Nitrate Capture Investigation in Plasma-Activated Water and Its Antifungal Effect on Cryptococcus pseudolongus Cells
Abstract
:1. Introduction
2. Results and Discussion
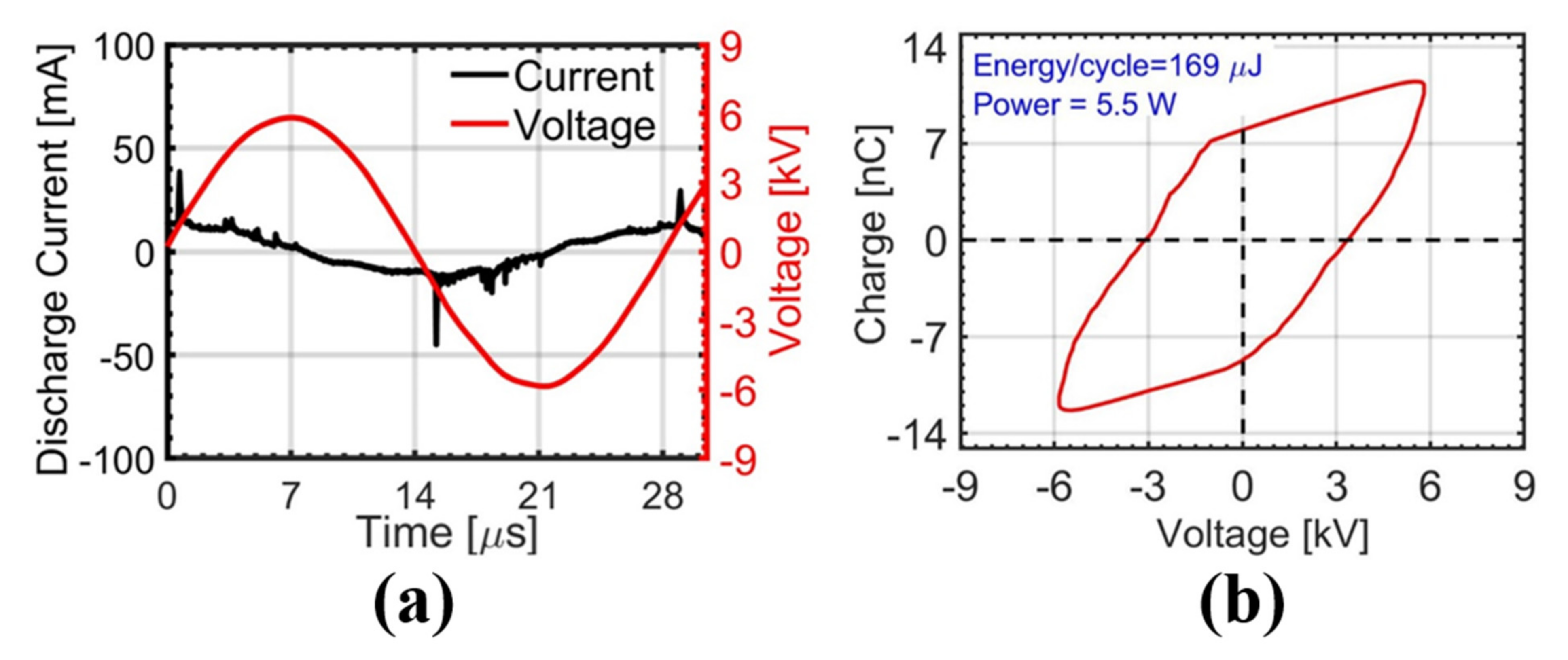
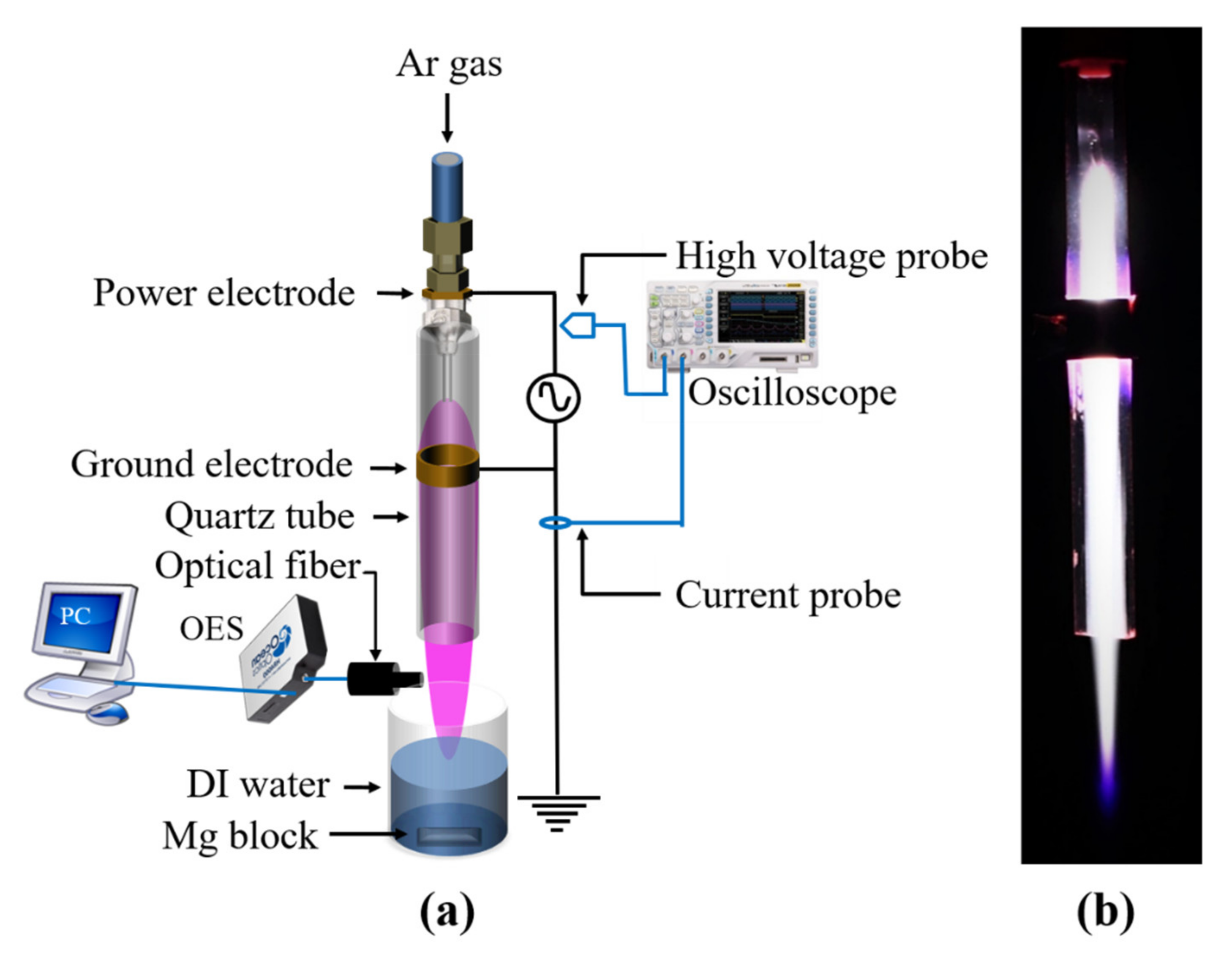
2.1. Electrical Characteristics of Atmospheric-Pressure Ar Plasma Discharge
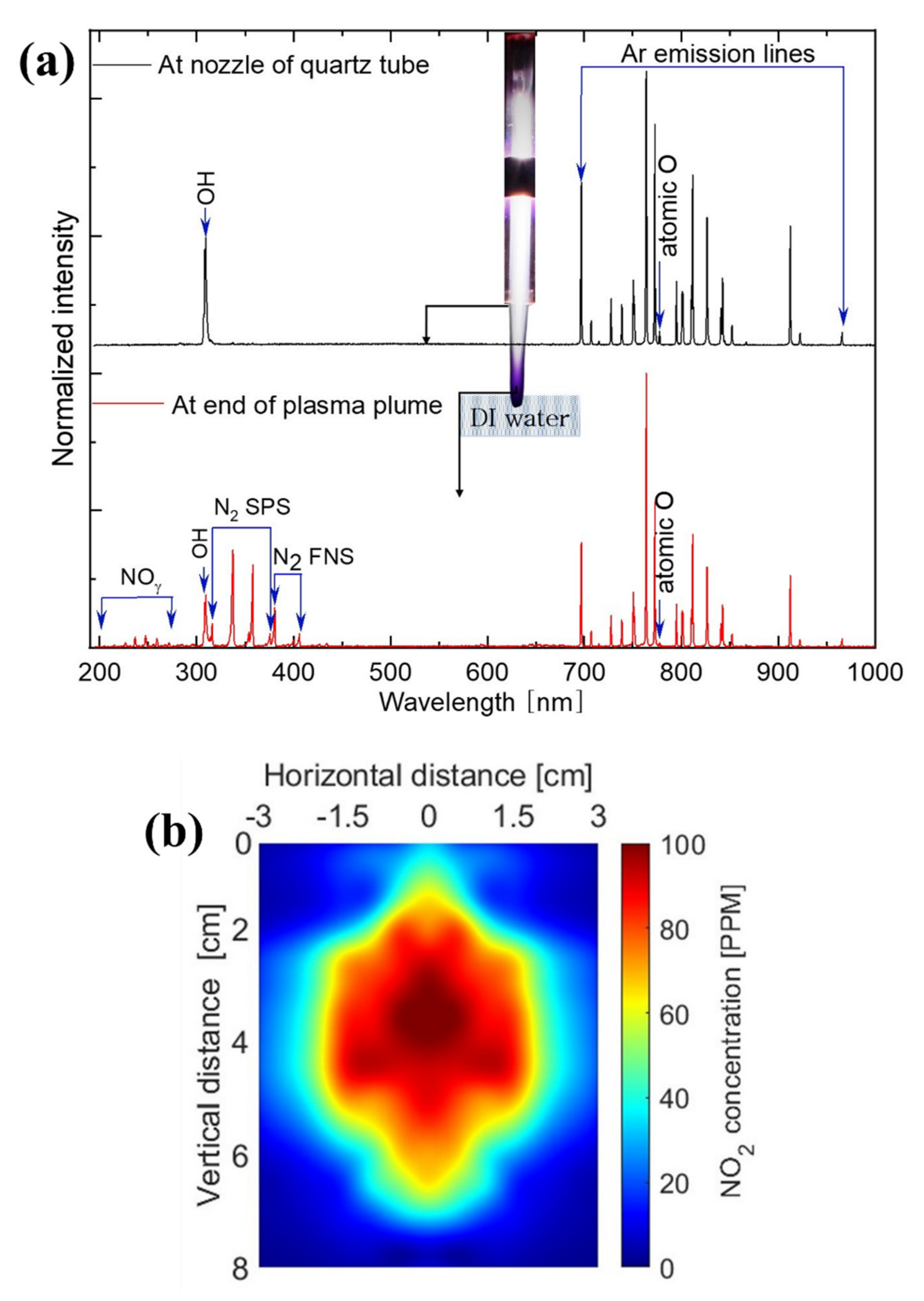
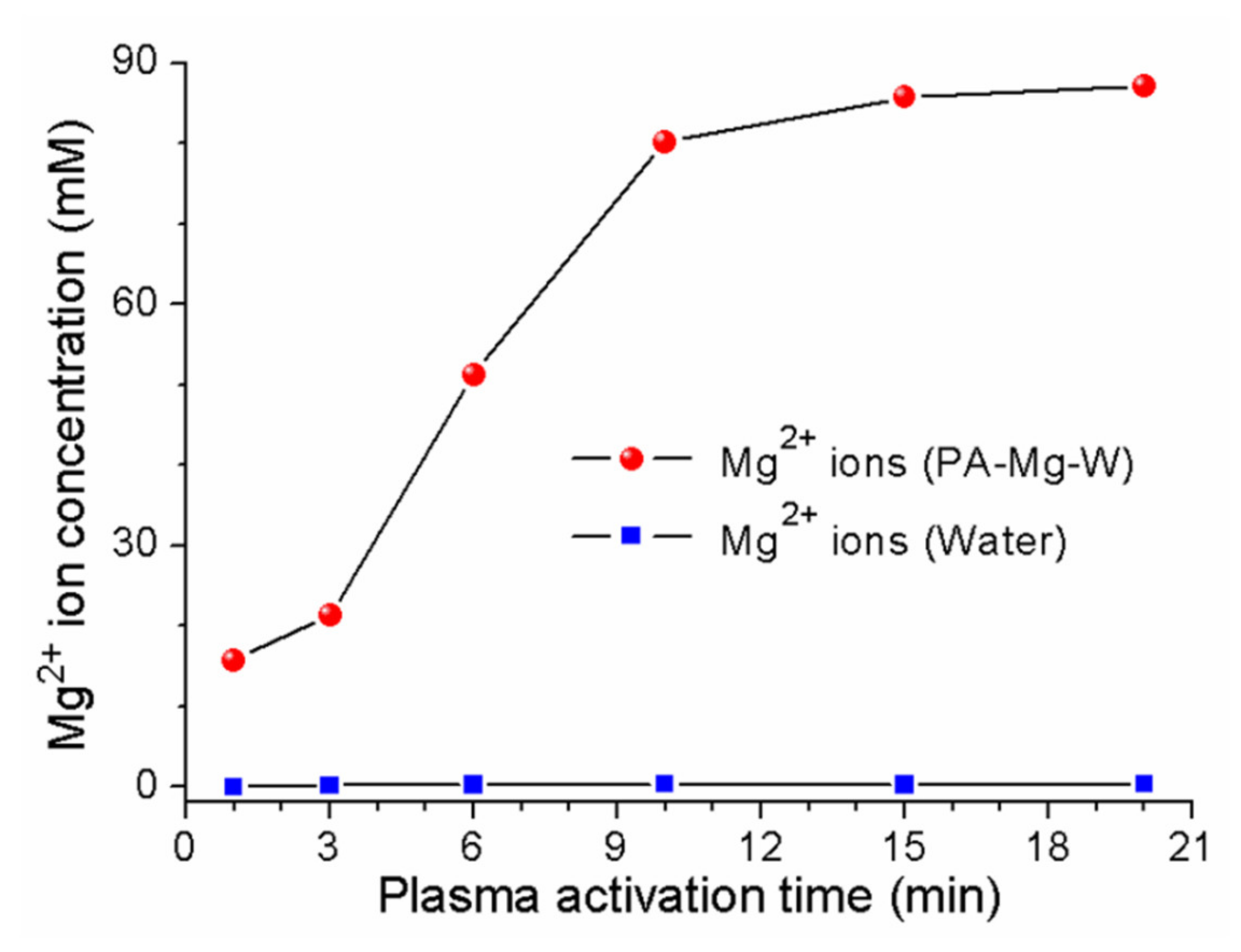
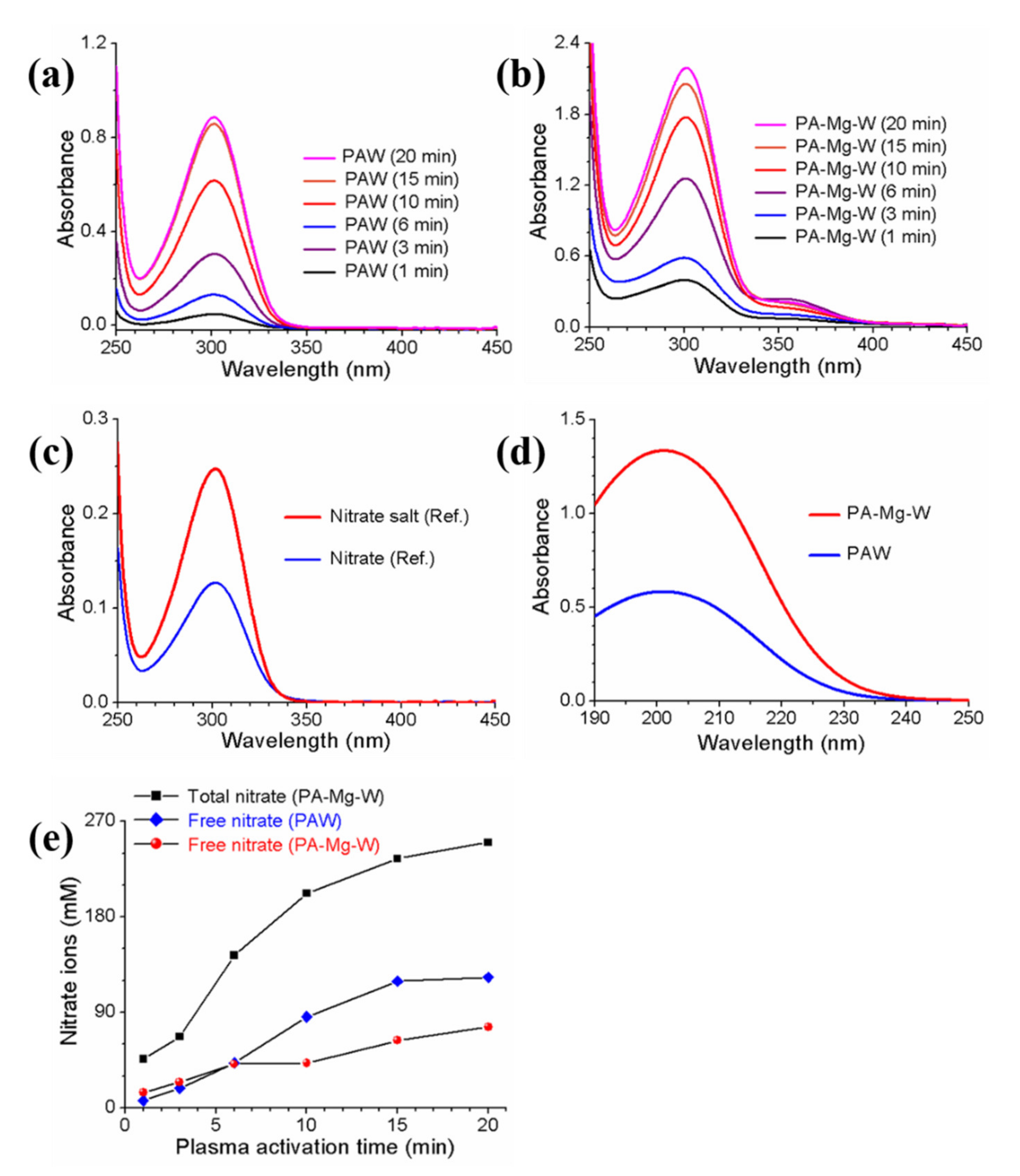
2.2. Analyses of Reactive Oxygen and Nitrogen Species Produced in Plasma Plume and Plasma-Activated Mg Water
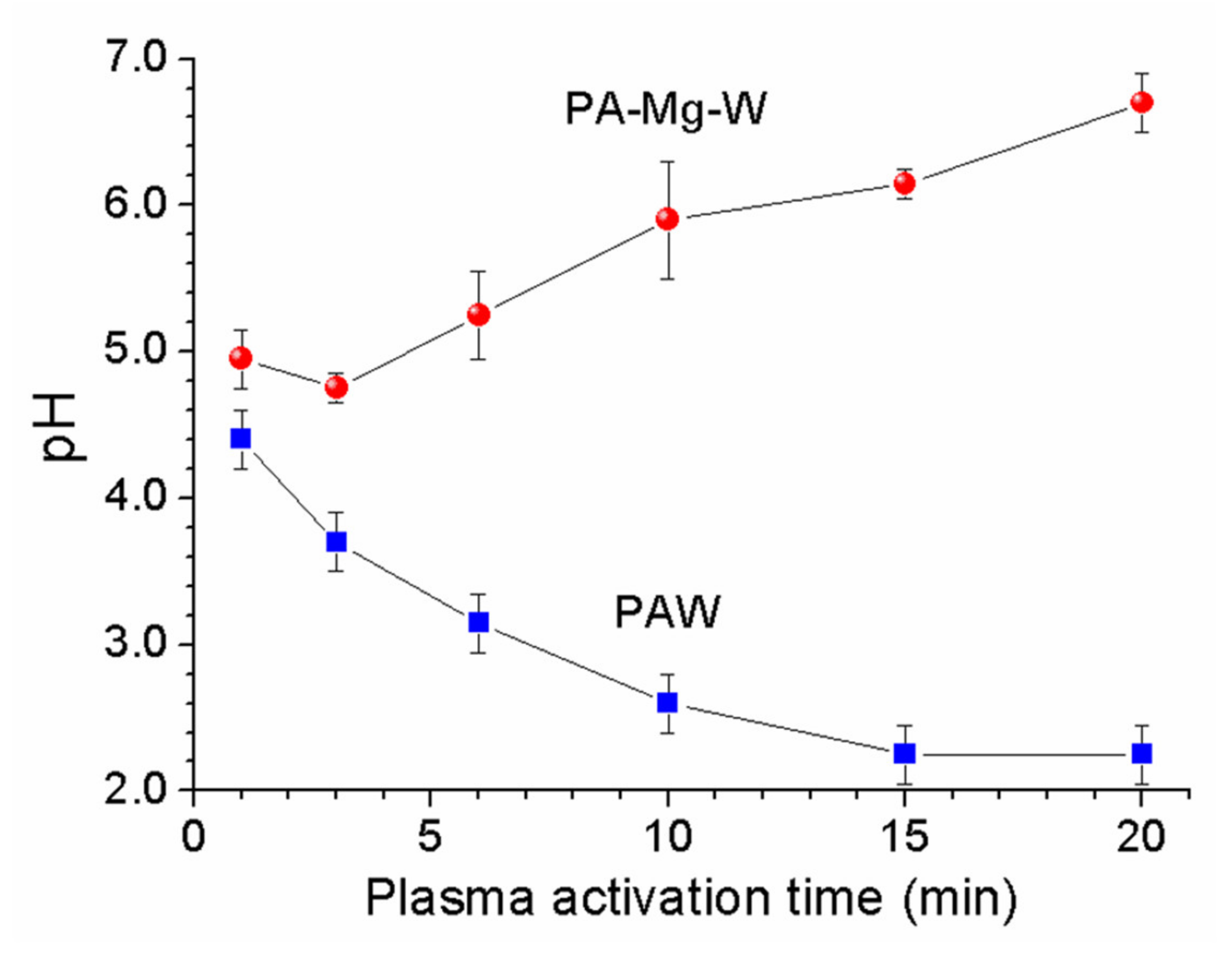
2.3. Optical Absorption Properties of Plasma-Activated Mg Water
2.4. Raman Spectroscopic Study of Plasma-Activated Mg Water
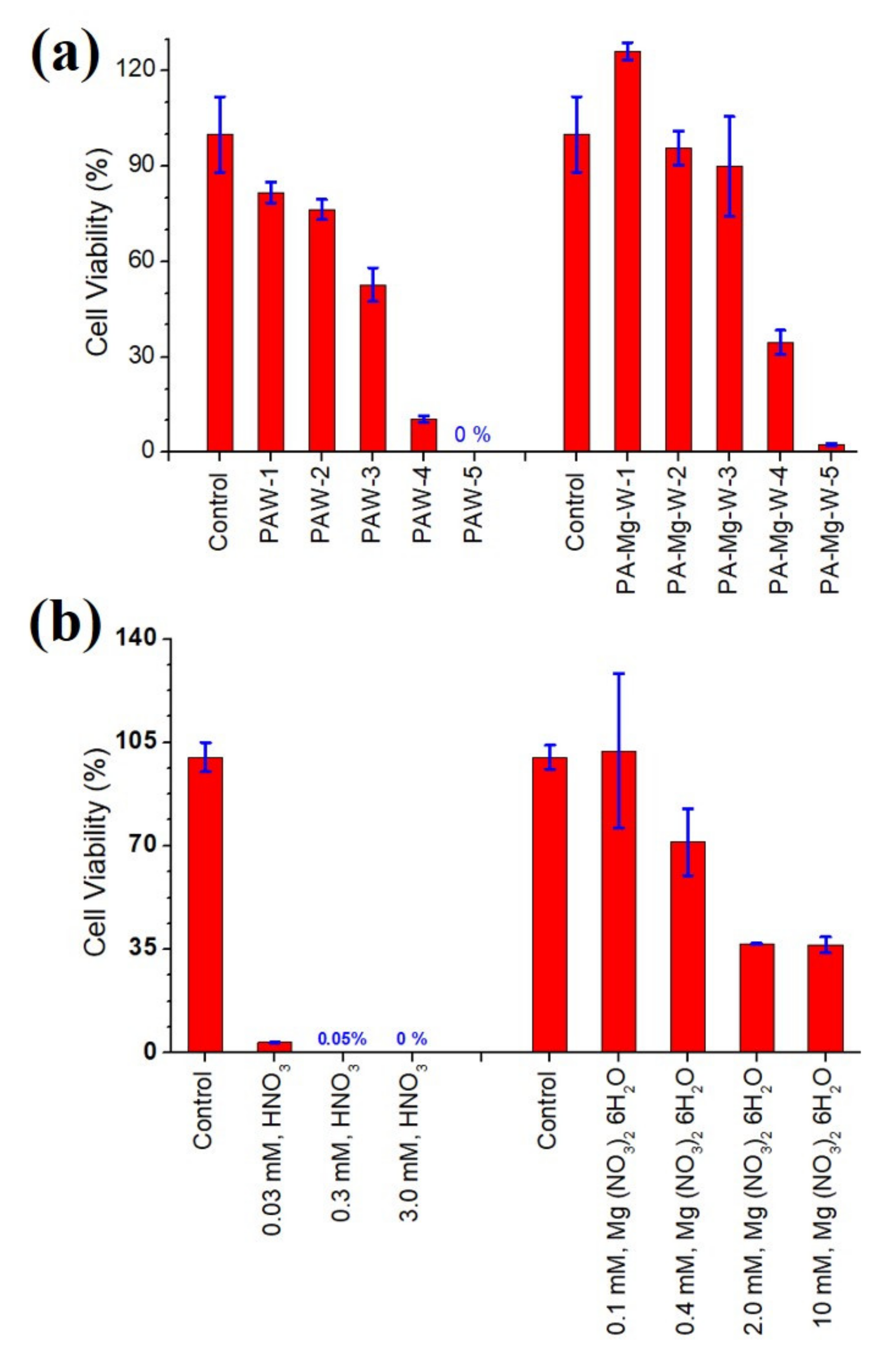
2.5. Antifungal Effects of Plasma-Activated Water on C. pseudolongus Cells
3. Materials and Experimental Methods
3.1. Characterizations of the Atmospheric-Pressure Ar Plasma Jet and Plasma-Activated Mg Water
3.2. Fungal Growth and Antifungal Test of Plasma-Activated Mg Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma-activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Lackmann, J.W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef] [Green Version]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.; Bai, N.; Zhou, H.; Wang, R.; Feng, H.; Zhu, W.; Zhang, J.; Fang, J. Inactivation of Bacillus subtilis spores in water by a direct-current, cold atmospheric-pressure air plasma microjet. Plasma Process. Polym. 2012, 9, 157–164. [Google Scholar] [CrossRef]
