Tryptophan Hydroxylase 2 Deficiency Modifies the Effects of Fluoxetine and Pargyline on the Behavior, 5-HT- and BDNF-Systems in the Brain of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Results
2.1. Effects of pCPA and Fluoxetine on Zebrafish Behavior in the Novel Tank Diving Test
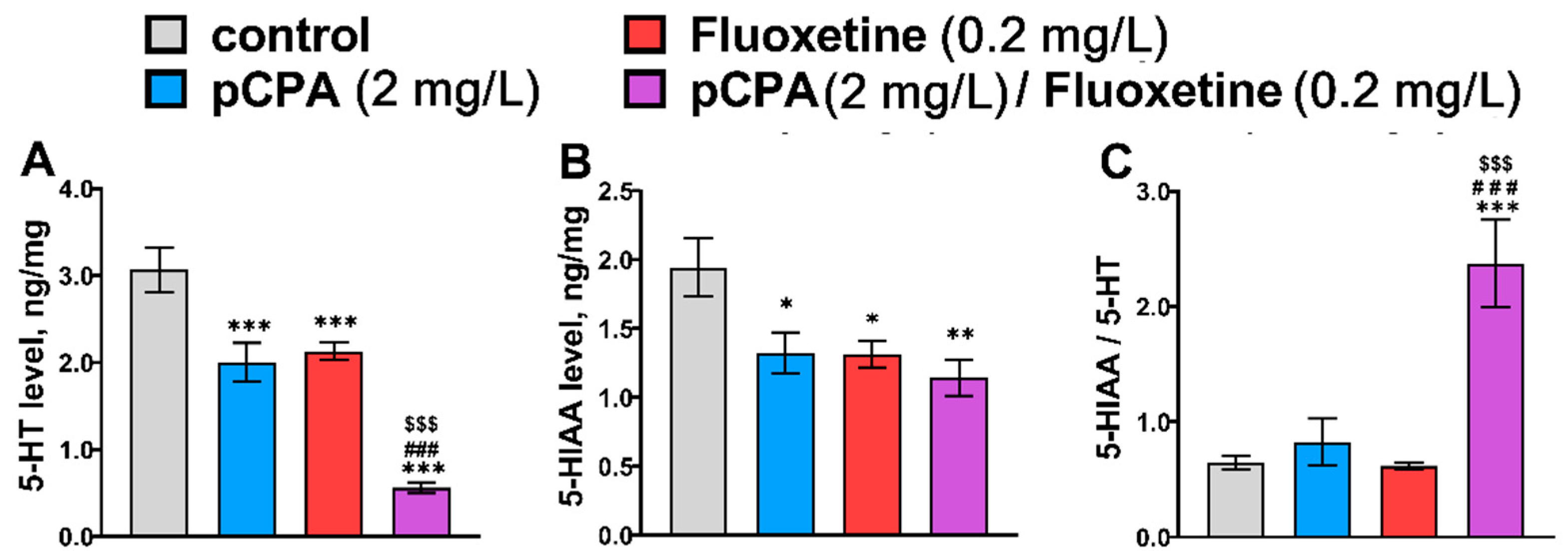
2.2. Effects of pCPA and Fluoxetine on 5-HT Metabolism in Zebrafish Brain
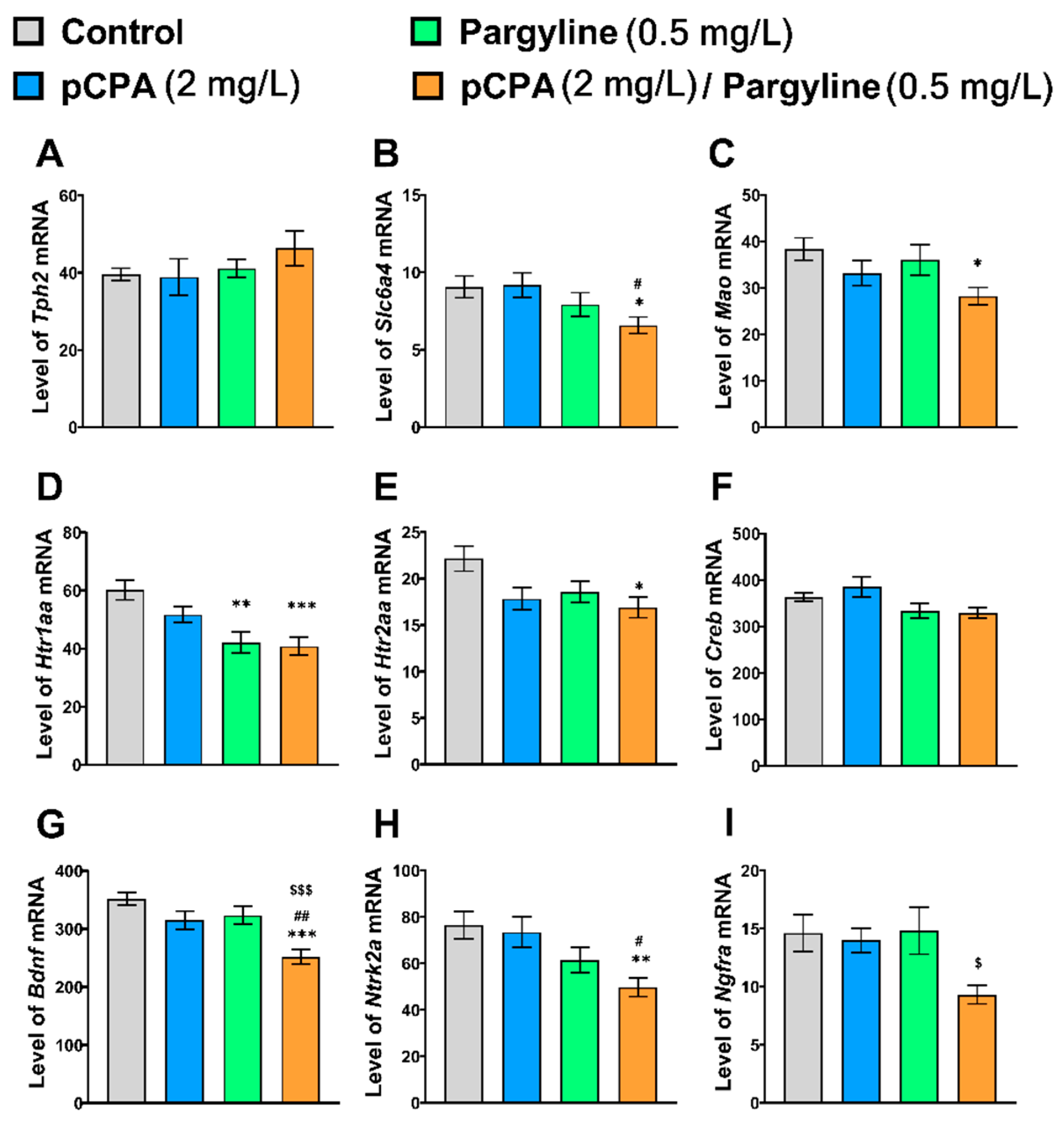
2.3. Effects of pCPA and Fluoxetine on mRNA Levels of Tph2, Slc6a4b, Mao, Htr1aa, Htr2aa, Creb, Bdnf, Ntrk2a and Ngfra Genes in Zebrafish Brain
2.4. Effects of pCPA and Pargyline on Zebrafish Behavior in the Novel Tank Diving Test
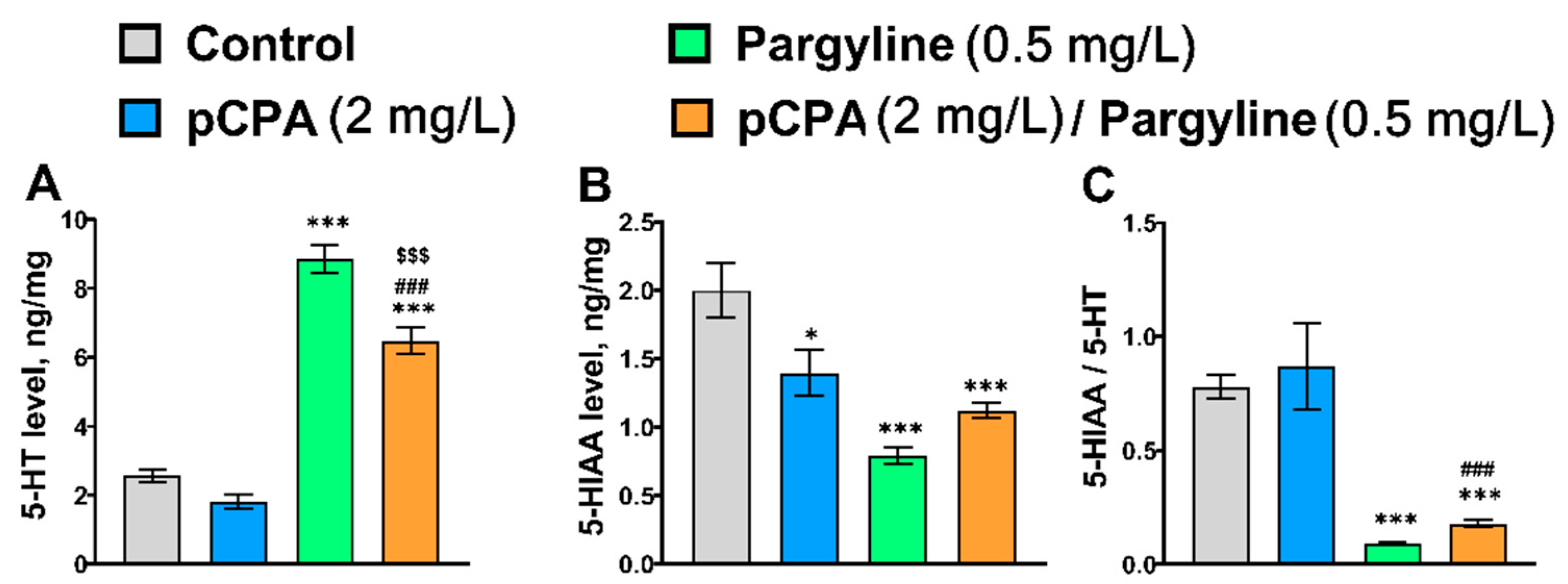
2.5. Effects of pCPA and Pargyline on 5-HT Metabolism in Zebrafish Brain
2.6. Effects of pCPA and Pargyline on the mRNA Levels of Tph2, Slc6a4b, Mao, Htr1aa, Htr2aa, Creb, Bdnf, Ntrk2a and Ngfra Genes in Zebrafish Brain
3. Discussion
4. Materials and Methods
4.1. Zebrafish
4.2. Drugs and Treatments
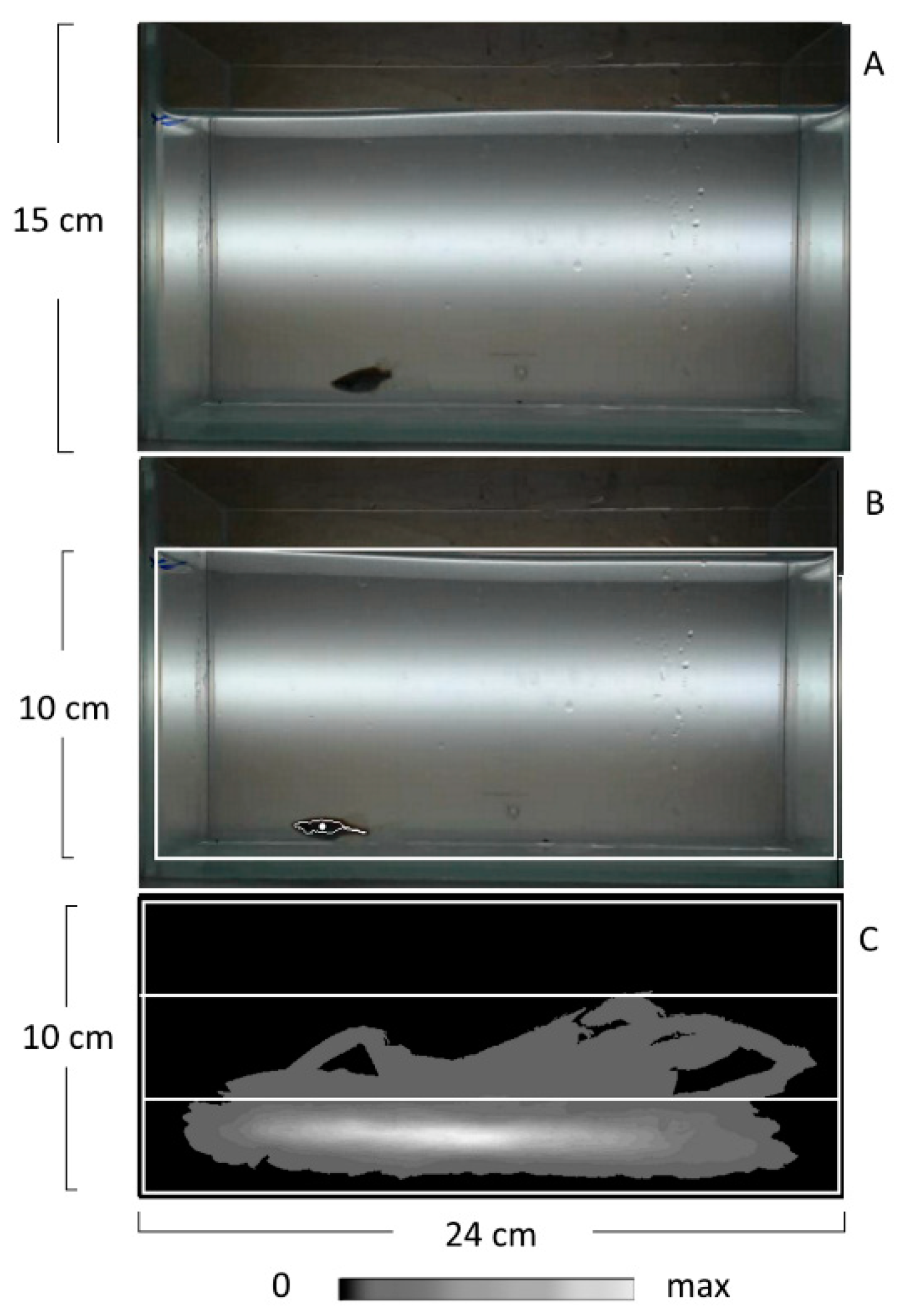
4.3. The Novel Tank Diving (NT) Test
4.4. 5-HT and 5-HIAA Concentrations Assay by HPLC
4.5. mRNA Level Assay by qPCR
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 26, 90. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Patten, S.B.; Vos, T.; Whiteford, H.A. The epidemiological modelling of major depressive disorder: Application for the Global Burden of Disease Study 2010. PLoS ONE 2013, 8, e69637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.A.; Salanti, G.; Atkinson, L.Z.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Chaimani, A.; Ogawa, Y.; Takeshima, N.; Hayasaka, Y.; et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: Protocol for a network meta-analysis. BMJ Open 2016, 6, e010919. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; El Mansari, M. Serotonin and beyond: Therapeutics for major depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120536. [Google Scholar] [CrossRef]
- Hale, M.W.; Raison, C.L.; Lowry, C.A. Integrative physiology of depression and antidepressant drug action: Implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol. Ther. 2013, 137, 108–118. [Google Scholar] [CrossRef]
