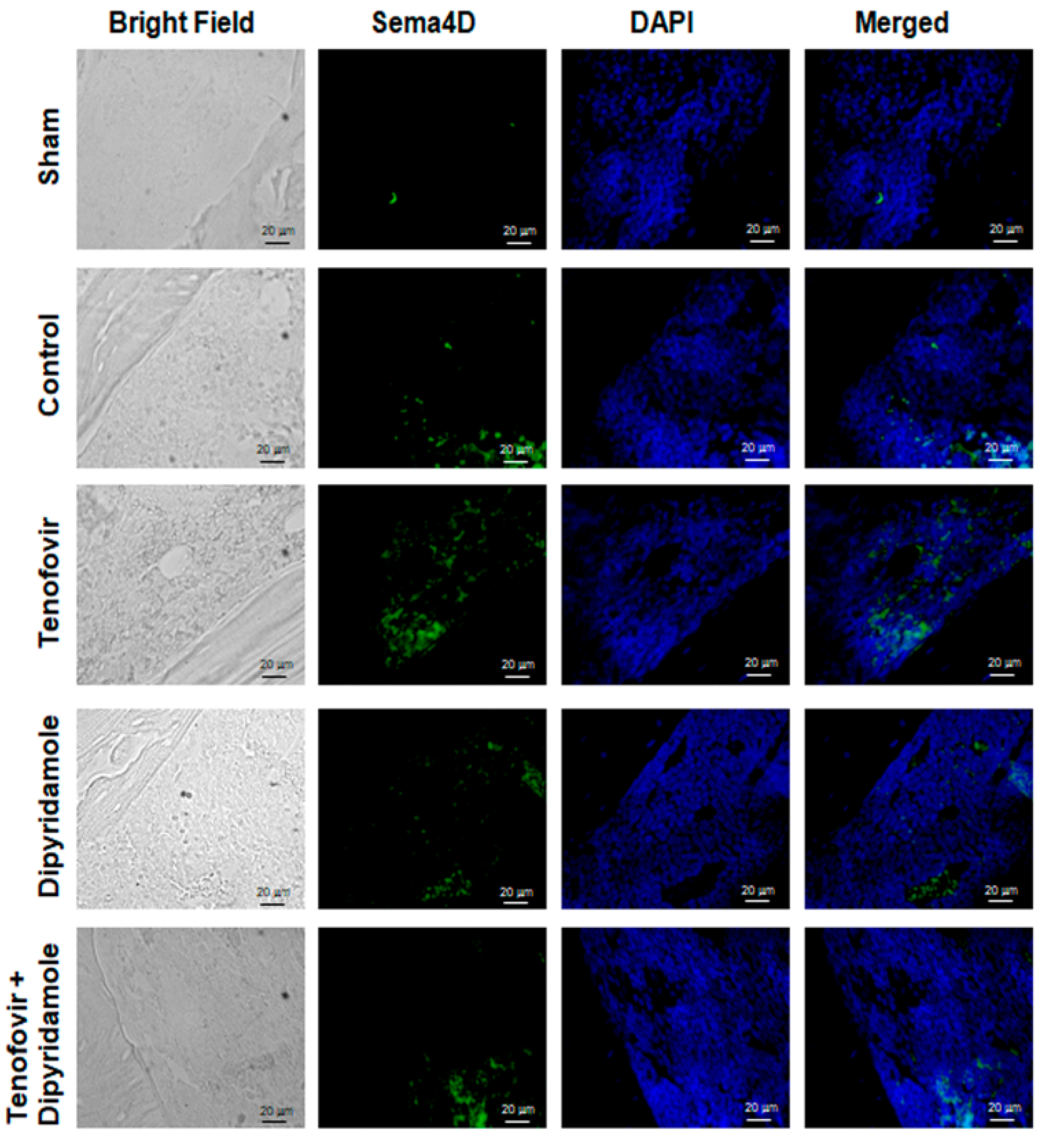
2.1. Mice Treated with Tenofovir Showed an Increased Expression of Sema4D
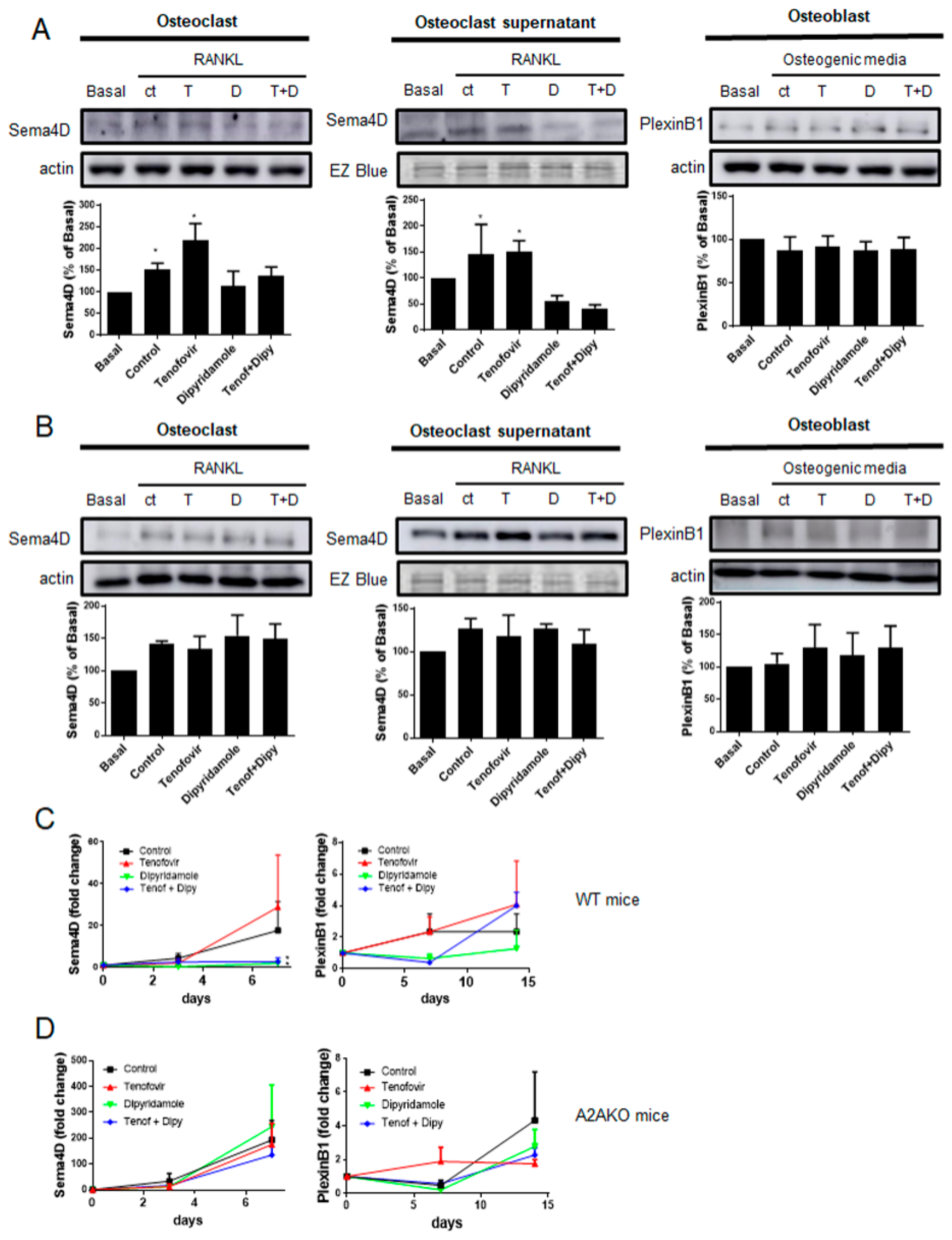
2.2. Tenofovir Increased Sema4D Expression and Secretion In Vitro
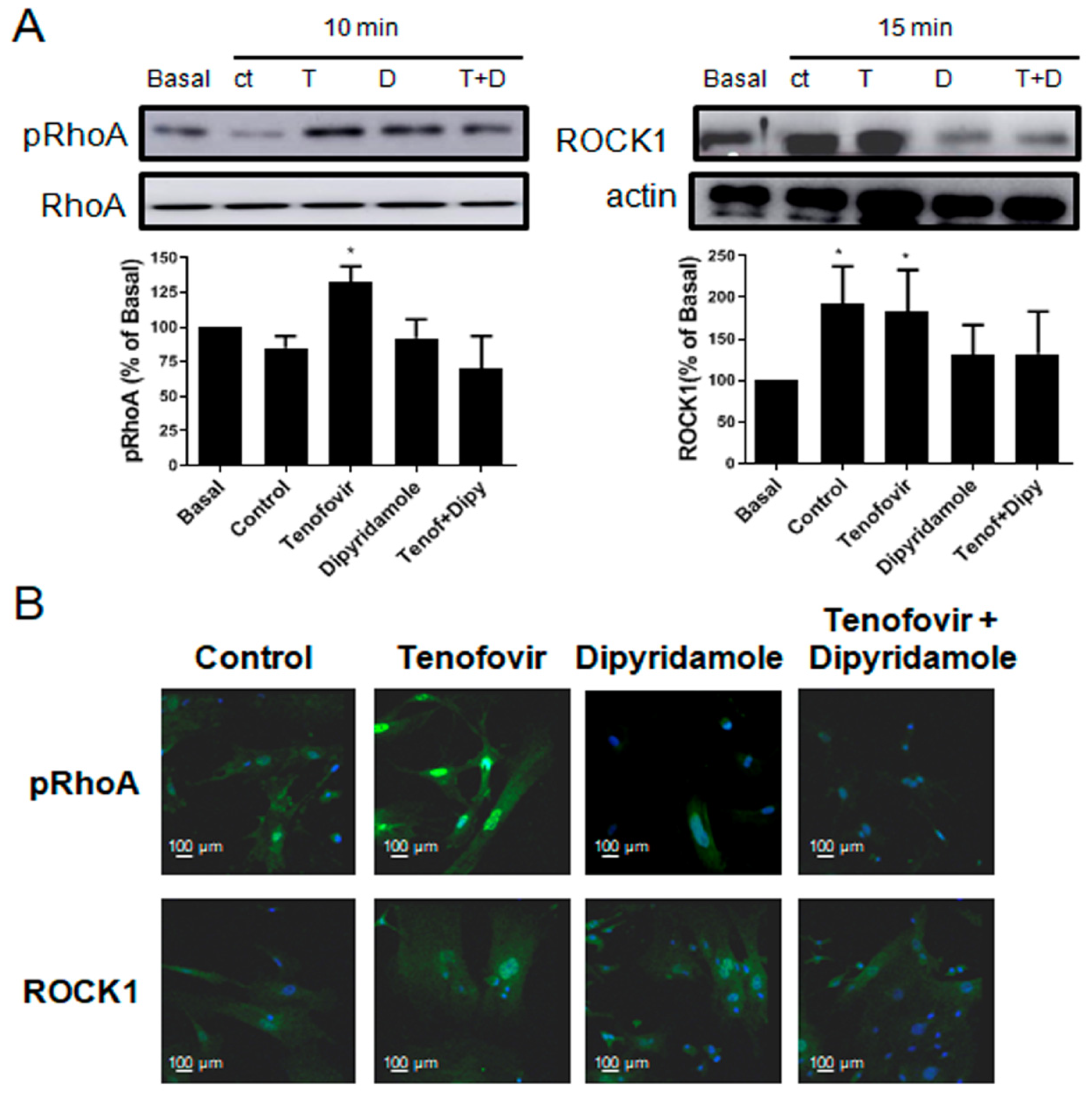
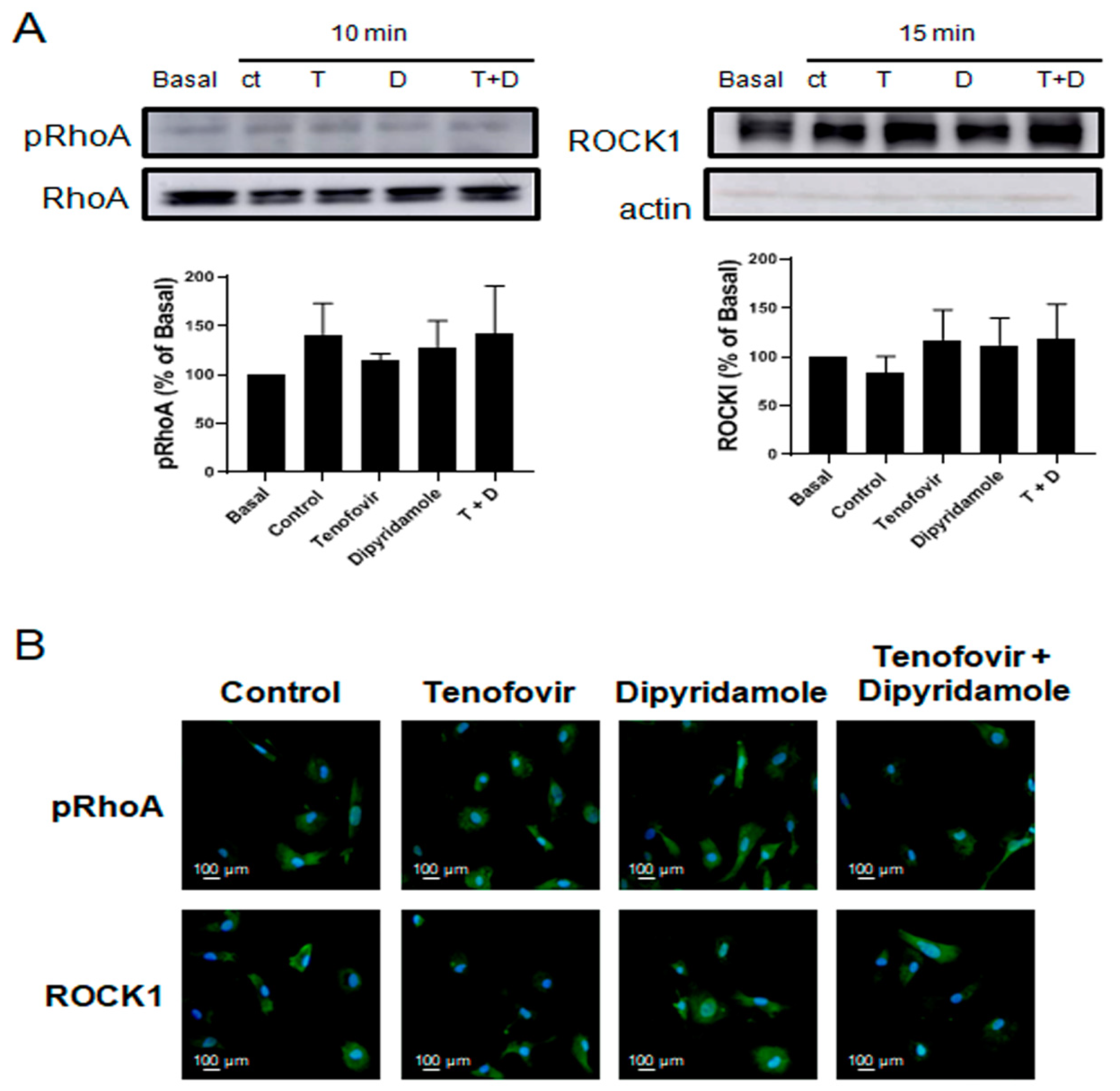
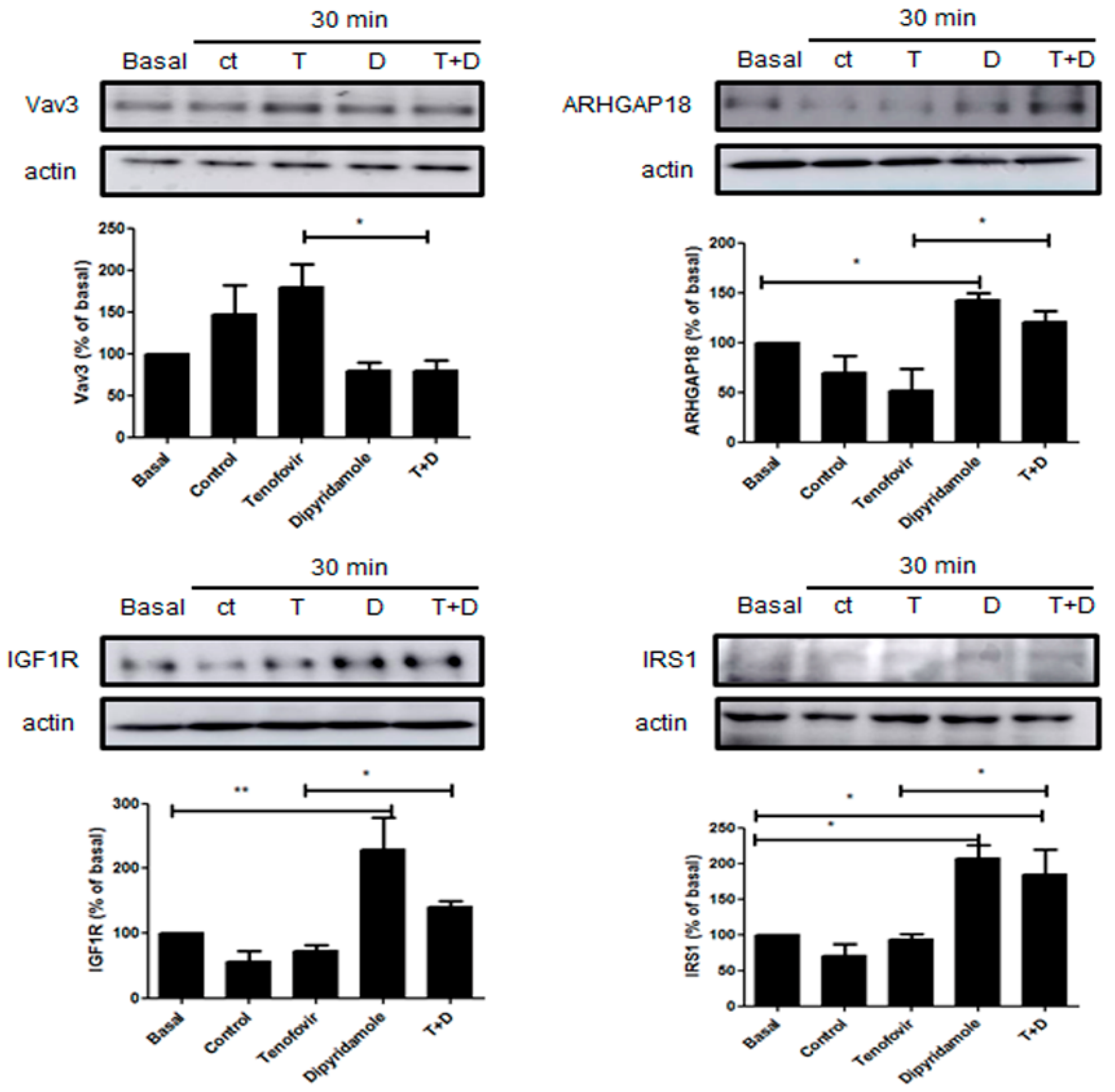
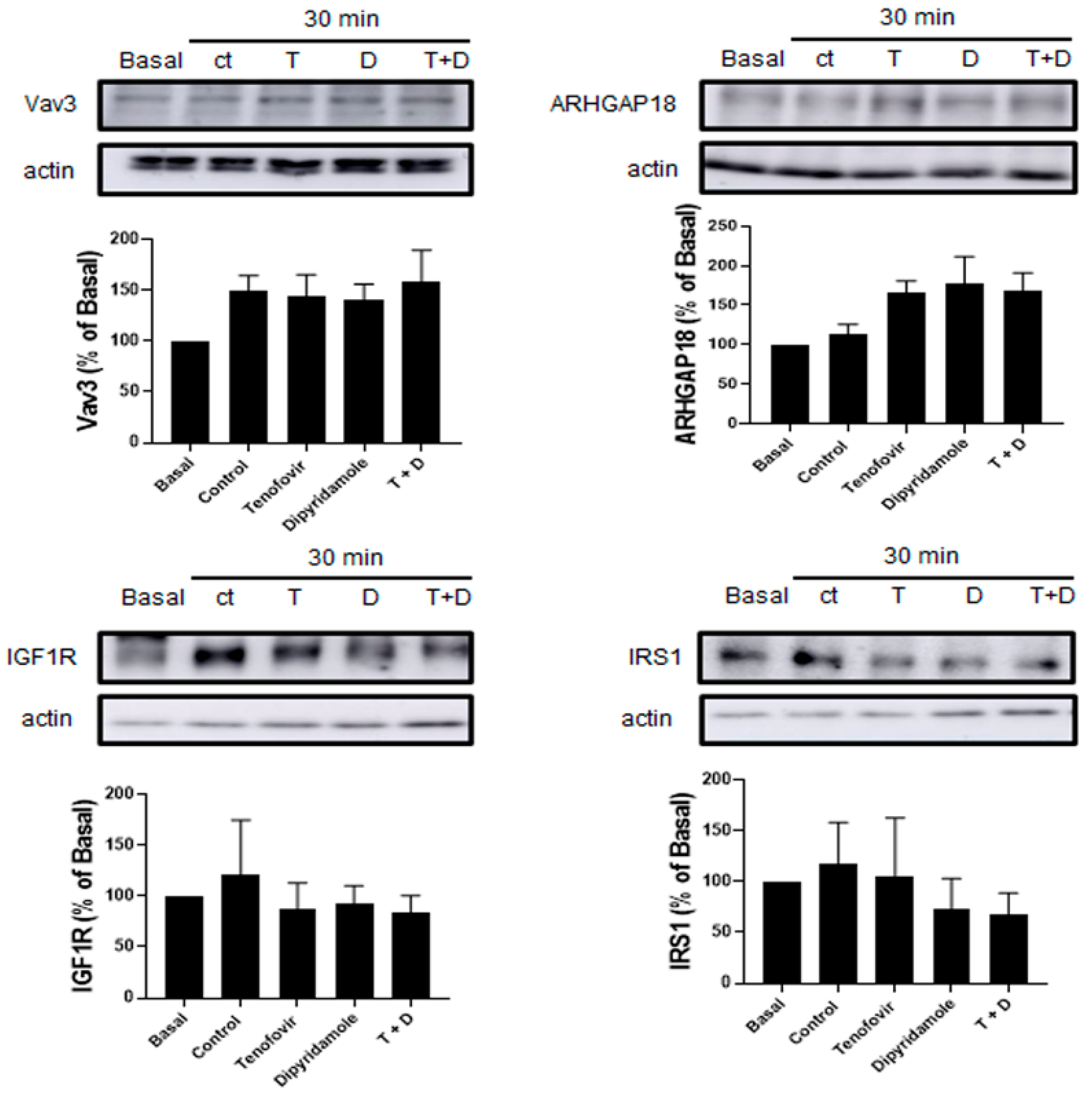
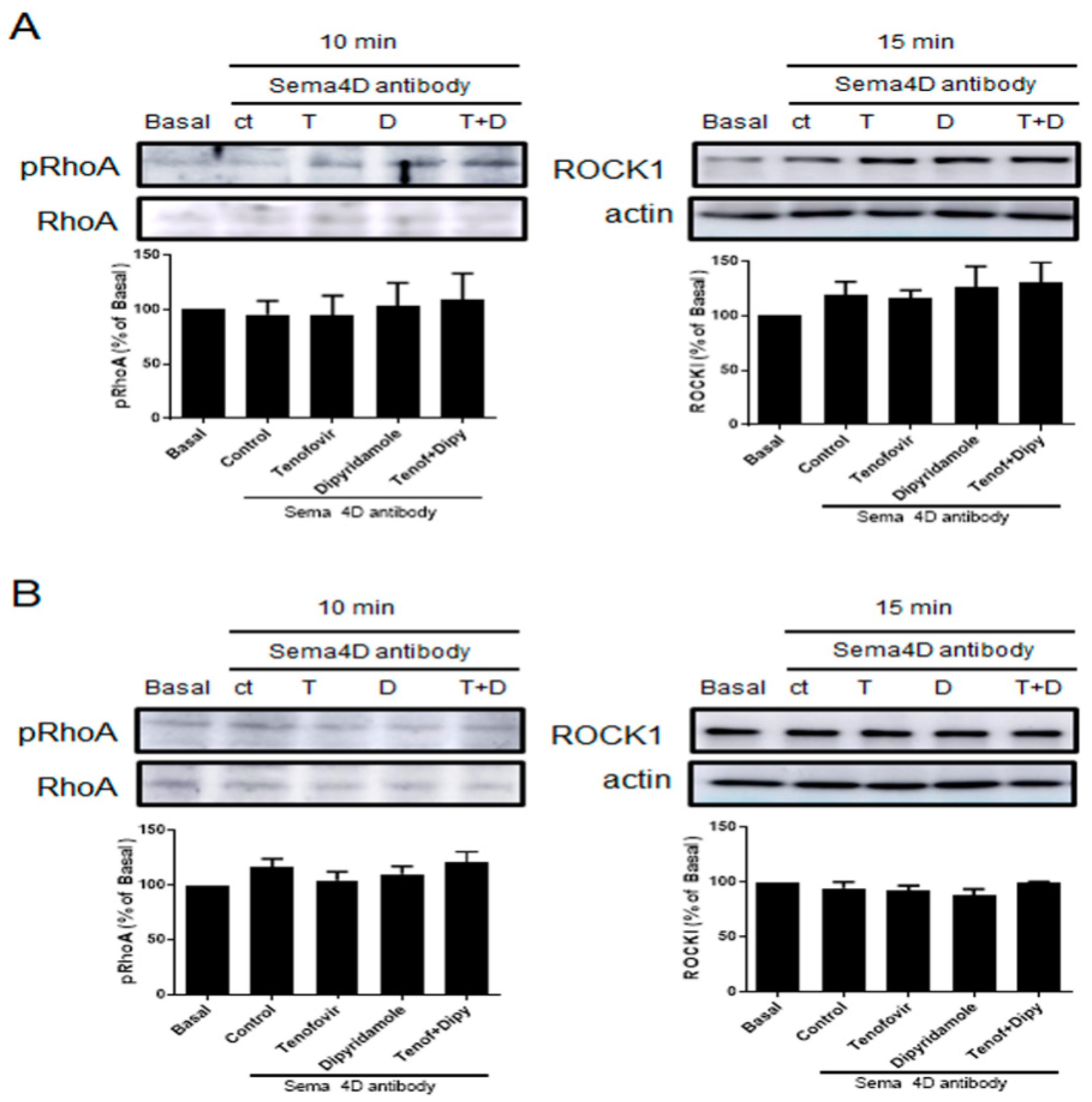
2.3. Phosphorylation of RhoA and ROCK1 Activation Were Increased by Tenofovir in Osteoblasts
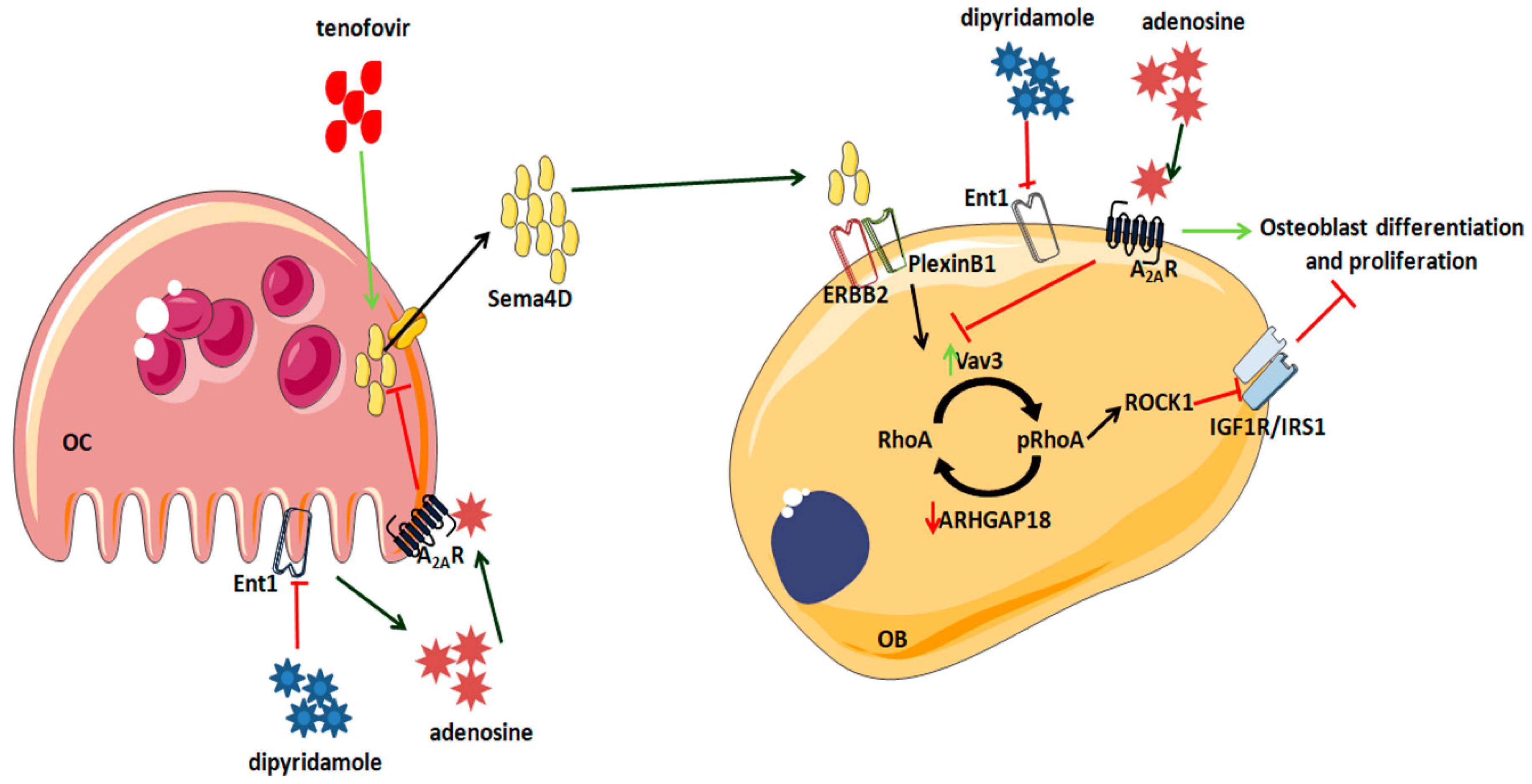
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. Histological Studies
4.4. Osteoclast Differentiation
4.5. Osteoblast Differentiation
4.6. Co-Culture of Osteoclast Precursors and Osteoblasts
4.7. Western Blot
4.8. Real-Time Quantitative RT-PCR
4.9. Immunocytochemistry
5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, C.M.; Mundy, G.R. Eph receptors and ephrin signaling pathways: A role in bone homeostasis. Int. J. Med. Sci. 2008, 5, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Mediero, A.; Ramkhelawon, B.; Perez-Aso, M.; Moore, K.J.; Cronstein, B.N. Netrin-1 is a critical autocrine/paracrine factor for osteoclast differentiation. J. Bone