Role of Stem Cells in the Ovarian Tissue Cryopreservation and Transplantation for Fertility Preservation
Abstract
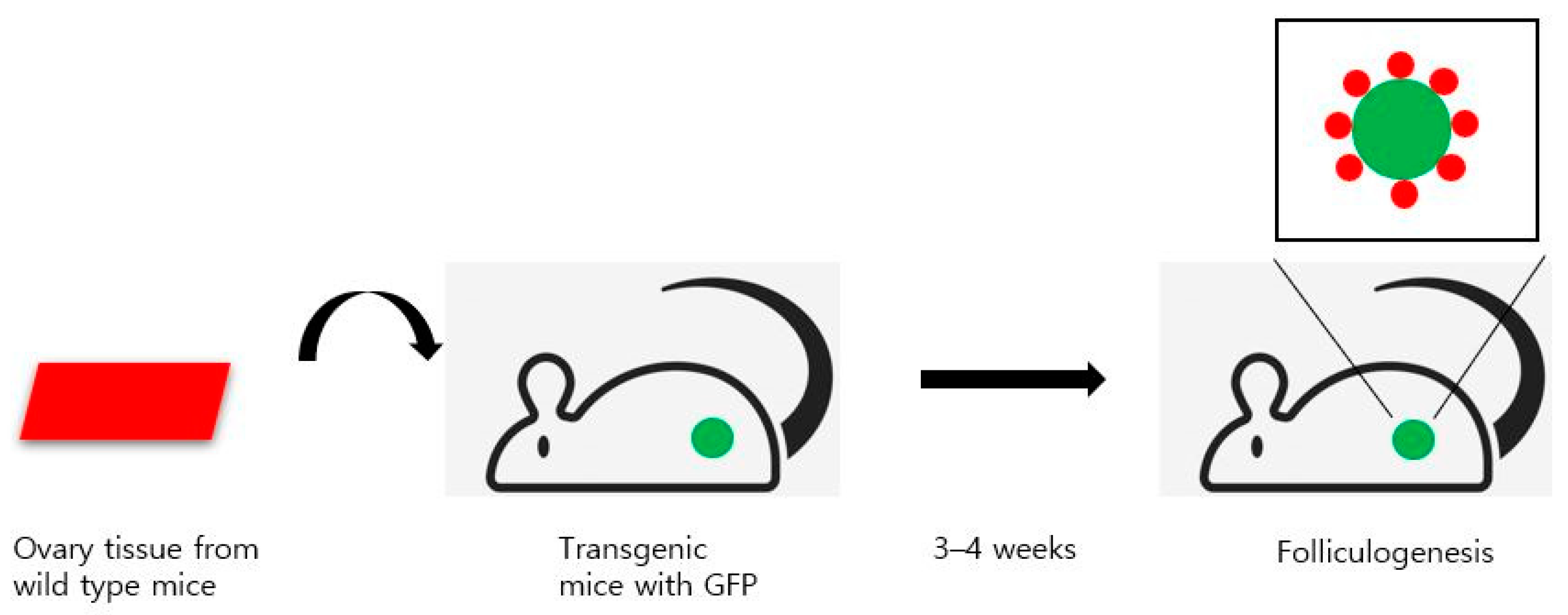
:1. Introduction
2. Identification and Isolation of Ovarian Stem Cell
3. Ovarian Tissue Cryopreservation and Transplantation
| Reference | Animal Model | Type of Stem Cells | Main Findings |
|---|---|---|---|
| [63] | SCID mice | Human ASCs | ASCs boosted vascularization in grafted ovarian tissue by secreting growth factor. |
| [64] | SCID mice | Human ASCs | The use of ASC in fibrin implant showed an increase in oxygenation. |
| [70] | Rat | Rat ASCs | Direct injection of ASCs into cryopreserved ovaries did not improve follicular survival. |
| [71] | Mice | Human MSCs from bone marrow | Human MSCs isolated from bone marrow increased the levels of VEGF, FGF2, and angiogenin, stimulated neovascularization, and increased blood perfusion of transplanted grafts. |
| [74] | SCID mice | Human ASCs | Prior to human ovary tissue transplantation in SCID mice, peritoneal transplantation was loaded with ASCs. Increased oxygenation, enhanced vascularization, increased primordial follicle survival rate, and decreased apoptosis rate were shown. |
4. Other Fertility Preservation Methods Using Stem Cells
4.1. Direct Injection of Stem Cells
4.2. Bone Marrow Transplantation
4.3. IVM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Chemotherapy and fertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2012, 26, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927–943. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444. [Google Scholar] [CrossRef]
- Cho, H.W.; Lee, S.; Min, K.J.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W.; Kim, T. Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity. Int. J. Mol. Sci. 2020, 21, 7792. [Google Scholar] [CrossRef]
- Carrillo, L.; Seidman, D.S.; Cittadini, E.; Meirow, D. The role of fertility preservation in patients with endometriosis. J. Assist. Reprod. Genet. 2016, 33, 317–323. [Google Scholar] [CrossRef]
- Llarena, N.C.; Falcone, T.; Flyckt, R.L. Fertility Preservation in Women with Endometriosis. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119873386. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Ozkavukcu, S.; Heytens, E.; Moy, F.; Oktay, K. Value of early referral to fertility preservation in young women with breast cancer. J. Clin. Oncol. 2010, 28, 4683–4686. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Heytens, E.; Moy, F.; Ozkavukcu, S.; Oktay, K. Determinants of access to fertility preservation in women with breast cancer. Fertil. Steril. 2011, 95, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, S.K.; Hwang, K.J.; Kim, T.; Kim, S.H. Fertility preservation for patients with gynecologic malignancies: The Korean Society for Fertility Preservation clinical guidelines. Clin. Exp. Reprod. Med. 2017, 44, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsepelidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum. Reprod. 2015, 30, 2107–2109. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, M.; Giudice, M.G.; Del Vento, F.; Wyns, C. Role of stem cells in fertility preservation: Current insights. Stem Cells Cloning 2019, 12, 27–48. [Google Scholar] [CrossRef] [Green Version]
- Van der Ven, H.; Liebenthron, J.; Beckmann, M.; Toth, B.; Korell, M.; Krussel, J.; Frambach, T.; Kupka, M.; Hohl, M.K.; Winkler-Crepaz, K.; et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: Tissue activity, pregnancy and delivery rates. Hum. Reprod. 2016, 31, 2031–2041. [Google Scholar] [CrossRef]