- Liu, F.; Sun, P.; Bai, N.; Tian, Y.; Zhou, H.; Wei, S.; Zhou, Y.; Zhang, J.; Zhu, W.; Becker, K.; et al. Inactivation of bacteria in an aqueous environment by a direct-current, cold-atmospheric-pressure air plasma microjet. Plasma Process. Polym. 2010, 7, 231–236. [Google Scholar] [CrossRef]
- Puac, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Li, Y.; Kang, M.H.; Uhm, H.S.; Lee, G.J.; Choi, E.H.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma-activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.J.; Kwon, Y.W.; Kim, Y.H.; Choi, E.H. Raman spectroscopic study of plasma-treated salmon DNA. Appl. Phys. Lett. 2013, 102, 021911. [Google Scholar]
- Noh, H.; Kim, J.E.; Kim, J.Y.; Kim, S.H.; Han, I.; Lim, J.; Ki, S.H.; Choi, E.H.; Lee, G.J. Spore viability and cell wall integrity of Cordyceps pruinosa treated with an electric shock-free, atmospheric-pressure air plasma jet. Appl. Sci. 2019, 9, 3921. [Google Scholar] [CrossRef] [Green Version]
- Ki, S.H.; Noh, H.; Ahn, G.R.; Kim, S.H.; Kaushik, N.K.; Choi, E.H.; Lee, G.J. Influence of nonthermal atmospheric plasma-activated water on the structural, optical, and biological properties of Aspergillus brasiliensis spores. Appl. Sci. 2020, 10, 6378. [Google Scholar] [CrossRef]