Min. Res. 2015, 30, 837–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togari, A.; Mogi, M.; Arai, M.; Yamamoto, S.; Koshihara, Y. Expression of mRNA for axon guidance molecules, such as semaphorin-III, netrins and neurotrophins, in human osteoblasts and osteoclasts. Brain Res. 2000, 878, 204–209. [Google Scholar] [CrossRef]
- Negishi-Koga, T.; Shinohara, M.; Komatsu, N.; Bito, H.; Kodama, T.; Friedel, R.H.; Takayanagi, H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011, 17, 1473–1480. [Google Scholar] [CrossRef]
- Dacquin, R.; Domenget, C.; Kumanogoh, A.; Kikutani, H.; Jurdic, P.; Machuca-Gayet, I. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS ONE 2011, 6, e26627. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 2013, 92, 860–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikutani, H.; Suzuki, K.; Kumanogoh, A. Immune semaphorins: Increasing members and their diverse roles. Adv. Immunol. 2007, 93, 121–143. [Google Scholar] [PubMed]
- Maleki, K.T.; Cornillet, M.; Björkström, N.K. Soluble SEMA4D/CD100: A novel immunoregulator in infectious and inflammatory diseases. Clin. Immunol. 2016, 163, 52–59. [Google Scholar] [CrossRef]
