The Secretome of Human Neonatal Mesenchymal Stem Cells Modulates Doxorubicin-Induced Cytotoxicity: Impact in Non-Tumor Cells
Abstract
:1. Introduction
2. Results
2.1. MSC Conditioned Media Encloses Proteins Involved in Cytoprotection
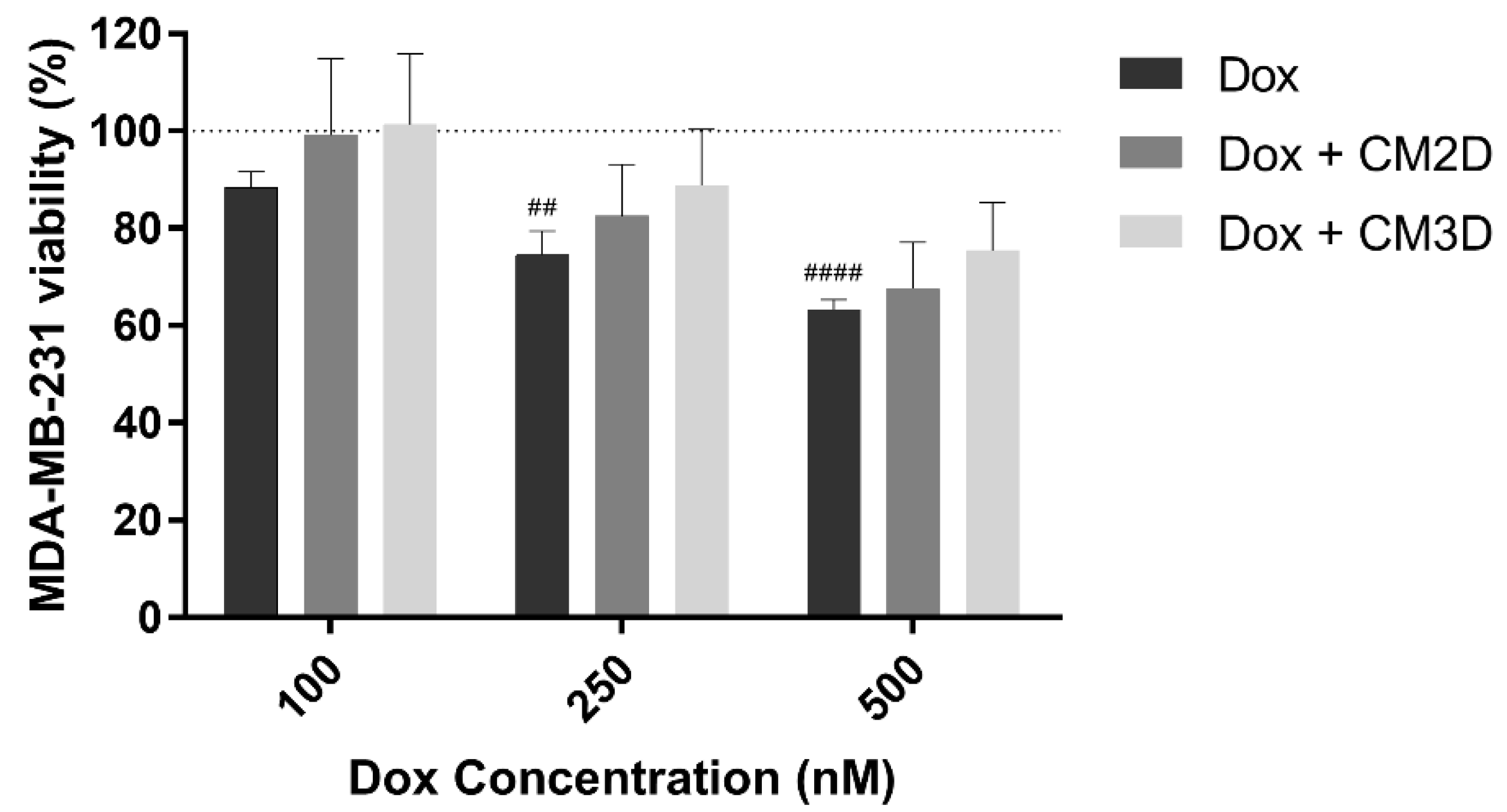
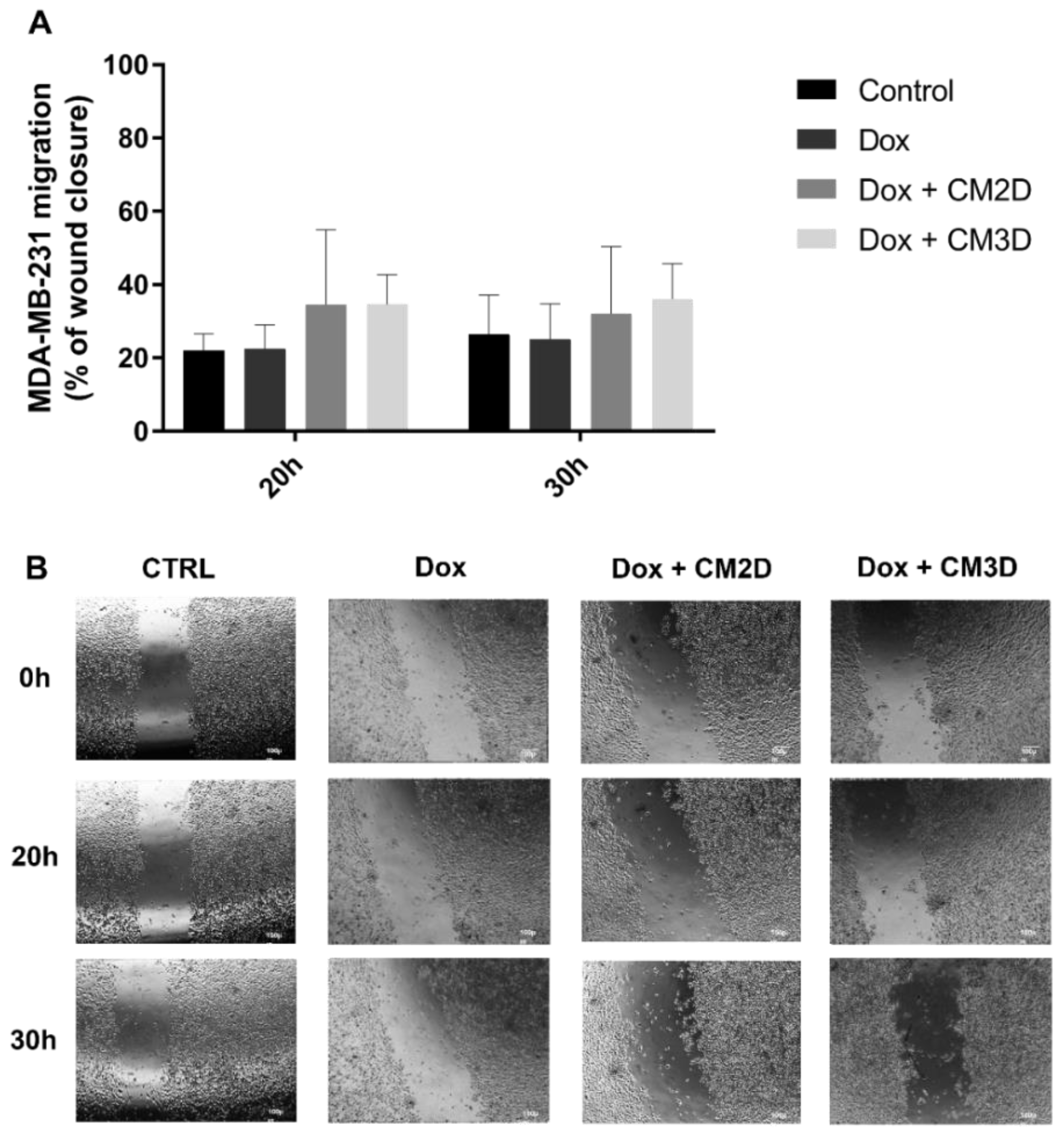