- Lamichhane, P.; Paneru, R.; Nguyen, L.N.; Lim, J.S.; Bhartiya, P.; Adhikari, B.C.; Mumtaz, S.; Choi, E.H. Plasma-assisted nitrogen fixation in water with various metals. React. Chem. Eng. 2020, 5, 2053–2057. [Google Scholar] [CrossRef]
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-temperature plasma-assisted nitrogen fixation for corn plant growth and development. Int. J. Mol. Sci. 2021, 22, 5360. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.W.; Kim, S.H. Pathological properties of Cryptococcus pseudolongus on the mycelia and fruit body of Lentinula edodes. Mycobiology 2021, 49, 173–182. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ramírez, A.; Cotrino, J.; Lambert, R.M.; González-Elipe, A.R. Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 2015, 24, 065011. [Google Scholar] [CrossRef]
- Wang, Y.; Craven, M.; Yu, X.; Ding, J.; Bryant, P.; Huang, J.; Tu, X. Plasma-enhanced catalytic synthesis of ammonia over a Ni/Al2O3 catalyst at near-room temperature: Insights into the importance of the catalyst surface on the reaction mechanism. ACS Catal. 2019, 9, 10780–10793. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Shao, T.; Zhang, C.; Li, W.; Yan, P.; Che, X.; Schamiloglu, E. Experimental study of Q-V Lissajous figures in nanosecond-pulse surface discharges. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 1101–1111. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, I.H.; Kim, D.; Kim, S.H.; Kwon, Y.W.; Han, G.H.; Cho, G.; Choi, E.H.; Lee, G.J. Effects of reactive oxygen species on the biological, structural, and optical properties of Cordyceps pruinosa spores. RSC Adv. 2016, 6, 30699–30709. [Google Scholar] [CrossRef]