- Köhler, S.; Cierpinsky, K.; Kronenberg, G.; Adli, M. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J. Psychopharmacol. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Olivier, B. Serotonin: A never-ending story. Eur. J. Pharmacol. 2015, 753, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Walker, F.R. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: Do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 2013, 67, 304–317. [Google Scholar] [CrossRef]
- Crisafulli, C.; Fabbri, C.; Porcelli, S.; Drago, A.; Spina, E.; De Ronchi, D.; Serretti, A. Pharmacogenetics of antidepressants. Front. Pharmacol. 2011, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- El-Hage, W.; Leman, S.; Camus, V.; Belzung, C. Mechanisms of antidepressant resistance. Front. Pharmacol. 2013, 4, 146. [Google Scholar] [CrossRef] [Green Version]
- Levinstein, M.R.; Samuels, B.A. Mechanisms underlying the antidepressant response and treatment resistance. Front. Behav. Neurosci. 2014, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathopysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Blier, P.; de Montigny, C. Serotonin and drug induced therapeutic responses in major depression, obsessive compulsive and panic disorders. Neuropsychopharmacology 1999, 21 (Suppl. S2), 91–98. [Google Scholar] [CrossRef] [Green Version]
- Bazhenova, E.Y.; Sinyakova, N.A.; Kulikova, E.A.; Kazarinova, I.A.; Bazovkina, D.V.; Gainetdinov, R.R.; Kulikov, A.V. No effect of C1473G polymorphism in the tryptophan hydroxylase 2 gene on the response of the brain serotonin system to chronic fluoxetine treatment in mice. Neurosci. Lett. 2017, 653, 264–268. [Google Scholar] [CrossRef]
- Siesser, W.B.; Sachs, B.D.; Ramsey, A.J.; Sotnikova, T.D.; Beaulieu, J.M.; Zhang, X.; Caron, M.G.; Gainetdinov, R.R. Chronic SSRI treatment exacerbates serotonin deficiency in humanized Tph2 mutant mice. ACS Chem. Neurosci. 2013, 4, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Koe, B.K.; Weissman, A. p-Chlorophenylalanine: A specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 1966, 154, 499–516. [Google Scholar] [PubMed]
- Kulikov, A.V.; Osipova, D.V.; Naumenko, V.S.; Terenina, E.; Mormède, P.; Popova, N.K. A pharmacological evidence of positive association between mouse intermale aggression and brain serotonin metabolism. Behav. Brain Res. 2012, 233, 113–119. [Google Scholar] [CrossRef]
- Alenina, N.; Kikic, D.; Todiras, M.; Mosienko, V.; Qadri, F.; Plehm, R.; Boyé, P.; Vilianovitch, L.; Sohr, R.; Tenner, K.; et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl. Acad. Sci. USA 2009, 106, 10332–10337. [Google Scholar] [CrossRef] [Green Version]