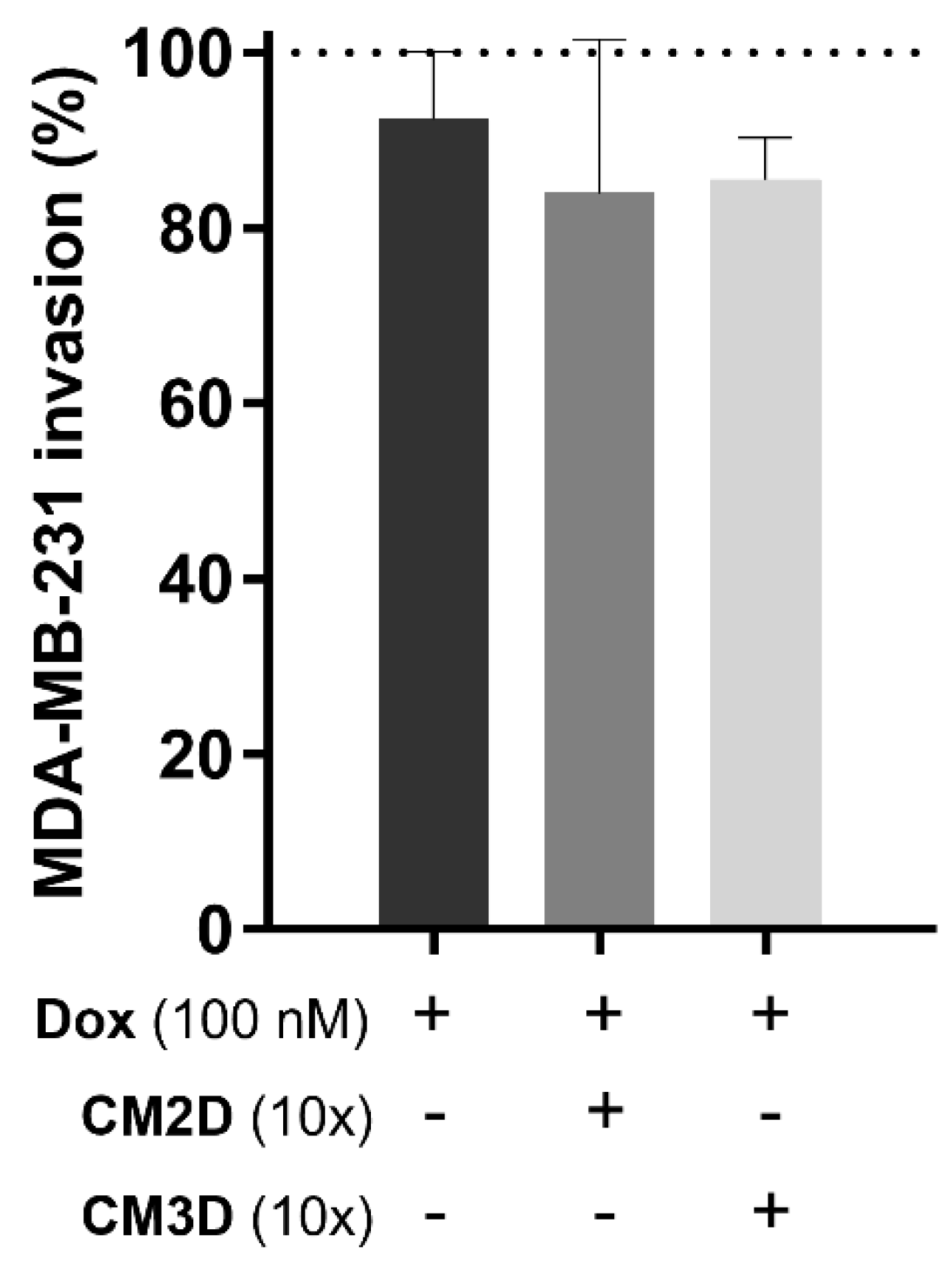
2.2. The Effect of Doxorubicin in the Viability, Migration and Invasion of MDA-MB-231 Cells Is Maintained When Co-Administered with MSC Secretome