- Jensen, A.K.; Kristensen, S.G.; Macklon, K.T.; Jeppesen, J.V.; Fedder, J.; Ernst, E.; Andersen, C.Y. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum. Reprod. 2015, 30, 2838–2845. [Google Scholar] [CrossRef] [Green Version]
- Rivas Leonel, E.C.; Lucci, C.M.; Amorim, C.A. Cryopreservation of Human Ovarian Tissue: A Review. Transfus. Med. Hemother. 2019, 46, 173–181. [Google Scholar] [CrossRef]
- Pacheco, F.; Oktay, K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod. Sci. 2017, 24, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Green, S.H.; Zuckerman, S. Quantitative aspects of the growth of the human ovum and follicle. J. Anat. 1951, 85, 373–375. [Google Scholar] [PubMed]
- Anderson, L.D.; Hirshfield, A.N. An overview of follicular development in the ovary: From embryo to the fertilized ovum in vitro. Md. Med. J. 1992, 41, 614–620. [Google Scholar] [PubMed]
- Porras-Gomez, T.J.; Moreno-Mendoza, N. Neo-oogenesis in mammals. Zygote 2017, 25, 404–422. [Google Scholar] [CrossRef]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ozakpinar, O.B.; Maurer, A.M.; Ozsavci, D. Ovarian stem cells: From basic to clinical applications. World J. Stem Cells 2015, 7, 757–768. [Google Scholar] [CrossRef]
- Johnson, J.; Bagley, J.; Skaznik-Wikiel, M.; Lee, H.J.; Adams, G.B.; Niikura, Y.; Tschudy, K.S.; Tilly, J.C.; Cortes, M.L.; Forkert, R.; et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 2005, 122, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggan, K.; Jurga, S.; Gosden, R.; Min, I.M.; Wagers, A.J. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature 2006, 441, 1109–1114. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Kang, J.X.; Xie, W.; Li, X.; Wu, C.; Xu, B.; Wu, J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol. Hum. Reprod. 2014, 20, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Yu, M.; Hu, Y.; Qiu, P.; Liu, W.; Zheng, W.; Peng, S.; Hua, J. Location and characterization of female germline stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif. 2013, 46, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, J.; Li, X.; Wang, H.; Liu, G.; Xu, B.; Mei, X.; Hua, X.; Wu, J. Characteristics of Female Germline Stem Cells from Porcine Ovaries at Sexual Maturity. Cell Transplant. 2018, 27, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- White, Y.A.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Li, C.H.; Wang, X.Y.; He, D.J.; Zheng, P. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol. Hum. Reprod. 2016, 22, 316–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Satirapod, C.; Ohguchi, Y.; Park, E.S.; Woods, D.C.; Tilly, J.L. Genetic studies in mice directly link oocytes produced during adulthood to ovarian function and natural fertility. Sci. Rep. 2017, 7, 10011. [Google Scholar] [CrossRef]
- Martin, J.J.; Woods, D.C.; Tilly, J.L. Implications and Current Limitations of Oogenesis from Female Germline or Oogonial Stem Cells in Adult Mammalian Ovaries. Cells 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Ozkavukcu, S.; Ku, S.Y. Current and Future Perspectives for Improving Ovarian Tissue Cryopreservation and Transplantation Outcomes for Cancer Patients. Reprod. Sci. 2021, 28, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.C.; Tilly, J.L. The next (re)generation of ovarian biology and fertility in women: Is current science tomorrow’s practice? Fertil. Steril. 2012, 98, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Horan, C.J.; Williams, S.A. Oocyte stem cells: Fact or fantasy? Reproduction 2017, 154, R23–R35. [Google Scholar] [CrossRef] [Green Version]
