The Anti-Inflammatory Effect of Hydrogen Gas Inhalation and Its Influence on Laser-Induced Choroidal Neovascularization in a Mouse Model of Neovascular Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Results
2.1. Color Fundus Photography (CFP) and Fluorescence Angiography (FA) Analysis of the Effects of Hydrogen Gas Inhalation on Laser-Induced CNV (n = 3)
2.2. Histology, Immunofluorescence Staining, and the Effect of Hydrogen as Inhalation on the Reduction in Phosphorylation of the VEGF Receptor
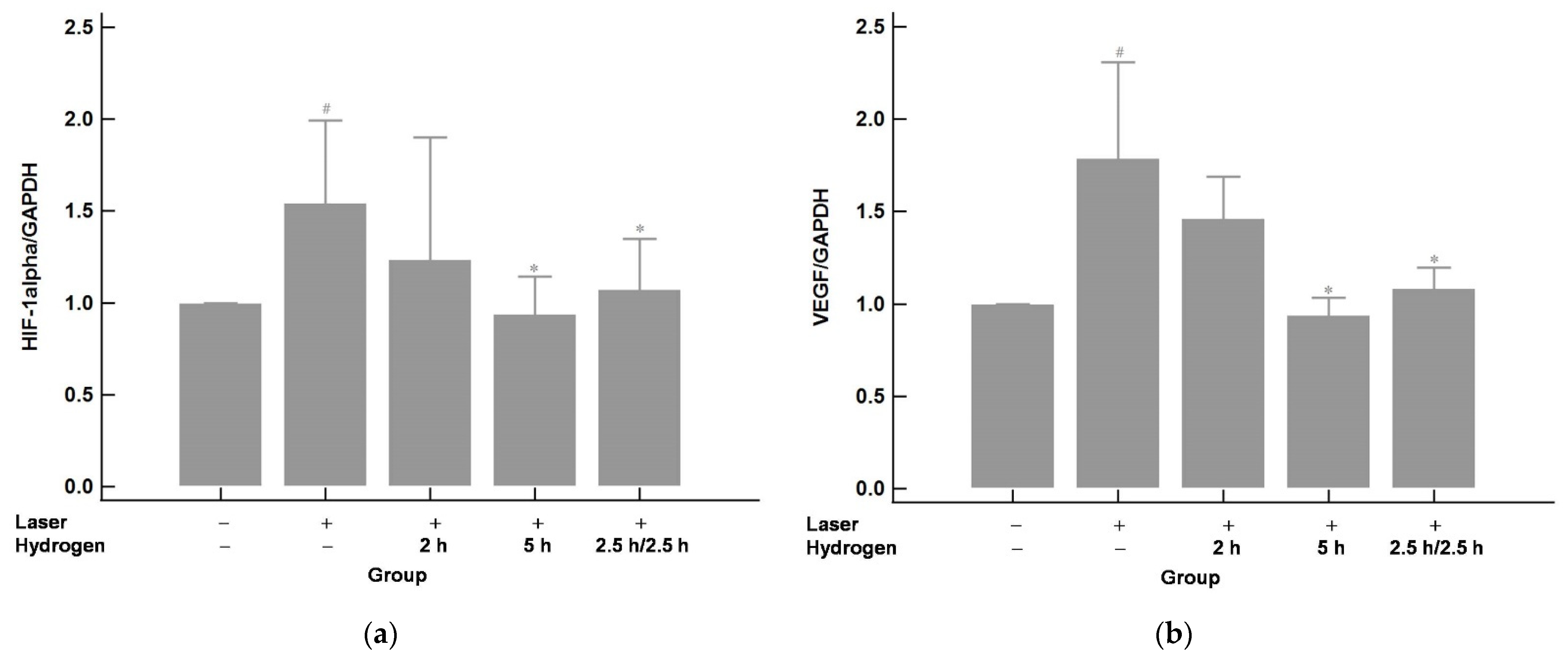
2.3. Hydrogen Gas Inhalation Downregulates the mRNA Expression of Hypoxia-Inducible Factor 1 alpha (HIF-1α) and Its Immediate Downstream Target VEGF in Laser-Induced CNV (n = 3)
2.4. Hydrogen Gas Inhalation Reduces the mRNA Levels of Tumor Necrosis Factor Alpha (TNF-α) and Interlukin-6 (IL-6) (n = 3)
3. Discussion
4. Materials and Methods
4.1. Animals
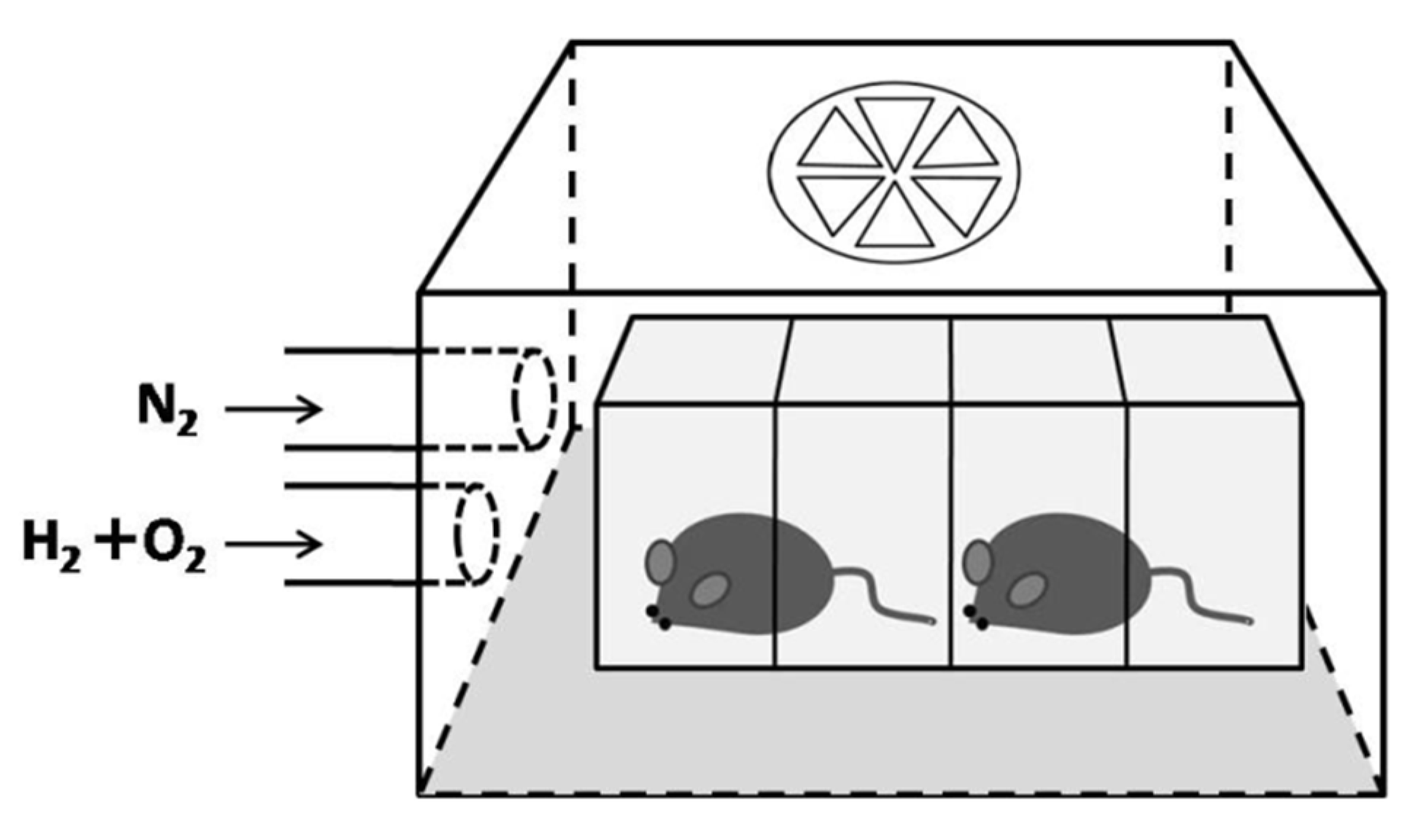
4.2. Hydrogen Inhalation
4.3. Laser-Induced CNV Model
4.4. Color Fundus Photography and Fluorescene Angiography
4.5. Histology and Immunofluorescence Staining
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) to Measure Transcription Levels
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apte, R.S. Age-Related Macular Degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013, 380, 2197–2223. [Google Scholar] [CrossRef]
- Vinding, T. Age-related macular degeneration. Macular changes, prevalence and sex ratio. Acta Ophthalmol. 2009, 67, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.R.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.; Owen, C.G. Age and Gender Variations in Age-related Macular Degeneration Prevalence in Populations of European Ancestry: A Meta-analysis. Ophthalmology 2012, 119, 571–580. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; He, Q.; Lu, M. Global, regional, and national burden of blindness and vision loss due to common eye diseases along with its attributable risk factors from 1990 to 2019: A systematic analysis from the global burden of disease study 2019. Aging 2021, 13, 19614–19642. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.N.Y.; Lim, S.C.; Jonas, J.B.; Sabanayagam, C. Obesity and risk of age-related eye diseases: A systematic review of prospective population-based studies. Int. J. Obes. 2021, 45, 1863–1885. [Google Scholar] [CrossRef] [PubMed]
- Roddy, G.W. Metabolic syndrome and the aging retina. Curr. Opin. Ophthalmol. 2021, 32, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Peto, T.; Bird, A.; VanNewkirk, M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss. Arch. Ophthalmol. 2001, 119, 1417–1436. [CrossRef] [Green Version]