- Lee, G.J.; Sim, G.B.; Choi, E.H.; Kwon, Y.W.; Kim, J.Y.; Jang, S.; Kim, S.H. Optical and structural properties of plasma-treated Cordyceps bassiana spores as studied by circular dichroism, absorption, and fluorescence spectroscopy. J. Appl. Phys. 2015, 117, 023303. [Google Scholar] [CrossRef]
- Atomic Spectra Database. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 1 February 2021).
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17, 063504. [Google Scholar] [CrossRef] [Green Version]
- Ershov, A.; Borysow, J. Dynamics of OH X2Π (v = 0) in high-energy atmospheric pressure electrical pulsed discharge. J. Phys. D Appl. Phys. 1995, 28, 68–74. [Google Scholar] [CrossRef]
- Rahman, A.; Yalin, A.; Surla, V.; Stan, O.; Hoshimiya, K.; Yu, Z.; Littlefield, E.; Collins, G. Absolute UV and VUV emission in the 110–400 nm region from 13.56 MHz driven hollow slot microplasmas operating in open air. Plasma Sources Sci. Technol. 2004, 13, 537–547. [Google Scholar] [CrossRef]
- Ghimire, B.; Lamichhane, P.; Lim, J.S.; Min, B.; Paneru, R.; Weltmann, K.D.; Choi, E.H. An atmospheric pressure plasma jet operated by injecting natural air. Appl. Phys. Lett. 2018, 113, 194101. [Google Scholar] [CrossRef]