- Silvestris, E.; D’Oronzo, S.; Cafforio, P.; Kardhashi, A.; Dellino, M.; Cormio, G. In Vitro Generation of Oocytes from Ovarian Stem Cells (OSCs): In Search of Major Evidence. Int. J. Mol. Sci. 2019, 20, 6225. [Google Scholar] [CrossRef] [Green Version]
- Hutt, K.J.; Albertini, D.F. Clinical applications and limitations of current ovarian stem cell research: A review. J. Exp. Clin. Assist. Reprod. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tilly, J.L. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle 2010, 9, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Karlikaya, G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N. Engl. J. Med. 2000, 342, 1919. [Google Scholar] [CrossRef]
- Silvestris, E.; De Palma, G.; Canosa, S.; Palini, S.; Dellino, M.; Revelli, A.; Paradiso, A.V. Human Ovarian Cortex biobanking: A Fascinating Resource for Fertility Preservation in Cancer. Int. J. Mol. Sci. 2020, 21, 3245. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Lee, S.; Song, J.Y.; Ku, S.Y.; Kim, S.H.; Kim, T. Fertility preservation in women with cancer. Clin. Exp. Reprod. Med. 2012, 39, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silber, S.J. Ovary cryopreservation and transplantation for fertility preservation. Mol. Hum. Reprod. 2012, 18, 59–67. [Google Scholar] [CrossRef]
- Kim, S.S. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J. Assist. Reprod. Genet. 2012, 29, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, W.H.; Kelsey, T.W.; Anderson, R.A. Ovarian cryopreservation: Experimental or established and a cure for the menopause? Reprod. Biomed. Online 2012, 25, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Dath, C.; Dethy, A.; Van Langendonckt, A.; Van Eyck, A.S.; Amorim, C.A.; Luyckx, V.; Donnez, J.; Dolmans, M.M. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum. Reprod. 2011, 26, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Cho, H.W.; Kim, B.; Lee, J.K.; Kim, T. The Effectiveness of Anti-Apoptotic Agents to Preserve Primordial Follicles and Prevent Tissue Damage during Ovarian Tissue Cryopreservation and Xenotransplantation. Int. J. Mol. Sci. 2021, 22, 2534. [Google Scholar] [CrossRef]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Perez, G.I.; Fuks, Z.; Haimovitz-Friedman, A.; Nguyen, H.; Bose, M.; Ilagan, A.; Hunt, P.A.; Morgan, W.F.; Tilly, J.L.; et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat. Med. 2002, 8, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, Z.; Wu, F.; Chen, W.; Xie, S.; Liu, J.; Huang, X.; Zhou, Y. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil. Steril. 2014, 102, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tzeng, C.R.; Wang, C.W.; Hsu, M.I.; Tan, S.J.; Chen, C.H. Antiapoptotic agent sphingosine-1-phosphate protects vitrified murine ovarian grafts. Reprod. Sci. 2014, 21, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Guzel, Y.; Bildik, G.; Dilege, E.; Oktem, O. Sphingosine-1-phosphate reduces atresia of primordial follicles occurring during slow-freezing and thawing of human ovarian cortical strips. Mol. Reprod. Dev. 2018, 85, 858–864. [Google Scholar] [CrossRef]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J. Ovarian Res. 2018, 11, 33. [Google Scholar] [CrossRef]