- Gutknecht, L.; Waider, J.; Kraft, S.; Kriegebaum, C.; Holtmann, B.; Reif, A.; Schmitt, A.; Lesch, K.P. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural Transm. 2008, 115, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Savelieva, K.V.; Zhao, S.; Pogorelov, V.M.; Rajan, I.; Yang, Q.; Cullinan, E.; Lanthorn, T.H. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE 2008, 3, e3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulikov, A.V.; Gainetdinov, R.R.; Ponimaskin, E.; Kalueff, A.V.; Naumenko, V.S.; Popova, N.K. Interplay between the key proteins of serotonin system in SSRI antidepressants efficacy. Expert Opin. Ther. Targets 2018, 22, 319–330. [Google Scholar] [CrossRef]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef]
- Guzzetti, S.; Calcagno, E.; Canetta, A.; Sacchetti, G.; Fracasso, C.; Caccia, S.; Cervo, L.; Invernizzi, R.W. Strain differences in paroxetine-induced reduction of immobility time in the forced swimming test in mice: Role of serotonin. Eur. J. Pharmacol. 2008, 594, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Tikhonova, M.A.; Osipova, D.V.; Kulikov, V.A.; Popova, N.K. Association between tryptophan hydroxylase-2 genotype and the antidepressant effect of citalopram and paroxetine on immobility time in the forced swim test in mice. Pharmacol. Biochem. Behav. 2011, 99, 683–687. [Google Scholar] [CrossRef]
- Siesser, W.B.; Zhang, X.; Jacobsen, J.P.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase 2 genotype determines brain serotonin synthesis but not tissue content in C57Bl/6J and BALB/cJ congenic mice. Neurosci. Lett. 2010, 481, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Kulikova, E.A.; Kulikov, A.V. Tryptophan hydroxylase 2 as a therapeutic target for psychiatric disorders: Focus on animal models. Expert Opin. Ther. Targets 2019, 23, 655–667. [Google Scholar] [CrossRef]
- Gaspar, P.; Lillesaar, C. Probing the diversity of serotonin neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2382–2394. [Google Scholar] [CrossRef] [Green Version]