- Age-Related Eye Disease Study 2 Research Group Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration. JAMA 2013, 309, 2005–2015. [CrossRef] [PubMed]
- E Clemons, T.; Milton, R.C.; Klein, R.; Seddon, J.M.; Ferris, F. Age-Related Eye Disease Study Research Group Risk Factors for the Incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology 2005, 112, 533–539.e1. [Google Scholar] [CrossRef] [Green Version]
- A Chapman, N.; Jacobs, R.J.; Braakhuis, A.J. Role of diet and food intake in age-related macular degeneration: A systematic review. Clin. Exp. Ophthalmol. 2019, 47, 106–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, Y.; Maddula, S.; Ambati, B.K. Mediators of ocular angiogenesis. J. Genet. 2009, 88, 495–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwesinger, C.; Yee, C.; Rohan, R.M.; Joussen, A.M.; Fernandez, A.; Meyer, T.N.; Poulaki, V.; Ma, J.J.; Redmond, T.M.; Liu, S.; et al. Intrachoroidal Neovascularization in Transgenic Mice Overexpressing Vascular Endothelial Growth Factor in the Retinal Pigment Epithelium. Am. J. Pathol. 2001, 158, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Zachary, I. Neuroprotective Role of Vascular Endothelial Growth Factor: Signalling Mechanisms, Biological Function, and Therapeutic Potential. Neurosignals 2005, 14, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Sene, A.; Chin-Yee, D.; Apte, R.S. Seeing through VEGF: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 2015, 21, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Long, P.; Yan, W.; He, M.; Zhang, Q.; Wang, Z.; Li, M.; Xue, J.; Chen, T.; An, J.; Zhang, Z. Protective effects of hydrogen gas in a rat model of branch retinal vein occlusion via decreasing VEGF-α expression. BMC Ophthalmol. 2019, 19, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Wu, J.; Chen, Z.; Xia, F.; Sun, Q.; Liu, L. Postconditioning with inhaled hydrogen promotes survival of retinal ganglion cells in a rat model of retinal ischemia/reperfusion injury. Brain Res. 2016, 1632, 82–90. [Google Scholar] [CrossRef]
- Yan, W.; Chen, T.; Long, P.; Zhang, Z.; Liu, Q.; Wang, X.; An, J.; Zhang, Z. Effects of Post-Treatment Hydrogen Gas Inhalation on Uveitis Induced by Endotoxin in Rats. Med Sci. Monit. 2018, 24, 3840–3847. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 1–25. [Google Scholar] [CrossRef]