- Blumenfeld, Z. Fertility Preservation Using GnRH Agonists: Rationale, Possible Mechanisms, and Explanation of Controversy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119870163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenfeld, Z. GnRH-agonists in fertility preservation. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 2007, 12, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Clowse, M.E.; Behera, M.A.; Anders, C.K.; Copland, S.; Coffman, C.J.; Leppert, P.C.; Bastian, L.A. Ovarian preservation by GnRH agonists during chemotherapy: A meta-analysis. J. Womens Health 2009, 18, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Blumenfeld, Z.; Eckman, A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J. Natl. Cancer Inst. Monogr. 2005, 34, 40–43. [Google Scholar] [CrossRef] [Green Version]
- Lambertini, M.; Horicks, F.; Del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat. Rev. 2019, 72, 65–77. [Google Scholar] [CrossRef] [Green Version]
- de Pedro, M.; Otero, B.; Martín, B. Fertility preservation and breast cancer: A review. Ecancermedicalscience 2015, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. Anti-Mullerian Hormone in Fertility Preservation: Clinical and Therapeutic Applications. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119854755. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Hwu, Y.M.; Lu, C.H.; Chang, H.H.; Hsieh, C.E.; Lee, R.K. VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues. Int. J. Mol. Sci. 2016, 17, 1237. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.K.; Shin, D.H.; Kim, B.Y.; Yoon, S.Y.; Yoon, T.K.; Lee, W.S.; Chung, H.M.; Lee, D.R. Effect of Human Endothelial Progenitor Cell (EPC)- or Mouse Vascular Endothelial Growth Factor-Derived Vessel Formation on the Survival of Vitrified/Warmed Mouse Ovarian Grafts. Reprod. Sci. 2014, 21, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Ryu, K.J.; Kim, B.; Kang, D.; Kim, Y.Y.; Kim, T. Comparison between Slow Freezing and Vitrification for Human Ovarian Tissue Cryopreservation and Xenotransplantation. Int. J. Mol. Sci. 2019, 20, 3346. [Google Scholar] [CrossRef] [Green Version]
- Manavella, D.D.; Cacciottola, L.; Payen, V.L.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. Mol. Hum. Reprod. 2019, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Manavella, D.D.; Cacciottola, L.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: A potential way to improve ovarian tissue transplantation. Hum. Reprod. 2018, 33, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.H.; Kim, S.Y.; Kim, Y.J.; Kim, S.J.; Lee, J.B.; Bae, Y.C.; Sung, S.M.; Jung, J.S. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell. Physiol. Biochem. 2006, 17, 279–290. [Google Scholar] [CrossRef]
- Schubert, T.; Xhema, D.; Veriter, S.; Schubert, M.; Behets, C.; Delloye, C.; Gianello, P.; Dufrane, D. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 2011, 32, 8880–8891. [Google Scholar] [CrossRef]
- Lafosse, A.; Desmet, C.; Aouassar, N.; Andre, W.; Hanet, M.S.; Beauloye, C.; Vanwijck, R.; Poirel, H.A.; Gallez, B.; Dufrane, D. Autologous Adipose Stromal Cells Seeded onto a Human Collagen Matrix for Dermal Regeneration in Chronic Wounds: Clinical Proof of Concept. Plast. Reconstr. Surg. 2015, 136, 279–295. [Google Scholar] [CrossRef]