- Ch’ng, E.S.; Kumanogoh, A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol. Cancer 2010, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabbert-de Ponnat, I.; Marie-Cardine, A.; Pasterkamp, R.J.; Schiavon, V.; Tamagnone, L.; Thomasset, N.; Bensussan, A.; Boumsell, L. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int. Immunol. 2005, 17, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, C.; Elhabazi, A.; Bismuth, G.; Bensussan, A.; Boumsell, L. CD100 is associated with CD45 at the surface of human T lymphocytes. Role in T cell homotypic adhesion. J. Immunol. 1996, 157, 5262–5268. [Google Scholar]
- Kumanogoh, A.; Kikutani, H. Immune semaphorins: A new area of semaphorin research. J. Cell Sci. 2003, 116, 3463–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, I.; Kumanogoh, A.; Suzuki, K.; Akahani, S.; Noda, K.; Kikutani, H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: Implications for the regulation of immune and inflammatory responses. Int. Immunol. 2003, 15, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kumanogoh, A.; Watanabe, C.; Shi, W.; Yoshida, K.; Kikutani, H. Functional soluble CD100/sema4D released from activated lymphocytes: Possible role in normal and pathologic immune responses. Blood 2001, 97, 3498–3504. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Ogata, A.; Kang, S.; Ebina, K.; Shi, K.; Nojima, S.; Kimura, T.; Ito, D.; Morimoto, K.; Nishide, M.; et al. Semaphorin 4D Contributes to Rheumatoid Arthritis by Inducing Inflammatory Cytokine Production: Pathogenic and Therapeutic Implications. Arthritis Rheumatol. 2015, 67, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, E.M.; Milush, J.M.; Ho, E.L.; Batista, M.D.; Holditch, S.J.; Keh, C.E.; Norris, P.J.; Keating, S.M.; Deeks, S.G.; Hunt, P.W.; et al. Expansion of CD8+ T cells lacking Sema4D/CD100 during HIV-1 infection identifies a subset of T cells with decreased functional capacity. Blood 2012, 119, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Correa-Rocha, R.; Lopez-Abente, J.; Gutierrez, C.; Perez-Fernandez, V.A.; Prieto-Sanchez, A.; Moreno-Guillen, S.; Muñoz-Fernandez, M.A.; Pion, M. CD72/CD100 and PD-1/PD-L1 markers are increased on T and B cells in HIV-1+ viremic individuals, and CD72/CD100 axis is correlated with T-cell exhaustion. PLoS ONE 2018, 13, e0203419. [Google Scholar] [CrossRef]