- Killingsworth, M.C. Angiogenesis in early choroidal neovascularization secondary to age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 233, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Green, W. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Rationale for Combination Therapies for Choroidal Neovascularization. Am. J. Ophthalmol. 2006, 141, 149–156. [Google Scholar] [CrossRef]
- Campa, C.; Costagliola, C.; Incorvaia, C.; Sheridan, C.; Semeraro, F.; De Nadai, K.; Sebastiani, A.; Parmeggiani, F. Inflammatory Mediators and Angiogenic Factors in Choroidal Neovascularization: Pathogenetic Interactions and Therapeutic Implications. Mediat. Inflamm. 2010, 2010, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, P.F.; Sippy, B.D.; Lambert, H.M.; Thach, A.B.; Hinton, D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 855–868. [Google Scholar]
- Ishibashi, T.; Hata, Y.; Yoshikawa, H.; Nakagawa, K.; Sueishi, K.; Inomata, H. Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 159–167. [Google Scholar] [CrossRef]
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular Endothelial Growth Factor Expression in the Retinal Pigment Epithelium Is Essential for Choriocapillaris Development and Visual Function. Am. J. Pathol. 2005, 167, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, C.M.; Pate, S.; Hiscott, P.; Wong, D.; Pattwell, D.M.; Kent, D. Expression of hypoxia-inducible factor−1α and −2α in human choroidal neovascular membranes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Vadlapatla, A.D.V.A.A.K.M.R.K.; Vadlapudi, A.D.; Mitra, A.K. Hypoxia-Inducible Factor-1 (HIF-1): A Potential Target for Intervention in Ocular Neovascular Diseases. Curr. Drug Targets 2013, 14, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Brijesh, T.; Shorya, A. Macular Atrophy Progression and 7-Year Vision Outcomes in Subjects From the ANCHOR, MARINA, and HORIZON Studies: The SEVEN-UP Study. Am. J. Ophthalmol. 2015, 162, 200. [Google Scholar] [CrossRef]
- Krebs, I.; Glittenberg, C.; Ansari-Shahrezaei, S.; Hagen, S.; Steiner, I.; Binder, S. Non-responders to treatment with antagonists of vascular endothelial growth factor in age-related macular degeneration. Br. J. Ophthalmol. 2013, 97, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.M.; Harding, S.P.; Johnston, R.L.; Kelly, S.P.; Lotery, A.J.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef]