- Lee, G.J.; Choi, M.A.; Kim, D.; Kim, J.Y.; Ghimire, B.; Choi, E.H.; Kim, S.H. Influence of plasma-generated reactive species on the plasmid DNA structure and plasmid-mediated transformation of Escherichia coli cells. J. Appl. Phys. 2017, 122, 103303. [Google Scholar] [CrossRef]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Mol. Biol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/ radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- De Grey, A.D.N.J. : The forgotten radical. DNA Cell Biol. 2002, 21, 251–257. [Google Scholar] [CrossRef]
- Schmidt-Bleker, A.; Winter, J.; Bosel, A.; Reuter, S.; Weltmann, K.D. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Sci. Technol. 2015, 25, 015005. [Google Scholar] [CrossRef]
- Schmidt-Bleker, A.; Winter, J.; Iseni, S.; Dunnbier, M.; Weltmann, K.D.; Reuter, S. Reactive species output of a plasma jet with a shielding gas device-combination of FTIR absorption spectroscopy and gas phase modelling. J. Phys. D Appl. Phys. 2014, 47, 145201. [Google Scholar] [CrossRef]
- Uhm, H.S. Generation of various radicals in nitrogen plasma and their behavior in media. Phys. Plasmas 2015, 22, 123506. [Google Scholar] [CrossRef]
- Uhm, H.S.; Na, Y.H.; Lee, C.B.; Choi, E.H.; Cho, G. Dissociation and excitation coefficients of nitrogen molecules and radical generation in nitrogen plasma. Curr. Appl. Phys. 2014, 14, S162–S166. [Google Scholar] [CrossRef]
- Lee, G.; Park, J. Reaction of zero-valent magnesium with water: Potential applications in environmental remediation. Geochim. Cosmochim. Acta 2013, 102, 162–174. [Google Scholar] [CrossRef]