- Panula, P.; Chen, Y.C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Fursenko, D.V.; Bazhenova, E.Y.; Kulikov, A.V. Decrease in the Activity of Striatal-enriched Protein-tyrosine-Phosphatase (STEP) in the Brain of Danio rerio Treated with p-Chlorophenylalanine and Pargyline. Mol. Biol. 2021, 55, 573–578. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Kulikov, A.V.; Sinyakova, N.A.; Kulikova, E.A.; Khomenko, T.M.; Salakhutdinov, N.F.; Kulikov, V.A.; Volcho, K.P. Effects of acute and chronic treatment of novel psychotropic drug, 8-(trifluoromethyl)-1,2,3,4,5-benzopentathiepin-6-amine hydrochloride (TC-2153), on the behavior of zebrafish (Danio rerio): A comparison with fluoxetine. Lett. Drug Des. Discov. 2019, 16, 1321–1328. [Google Scholar] [CrossRef]
- Kulikova, E.A.; Bazovkina, D.V.; Evsyukova, V.S.; Kulikov, A.V. Acute Administration of Imipramine and Citalopram Increases Activity of Striatal-Enriched Tyrosine Protein Phosphatase (STEP) in Brain of Zebrafish Danio rerio. Bull. Exp. Biol. Med. 2021, 170, 627–630. [Google Scholar] [CrossRef]
- Maximino, C.; Puty, B.; Benzecry, R.; Araújo, J.; Lima, M.G.; de Jesus Oliveira Batista, E.; Renata de Matos Oliveira, K.; Crespo-Lopez, M.E.; Herculano, A.M. Role of serotonin in zebrafish (Danio rerio) anxiety: Relationship with serotonin levels, and effect of buspirone, WAY 100635, SB 224289, fluoxetine and parachlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology 2013, 71, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Sinyakova, N.A.; Kulikova, E.A.; Englevskii, N.A.; Kulikov, A.V. Effects of Fluoxetine and Potential Antidepressant 8-Trifluoromethyl 1,2,3,4,5-Benzopentathiepin-6-Amine Hydrochloride (TC-2153) on Behavior of Danio rerio Fish in the Novel Tank Test and Brain Content of Biogenic Amines and Their Metabolites. Bull. Exp. Biol. Med. 2018, 164, 620–623. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.E.; Ziani, P.R.; Fontana, B.D.; Duarte, T.; Stefanello, F.V.; Canzian, J.; Santos, A.R.S.; Rosemberg, D.B. Role of the serotonergic system in ethanol-induced aggression and anxiety: A pharmacological approach using the zebrafish model. Eur. Neuropsychopharmacol. 2020, 32, 66–76. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Zhang, X.; Rodriguiz, R.M.; Sotnikova, T.D.; Cools, M.J.; Wetsel, W.C.; Gainetdinov, R.R.; Caron, M.G. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. USA 2008, 105, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.M.; Cachat, J.; Gaikwad, S.; Robinson, K.S.; Gebhardt, M.; Kalueff, A.V. Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem. Int. 2013, 62, 893–902. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine inanimal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef] [Green Version]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.P.; et al. Neurogenesis-dependent and –independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Theodoridi, A.; Tsalafouta, A.; Pavlidis, M. Acute Exposure to Fluoxetine Alters Aggressive Behavior of Zebrafish and Expression of Genes Involved in Serotonergic System Regulation. Front. Neurosci. 2017, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine modulates the transcription of genes involved in serotonin, dopamine and adrenergic signalling in zebrafish embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef]
- Toth, M. Transcriptional regulation of the 5-HT2A receptor. Behav. Brain Res. 1996, 73, 183–186. [Google Scholar] [CrossRef]
- Van Oekelen, D.; Luyten, W.H.; Leysen, J.E. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003, 72, 2429–2449. [Google Scholar] [CrossRef]
- Roth, B.L.; Willins, D.L.; Kristiansen, K.; Kroeze, W.K. 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): Where structure meets function. Pharmacol. Ther. 1998, 79, 231–257. [Google Scholar] [CrossRef]
- Lesch, K.P.; Waider, J. Serotonin in the modulation of neural plasticity and networks: Implications for neurodevelopmental disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, N.K.; Naumenko, V.S. Neuronal and behavioral plasticity: The role of serotonin and BDNF systems tandem. Expert Opin. Ther. Targets 2019, 23, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, L.F.; Warren, B.L.; Parise, E.M.; Iñiguez, S.D.; Bolaños-Guzmán, C.A. Effects of psychotropic drugs on second messenger signaling and preference for nicotine in juvenile male mice. Psychopharmacology 2014, 231, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebb, A.L.; Robertson, H.A.; Denovan-Wright, E.M. Phosphodiesterase 10A inhibition is associated with locomotor and cognitive deficits and increased anxiety in mice. Eur. Neuropsychopharmacol. 2008, 18, 339–363. [Google Scholar] [CrossRef]
- Laifenfeld, D.; Karry, R.; Grauer, E.; Klein, E.; Ben-Shachar, D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol. Dis. 2005, 20, 432–441. [Google Scholar] [CrossRef]
- Rogóż, Z.; Kamińska, K.; Pańczyszyn-Trzewik, P.; Sowa-Kućma, M. Repeated co-treatment with antidepressants and risperidone increases BDNF mRNA and protein levels in rats. Pharmacol. Rep. 2017, 69, 885–893. [Google Scholar] [CrossRef]