- Apte, R.S.; Richter, J.; Herndon, J.; A Ferguson, T. Macrophages Inhibit Neovascularization in a Murine Model of Age-Related Macular Degeneration. PLoS Med. 2006, 3, e310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Outtz, H.H.; Tattersall, I.; Kofler, N.M.; Steinbach, N.; Kitajewski, J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood 2011, 118, 3436–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, E.; Anand, A.; Ambati, B.K.; Van Rooijen, N.; Ambati, J. Macrophage Depletion Inhibits Experimental Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3578–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Grossniklaus, H.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.; Elner, S.G.; Sternberg, P. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 2002, 8, 119–126. [Google Scholar]
- Leibovich, S.J.; Polverini, P.J.; Shepard, H.M.; Wiseman, D.; Shively, V.P.; Nuseir, N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nat. Cell Biol. 1987, 329, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Heidmann, D.G.; Suner, I.J.; Hernandez, E.P.; Monroy, D.; Csaky, K.G.; Cousins, S.W. Macrophage Depletion Diminishes Lesion Size and Severity in Experimental Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3586–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Kirchhof, B.; Koizumi, K.; Döhmen, S.; Adamis, A.P. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-α suppression. FASEB J. 2002, 16, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Poulaki, V.; Doehmen, S.; Welsandt, G.; Radetzky, S.; Lappas, A.; Kociok, N.; Kirchhof, B.; Joussen, A.M. Contribution of TNF-α to Leukocyte Adhesion, Vascular Leakage, and Apoptotic Cell Death in Endotoxin-Induced Uveitis In Vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2184–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, H. Roles of IL-6 in Ocular Inflammation: A Review. Ocul. Immunol. Inflamm. 2018, 26, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; George, S.; Rosner, B.; Rifai, N. Progression of Age-Related Macular Degeneration. Arch. Ophthalmol. 2005, 123, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Zhang, H.; Liu, Z.-L. Interleukin-6 receptor blockade suppresses subretinal fibrosis in a mouse model. Int. J. Ophthalmol. 2014, 7, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takeda, A.; Hasegawa, E.; Jo, Y.-J.; Arima, M.; Oshima, Y.; Ryoji, Y.; Nakazawa, T.; Yuzawa, M.; Nakashizuka, H.; et al. Interleukin-6 plays a crucial role in the development of subretinal fibrosis in a mouse model. Immunol. Med. 2018, 41, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, O.; Ye, Z.; Zhang, R.; Hu, H.; Zhang, N.; Huang, J.; Liu, W.; Sun, X. Inhalation of high concentrations of hydrogen ameliorates liver ischemia/reperfusion injury through A 2A receptor mediated PI3K-Akt pathway. Biochem. Pharmacol. 