- Tsukahara, H.; Ishida, T.; Mayumi, M. Gas-phase oxidation of nitric oxide: Chemical kinetics and rate constant. Nitric Oxide 1999, 3, 191–198. [Google Scholar] [CrossRef]
- Bibinov, N.; Knake, N.; Bahre, H.; Awakowicz, P.; Schulz-von der Gathen, V. Spectroscopic characterization of an atmospheric pressure μ-jet plasma source. J. Phys. D Appl. Phys. 2011, 44, 345204. [Google Scholar] [CrossRef]
- Deng, X.L.; Nikiforov, A.Y.; Vanraes, P.; Leys, C. Direct current plasma jet at atmospheric pressure operating in nitrogen and air. J. Appl. Phys. 2013, 113, 023305. [Google Scholar] [CrossRef] [Green Version]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Van Gils, C.A.J.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Fukuto, J.M.; Griscavage, J.M.; Rogers, N.E.; Byrns, R.E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: Comparison with enzymatically formed nitric oxide from L-arginine. Proc. Natl. Acad. Sci. USA 1993, 90, 8103−8107. [Google Scholar] [CrossRef] [Green Version]
- Pires, M.; Rossi, M.J.; Ross, D.S. Kinetic and mechanistic aspects of the NO oxidation by O2 in aqueous phase. Int. J. Chem. Kinet. 1994, 26, 1207–1227. [Google Scholar] [CrossRef]