- Si, Y.; Xue, X.; Liu, S.; Feng, C.; Zhang, H.; Zhang, S.; Ren, Y.; Ma, H.; Dong, Y.; Li, H.; et al. CRTC1 signaling involvement in depression-like behavior of prenatally stressed offspring rat. Behav. Brain Res. 2021, 399, 113000. [Google Scholar] [CrossRef]
- Song, L.; Che, W.; Min-Wei, W.; Murakami, Y.; Matsumoto, K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol. Biochem. Behav. 2006, 83, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Vinet, J.; Carra, S.; Blom, J.M.; Brunello, N.; Barden, N.; Tascedda, F. Chronic treatment with desipramine and fluoxetine modulate BDNF, CaMKKalpha and CaMKKbeta mRNA levels in the hippocampus of transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuropharmacology 2004, 47, 1062–1069. [Google Scholar] [CrossRef]
- Yan, L.; Xu, X.; He, Z.; Wang, S.; Zhao, L.; Qiu, J.; Wang, D.; Gong, Z.; Qiu, X.; Huang, H. Antidepressant-Like Effects and Cognitive Enhancement of Coadministration of Chaihu Shugan San and Fluoxetine: Dependent on the BDNF-ERK-CREB Signaling Pathway in the Hippocampus and Frontal Cortex. Biomed. Res. Int. 2020, 2020, 2794263. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; Puty, B.; Matos Oliveira, K.R.; Herculano, A.M. Behavioral and neurochemical changes in the zebrafish leopard strain. Genes Brain Behav. 2013, 12, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Tikhonova, M.A.; Kulikov, V.A. Automated measurement of spatial preference in the open field test with transmitted lighting. J. Neurosci. Methods 2008, 170, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Khotskin, N.V.; Plyusnina, A.V.; Kulikova, E.A.; Bazhenova, E.Y.; Fursenko, D.V.; Sorokin, I.E.; Kolotygin, I.; Mormede, P.; Terenina, E.E.; Shevelev, O.B.; et al. On association of the lethal yellow (AY) mutation in the agouti gene with the alterations in mouse brain and behavior. Behav. Brain Res. 2019, 359, 446–456. [Google Scholar] [CrossRef] [PubMed]



| Trait | pCPA (2 mg/L) | Fluoxetine (0.2 mg/L) | Interaction |
|---|---|---|---|
| Distance traveled | F1,36 < 1 | F1,36 = 20.0, p < 0.001 | F1,36 = 2.3, p = 0.14 |
| Distance from tank’s bottom | F1,36 = 28.0, p < 0.001 | F1,36 = 72.5, p < 0.001 | F1,36 = 10.9, p = 0.002 |
| Time spent in the lower third | F1,36 = 18.4, p < 0.001 | F1,36 = 64.0, p < 0.001 | F1,36 = 8.6, p = 0.006 |