- Vadasz, Z.; Elbirt, D.; Radian, S.; Bezalel-Rosenberg, S.; Mahlab-Guri, K.; Toubi, E.; Asherb, I.; Sthoeger, Z. Low levels of the immunoregulator Semaphorin 4D (CD100) in sera of HIV patients. Clin. Immunol. 2018, 191, 88–93. [Google Scholar] [CrossRef]
- Brown, T.T.; Ross, A.C.; Storer, N.; Labbato, D.; McComsey, G.A. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: Tenofovir versus non-tenofovir regimens. Antivir. Ther. 2011, 16, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Feig, J.L.; Mediero, A.; Corciulo, C.; Liu, H.; Zhang, J.; Perez-Aso, M.; Picard, L.; Wilder, T.; Cronstein, B. The antiviral drug tenofovir, an inhibitor of Pannexin-1-mediated ATP release, prevents liver and skin fibrosis by downregulating adenosine levels in the liver and skin. PLoS ONE 2017, 12, e0188135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasquez, S.; Eugenin, E.A. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front. Physiol. 2014, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conesa-Buendía, F.M.C.; Llamas-Granda, P.; Larrañaga-Vera, A.; Wilder, T.; Largo, R.; Herrero-Beaumont, G.; Cronstein, B.; Mediero, A. Tenofovir Causes Bone Loss via Decreased Bone Formation and Increased Bone Resorption, Which Can Be Counteracted by Dipyridamole in Mice. J. Bone Min. Res. 2019, 34, 923–938. [Google