- Lamichhane, P.; Ghimire, B.; Mumtaz, S.; Paneru, R.; Ki, S.H.; Choi, E.H. Control of hydrogen peroxide production in plasma-activated water by utilizing nitrification. J. Phys. D Appl. Phys. 2019, 52, 265206. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A.; Cunningham, M.M. Measurement of the absorption constants for nitrate in water between 270 and 335 nm. Environ. Sci. Technol. 1992, 26, 207–209. [Google Scholar] [CrossRef]
- Lanoul, A.; Coleman, T.; Asher, S.A. UV resonance Raman spectroscopic detection of nitrate and nitrite in waste water treatment processes. Anal. Chem. 2002, 74, 1458–1461. [Google Scholar] [PubMed]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Szabo, C.; Ohshima, H. DNA damage induced by peroxynitrite: Subsequent biological effects. Nitric Oxide 1997, 1, 373–385. [Google Scholar] [CrossRef]
- Carey, D.M.; Korenowski, G.M. Measurement of the Raman spectrum of liquid water. J. Chem. Phys. 1998, 108, 2669–2675. [Google Scholar] [CrossRef]
- Moskovits, M.; Michaelian, K.H. A reinvestigation of the Raman spectrum of water. J. Chem. Phys. 1978, 69, 2306–2311. [Google Scholar] [CrossRef]
- Xu, M.; Larentzos, J.P.; Roshdy, M.; Criscenti, L.J.; Allen, H.C. Aqueous divalent metal–nitrate interactions: Hydration versus ion pairing. Phys. Chem. Chem. Phys. 2008, 10, 4793–4801. [Google Scholar] [CrossRef]
- Fontana, M.D.; Mabrouk, K.B.; Kauffmann, T.H. Raman Spectroscopic Sensors for Inorganic Salts. In Spectroscopic Properties of Inorganic and Organometallic Compounds; Yarwood, J., Douthwaite, R., Duckett, S., Eds.; RSC Publishing: Cambridge, UK, 2013; Volume 44, pp. 40–67. [Google Scholar]