| Time spent in the upper third | F1,36 = 34.1, p < 0.001 | F1,36 = 92.1, p < 0.001 | F1,36 = 21.5, p < 0.001 |
| Trait | pCPA (2 mg/L) | Fluoxetine (0.2 mg/L) | Interaction |
|---|---|---|---|
| 5-HT | F1,36 = 54.2, p < 0.001 | F1,36 = 44.2, p < 0.001 | F1,36 = 2.0, p = 0.17 |
| 5-HIAA | F1,36 = 6.5, p = 0.015 | F1,36 = 6.9, p = 0.013 | F1,36 = 2.1, p = 0.16 |
| 5-HIAA/5-HT | F1,36 = 18.4, p < 0.001 | F1,36 = 11.3, p = 0.002 | F1,36 = 12.2, p = 0.0013 |
| Gene | pCPA (2 mg/L) | Fluoxetine (0.2 mg/L) | Interaction |
|---|---|---|---|
| Tph2 | F1,36 = 2.7, p = 0.11 | F1,36 = 27.0, p < 0.001 | F1,36 = 1.7, p = 0.20 |
| Slc6a4b | F1,36 = 3.5, p < 0.07 | F1,36 = 10.9, p = 0.002 | F1,36 < 1 |
| Mao | F1,36 < 1 | F1,36 = 7.8, p = 0.008 | F1,36 < 1 |
| Htr1aa | F1,36 < 1 | F1,36 < 1 | F1,36 = 1.0, p = 0.32 |
| Htr2aa | F1,36 < 1 | F1,36 = 12.7, p < 0.001 | F1,36 < 1 |
| Creb | F1,36 < 1 | F1,36 2.6, p = 0.12 | F1,36 < 1 |
| Bdnf | F1,36 = 1.9, p = 0.26 | F1,36 = 1.2, p = 0.28 | F1,36 = 1.6, p = 0.22 |
| Ntrk2a | F1,36 < 1 | F1,36 = 5.9, p = 0.02 | F1,36 < 1 |
| Ngfra | F1,36 = 1.2, p = 0.28 | F1,36 < 1 | F1,36 < 1 |
| Trait | pCPA (2 mg/L) | Pargyline (0.5 mg/L) | Interaction |
|---|---|---|---|
| Distance traveled | F1,36 = 1.3, p = 0.26 | F1,36 = 23.4, p < 0.001 | F1,36 = 16.5, p < 0.001 |
| Distance from tank’s bottom | F1,36 = 34.6, p < 0.001 | F1,36 = 67.1, p < 0.001 | F1,36 = 16.8, p = 0.002 |
| Time spent in the lower third | F1,36 = 25.8, p < 0.001 | F1,36 = 96.4, p < 0.001 | F1,36 = 15.9, p = 0.006 |
| Time spent in the upper third | F1,36 = 44.7, p < 0.001 | F1,36 = 74.5, p < 0.001 | F1,36 = 28.1, p < 0.001 |
| Trait | pCPA (2 mg/L) | Pargyline (0.5 mg/L) | Interaction |
|---|---|---|---|
| 5-HT | F1,36 = 24.3, p < 0.001 | F1,36 = 299.6, p < 0.001 | F1,36 = 6.7, p = 0.014 |
| 5-HIAA | F1,36 = 1.03, p = 0.32 | F1,36 = 31.0, p = 0.015 | F1,36 = 12.2, p = 0.0013 |
| 5-HIAA/5-HT | F1,36 < 1 | F1,36 = 57.0, p < 0.001 | F1,36 < 1 |
| Gene | pCPA (2 mg/L) | Pargyline (0.5 mg/L) | Interaction |
|---|---|---|---|
| Tph2 | F1,36 < 1 | F1,36 = 1.6, p = 0.22 | F1,36 < 1 |
| Slc6a4b | F1,36 < 1 | F1,36 = 7.0, p = 0.012 | F1,36 = 1.0, p = 0.32 |
| Mao | F1,36 = 6.2, p = 0.018 | F1,36 = 1.9, p = 0.26 | F1,36 < 1 |
| Htr1aa | F1,36 = 2.2, p = 0.14 | F1,36 = 19.4, p < 0.001 | F1,36 = 1.2, p = 0.28 |