- Van Eyck, A.S.; Jordan, B.F.; Gallez, B.; Heilier, J.F.; Van Langendonckt, A.; Donnez, J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil. Steril. 2009, 92, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Damous, L.L.; Nakamuta, J.S.; de Carvalho, A.E.; Soares, J.M., Jr.; de Jesus Simoes, M.; Krieger, J.E.; Baracat, E.C. Adipose tissue-derived stem cell therapy in rat cryopreserved ovarian grafts. Stem Cell Res. Ther. 2015, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Yin, T.; Yan, J.; Yan, L.; Jin, C.; Lu, C.; Wang, T.; Zhu, X.; Zhi, X.; Wang, J.; et al. Mesenchymal Stem Cells Enhance Angiogenesis and Follicle Survival in Human Cryopreserved Ovarian Cortex Transplantation. Cell Transplant. 2015, 24, 1999–2010. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.M.; Yan, J.; Li, R.; Li, M.; Yan, L.Y.; Wang, T.R.; Zhao, H.C.; Zhao, Y.; Yu, Y.; Qiao, J. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum. Reprod. 2013, 28, 2784–2793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xia, X.; Yan, J.; Yan, L.; Lu, C.; Zhu, X.; Wang, T.; Yin, T.; Li, R.; Chang, H.M.; et al. Mesenchymal stem cell-derived angiogenin promotes primodial follicle survival and angiogenesis in transplanted human ovarian tissue. Reprod. Biol. Endocrinol. 2017, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Manavella, D.D.; Cacciottola, L.; Pomme, S.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum. Reprod. 2018, 33, 1107–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, Z.; Wu, M.; Zhang, J.; Cheng, J.; Luo, A.; Shen, W.; Fang, L.; Zhou, S.; Wang, S. Intraovarian Transplantation of Female Germline Stem Cells Rescue Ovarian Function in Chemotherapy-Injured Ovaries. PLoS ONE 2015, 10, e0139824. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Morimoto, H.; Shinohara, T. Fertility of Male Germline Stem Cells Following Spermatogonial Transplantation in Infertile Mouse Models. Biol. Reprod. 2016, 94, 112. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, S.; Buigues, A.; Diaz-Garcia, C.; Romeu, M.; Martinez, S.; Gomez-Segui, I.; Simon, C.; Hsueh, A.J.; Pellicer, A. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil. Steril. 2018, 109, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Segers, I.; Mateizel, I.; Van Moer, E.; Smitz, J.; Tournaye, H.; Verheyen, G.; De Vos, M. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: A promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J. Assist. Reprod. Genet. 2015, 32, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Fouks, Y.; Hamilton, E.; Cohen, Y.; Hasson, J.; Kalma, Y.; Azem, F. In-vitro maturation of oocytes recovered during cryopreservation of pre-pubertal girls undergoing fertility preservation. Reprod. Biomed. Online 2020, 41, 869–873. [Google Scholar] [CrossRef]
- Xia, X.; Wang, T.; Yin, T.; Yan, L.; Yan, J.; Lu, C.; Zhao, L.; Li, M.; Zhang, Y.; Jin, H.; et al. Mesenchymal Stem Cells Facilitate In Vitro Development of Human Preantral Follicle. Reprod. Sci. 2015, 22, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, Z.; Yazdekhasti, H.; Noori Mugahi, S.M.H.; Abbasi, M.; Kazemnejad, S.; Shirazi, A.; Majidi, M.; Zarnani, A.H. Mouse preantral follicle growth in 3D co-culture system using human menstrual blood mesenchymal stem cell. Reprod. Biol. 2018, 18, 122–131. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Kim, S.; Lee, S. Role of Stem Cells in the Ovarian Tissue Cryopreservation and Transplantation for Fertility Preservation. Int. J. Mol. Sci. 2021, 22, 12482. https://doi.org/10.3390/ijms222212482
Kim JM, Kim S, Lee S. Role of Stem Cells in the Ovarian Tissue Cryopreservation and Transplantation for Fertility Preservation. International Journal of Molecular Sciences. 2021; 22(22):12482. https://doi.org/10.3390/ijms222212482
Chicago/Turabian StyleKim, Jeong Min, Seongmin Kim, and Sanghoon Lee. 2021. "Role of Stem Cells in the Ovarian Tissue Cryopreservation and Transplantation for Fertility Preservation" International Journal of Molecular Sciences 22, no. 22: 12482. https://doi.org/10.3390/ijms222212482