- Koussinsa, F.; Bertin, F. Raman microspectrometric study of the dissolution layer of M(NO3)2⋅nH2O crystals (M = Mg, Ca, Zn and Cd) in their undersaturated aqueous solutions. J. Raman Spectrosc. 1991, 22, 169–176. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. BioEssays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Miller, A.J. Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 659–688. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.J.; Lamichhane, P.; Ahn, S.J.; Kim, S.H.; Yewale, M.A.; Choong, C.E.; Jang, M.; Choi, E.H. Nitrate Capture Investigation in Plasma-Activated Water and Its Antifungal Effect on Cryptococcus pseudolongus Cells. Int. J. Mol. Sci. 2021, 22, 12773. https://doi.org/10.3390/ijms222312773
Lee GJ, Lamichhane P, Ahn SJ, Kim SH, Yewale MA, Choong CE, Jang M, Choi EH. Nitrate Capture Investigation in Plasma-Activated Water and Its Antifungal Effect on Cryptococcus pseudolongus Cells. International Journal of Molecular Sciences. 2021; 22(23):12773. https://doi.org/10.3390/ijms222312773
Chicago/Turabian StyleLee, Geon Joon, Pradeep Lamichhane, Seong Jae Ahn, Seong Hwan Kim, Manesh Ashok Yewale, Choe Earn Choong, Min Jang, and Eun Ha Choi. 2021. "Nitrate Capture Investigation in Plasma-Activated Water and Its Antifungal Effect on Cryptococcus pseudolongus Cells" International Journal of Molecular Sciences 22, no. 23: 12773. https://doi.org/10.3390/ijms222312773