| Htr2aa | F1,36 = 6.2, p = 0.018 | F1,36 = 3.6, p = 0.07 | F1,36 = 1.3, p = 0.27 |
| Creb | F1,36 < 1 | F1,36 = 7.9, p = 0.008 | F1,36 < 1 |
| Bdnf | F1,36 = 15.6, p < 0.001 | F1,36 = 11.3, p = 0.002 | F1,36 = 1.6, p = 0.22 |
| Ntrk2a | F1,36 = 1.8, p = 0.19 | F1,36 = 12.4, p < 0.001 | F1,36 < 1 |
| Ngfra | F1,36 = 4.4, p = 0.042 | F1,36 = 2.4, p = 0.13 | F1,36 = 2.8, p = 0.10 |
| Gene | Primer Sequences | Annealing Temperatures, °C | Amplicon Size, bp |
|---|---|---|---|
| Polr2eb | F5′-GTGACGCAGGATGAATTGGA-3′ R5′-CACCAGGACTGTCAGGTCATT-3′ | 62 | 105 |
| Tph2 | F5′-TCTACTACAACCCTTACACGCAGA-3′ R5′-CGTCACAGACGGTGGTTAAG-3′ | 62 | 105 |
| Slc6a4b | F5′-ACCGCAAATCCAATGACCGAT-3′ R5′-CGCTCACGGGAACCTCTG-3′ | 63 | 144 |
| Mao | F5′-AAACCATGCACTTGATGACTGA-3′ R5′-TCATACTTGCCATACCCCCTG-3′ | 62 | 121 |
| Hr1aa | F5′-GCTGCACTTCTTCCATCCTG-3′ R5′-GGTTTCCTCCAACCCAACAT-3′ | 61 | 178 |
| Htr2aa | F5′-TTTGGCAGTGGTTTGTGAAC-3′ R5′-ATCCAGTGAGTGGCAGGTGT-3′ | 61 | 257 |
| Creb | F5′-GCTTTGAATCGCAGACATCA-3′ R5′-ATGGCATAATCGTGGTCGTT-3′ | 60 | 409 |
| Bdnf | F5′-TGCGAGTTATAGTGCCGCTT-3′ R5′-AGCCGCCGTTACTCTTTCTC-3′ | 63 | 313 |
| Ntrk2a | F5′-TATTCCCTTCAGCGTGTCTGG-3′ R5′-GCATGAAATGAGCAGATACGG-3′ | 62 | 233 |
| Ngfra | F5′-GATTTAGATCGTCTGTGGAGC-3′ R5′-AAAATGATGTACGCCAGGAG-3′ | 59 | 161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evsiukova, V.S.; Bazovkina, D.; Bazhenova, E.; Kulikova, E.A.; Kulikov, A.V. Tryptophan Hydroxylase 2 Deficiency Modifies the Effects of Fluoxetine and Pargyline on the Behavior, 5-HT- and BDNF-Systems in the Brain of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2021, 22, 12851. https://doi.org/10.3390/ijms222312851
Evsiukova VS, Bazovkina D, Bazhenova E, Kulikova EA, Kulikov AV. Tryptophan Hydroxylase 2 Deficiency Modifies the Effects of Fluoxetine and Pargyline on the Behavior, 5-HT- and BDNF-Systems in the Brain of Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2021; 22(23):12851. https://doi.org/10.3390/ijms222312851
Chicago/Turabian StyleEvsiukova, Valentina S., Daria Bazovkina, Ekaterina Bazhenova, Elizabeth A. Kulikova, and Alexander V. Kulikov. 2021. "Tryptophan Hydroxylase 2 Deficiency Modifies the Effects of Fluoxetine and Pargyline on the Behavior, 5-HT- and BDNF-Systems in the Brain of Zebrafish (Danio rerio)" International Journal of Molecular Sciences 22, no. 23: 12851. https://doi.org/10.3390/ijms222312851