2017, 130, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, X.; Zhai, X.; Shi, D.; Zhang, R.; Zhi, X.; Li, X.; Gu, Z.; Cao, L.; Weng, W.; et al. Inhalation of water electrolysis-derived hydrogen ameliorates cerebral ischemia—reperfusion injury in rats – A possible new hydrogen resource for clinical use. Neurosci. 2016, 335, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Iwanishi, H.; Fujita, N.; Tomoyose, K.; Okada, Y.; Yamanaka, O.; Flanders, K.C.; Saika, S. Inhibition of development of laser-induced choroidal neovascularization with suppression of infiltration of macrophages in Smad3-null mice. Lab. Investig. 2016, 96, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.R.; Choi, W.; Hong, H.K.; Kim, Y.; Hwang, Y.; Woo, S.J.; Oh, W.-Y. Imaging Laser-Induced Choroidal Neovascularization in the Rodent Retina Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Onami, H.; Nagai, N.; Kaji, H.; Nishizawa, M.; Sato, Y.; Osumi, N.; Nakazawa, T.; Abe, T. Transscleral Sustained Vasohibin-1 Delivery by a Novel Device Suppressed Experimentally-Induced Choroidal Neovascularization. PLoS ONE 2013, 8, e58580. [Google Scholar] [CrossRef] [PubMed]





| Primer | Primer Sequence (5′ -3′) | Primer Sequence (3′ -5′) | Product Length (bp) |
|---|---|---|---|
| HIF-1α | CCAGCAGACCCAGTTACAGA | TGAGTGCCACTGTATGCTGA | 20 |
| VEGF | CTGCTGTAACGATGAAGCCCTG | GCTGTAGGAAGCTCATCTCTCC | 22 |
| TNF-α | GGTGCCTATGTCTCAGCCTCTTTT | GCCATAGAACTGATGAGAGGGAG | 23 |
| IL-6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, I.-C.; Ko, W.-C.; Hsu, Y.-J.; Lin, Y.-R.; Chang, Y.-H.; Zong, X.-H.; Lai, P.-C.; Chang, D.-C.; Hung, C.-F. The Anti-Inflammatory Effect of Hydrogen Gas Inhalation and Its Influence on Laser-Induced Choroidal Neovascularization in a Mouse Model of Neovascular Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 12049. https://doi.org/10.3390/ijms222112049
Liang I-C, Ko W-C, Hsu Y-J, Lin Y-R, Chang Y-H, Zong X-H, Lai P-C, Chang D-C, Hung C-F. The Anti-Inflammatory Effect of Hydrogen Gas Inhalation and Its Influence on Laser-Induced Choroidal Neovascularization in a Mouse Model of Neovascular Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2021; 22(21):12049. https://doi.org/10.3390/ijms222112049
Chicago/Turabian StyleLiang, I-Chia, Wen-Chin Ko, Yu-Jou Hsu, Yi-Ru Lin, Yun-Hsiang Chang, Xv-Hui Zong, Pei-Chen Lai, Der-Chen Chang, and Chi-Feng Hung. 2021. "The Anti-Inflammatory Effect of Hydrogen Gas Inhalation and Its Influence on Laser-Induced Choroidal Neovascularization in a Mouse Model of Neovascular Age-Related Macular Degeneration" International Journal of Molecular Sciences 22, no. 21: 12049. https://doi.org/10.3390/ijms222112049