Scholar] [CrossRef]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone regeneration in critical bone defects using three-dimensionally printed beta-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 105, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Mediero, A.; Wilder, T.; Shah, L.; Cronstein, B.N. Adenosine A2A receptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis. FASEB J. 2018, 32, 3487–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Kumanogoh, A. Semaphorins in bone development, homeostasis, and disease. Semin. Cell Dev. Biol. 2013, 24, 163–171. [Google Scholar] [CrossRef]
- Brown, T.T.; Qaqish, R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic review. AIDS 2006, 20, 2165–2174. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Allshouse, A.A.; Jankowski, C.M.; MaWhinney, S.; Kohrt, W.M.; Campbell, T.B. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J. Acquir. Immune Defic. Syndr. 2013, 63, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starup-Linde, J.; Rosendahl, S.B.; Storgaard, M.; Langdahl, B. Management of Osteoporosis in Patients Living With HIV-A Systematic Review and Meta-analysis. J. Acquir. Immune Defic. Syndr. 2020, 83, 1–8. [Google Scholar] [CrossRef]
- Parperis, K.; Abdulqader, Y.; Myers, R.; Bhattarai, B.; Al-Ani, M. Rheumatic diseases in HIV-infected patients in the post-antiretroviral therapy era: A tertiary care center experience. Clin. Rheumatol. 2019, 38, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone—the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Callebaut, C.; Liu, Y.; Babusis, D.; Ray, A.; Miller, M.; Kitrinos, K. Viability of primary osteoblasts after treatment with tenofovir alafenamide: Lack of cytotoxicity at clinically relevant drug concentrations. PLoS ONE 2017, 12, e0169948. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, I.F.; Pham, L.; Mansky, L.M.; Gopalakrishnan, R.; Carlson, A.E.; Manskya, K.C. Tenofovir treatment of primary osteoblasts alters gene expression profiles: Implications for bone mineral density loss. Biochem. Biophys. Res. Commun. 2010, 394, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuklina, E.M.; Nekrasova, I.V.; Valieva, Y.V. Involvement of Semaphorin (Sema4D) in T-Dependent Activation of B Cells. Bull. Exp. Biol. Med. 2017, 163, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Kruppke, B.; Ray, S.; Alt, V.; Rohnke, M.; Kern, C.; Kampschulte, M.; Heinemann, C.; Budak, M.; Adam, J.; Döhner, N.; et al. Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects-In Vitro to In Vivo Translation. Molecules 2020, 25, 5103. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamas-Granda, P.; Martin-Rodríguez, L.; Largo, R.; Herrero-Beaumont, G.; Mediero, A. Tenofovir Modulates Semaphorin 4D Signaling and Regulates Bone Homeostasis, Which Can Be Counteracted by Dipyridamole and Adenosine A2A Receptor. Int. J. Mol. Sci. 2021, 22, 11490. https://doi.org/10.3390/ijms222111490
Llamas-Granda P, Martin-Rodríguez L, Largo R, Herrero-Beaumont G, Mediero A. Tenofovir Modulates Semaphorin 4D Signaling and Regulates Bone Homeostasis, Which Can Be Counteracted by Dipyridamole and Adenosine A2A Receptor. International Journal of Molecular Sciences. 2021; 22(21):11490. https://doi.org/10.3390/ijms222111490
Chicago/Turabian StyleLlamas-Granda, Patricia, Laura Martin-Rodríguez, Raquel Largo, Gabriel Herrero-Beaumont, and Aránzazu Mediero. 2021. "Tenofovir Modulates Semaphorin 4D Signaling and Regulates Bone Homeostasis, Which Can Be Counteracted by Dipyridamole and Adenosine A2A Receptor" International Journal of Molecular Sciences 22, no. 21: 11490. https://doi.org/10.3390/ijms222111490