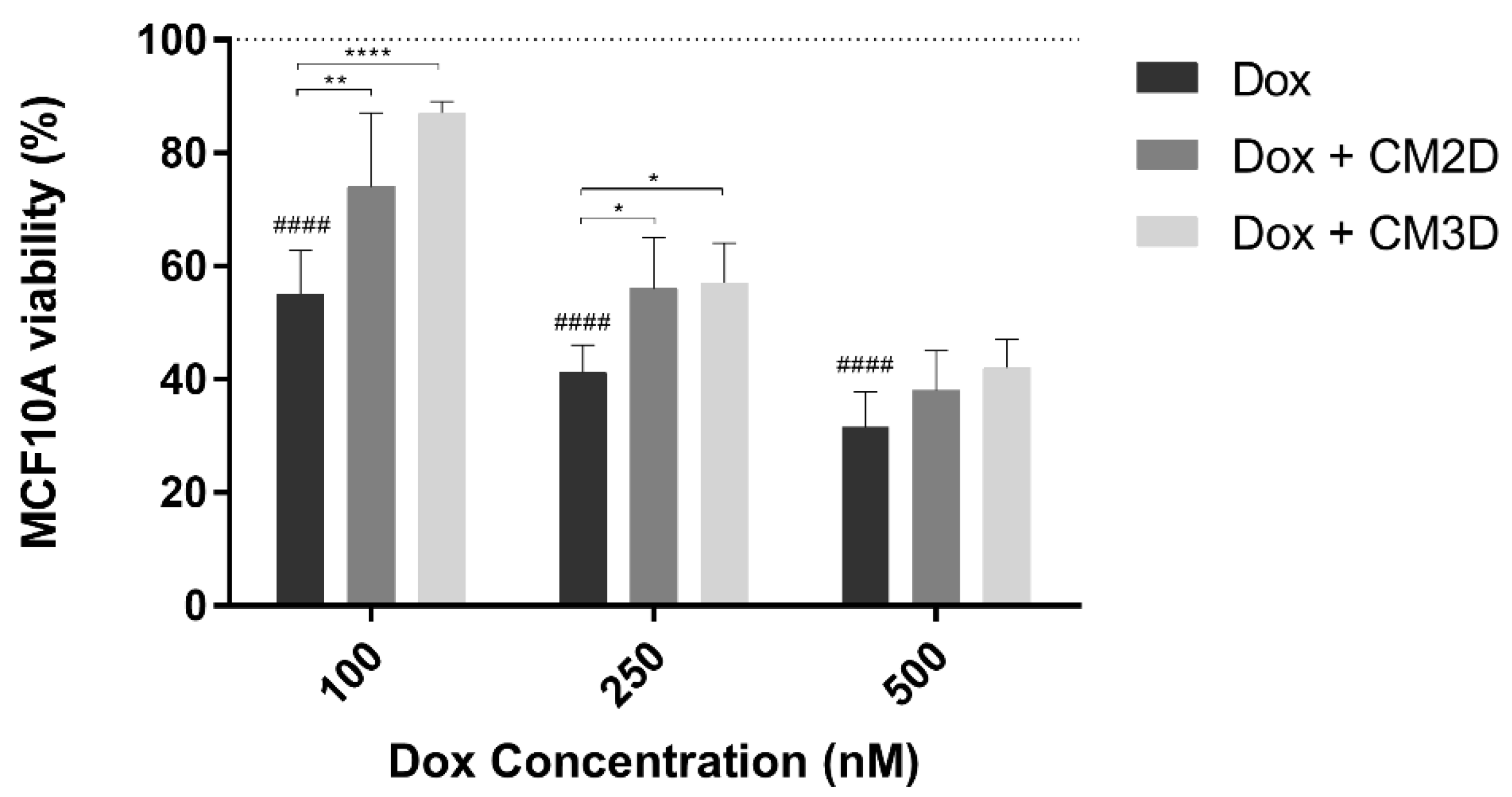
2.3. MSC Secretome Ameliorates the Cytotoxic Effect of Doxorubicin in Non-Tumor Breast Epithelial Cells
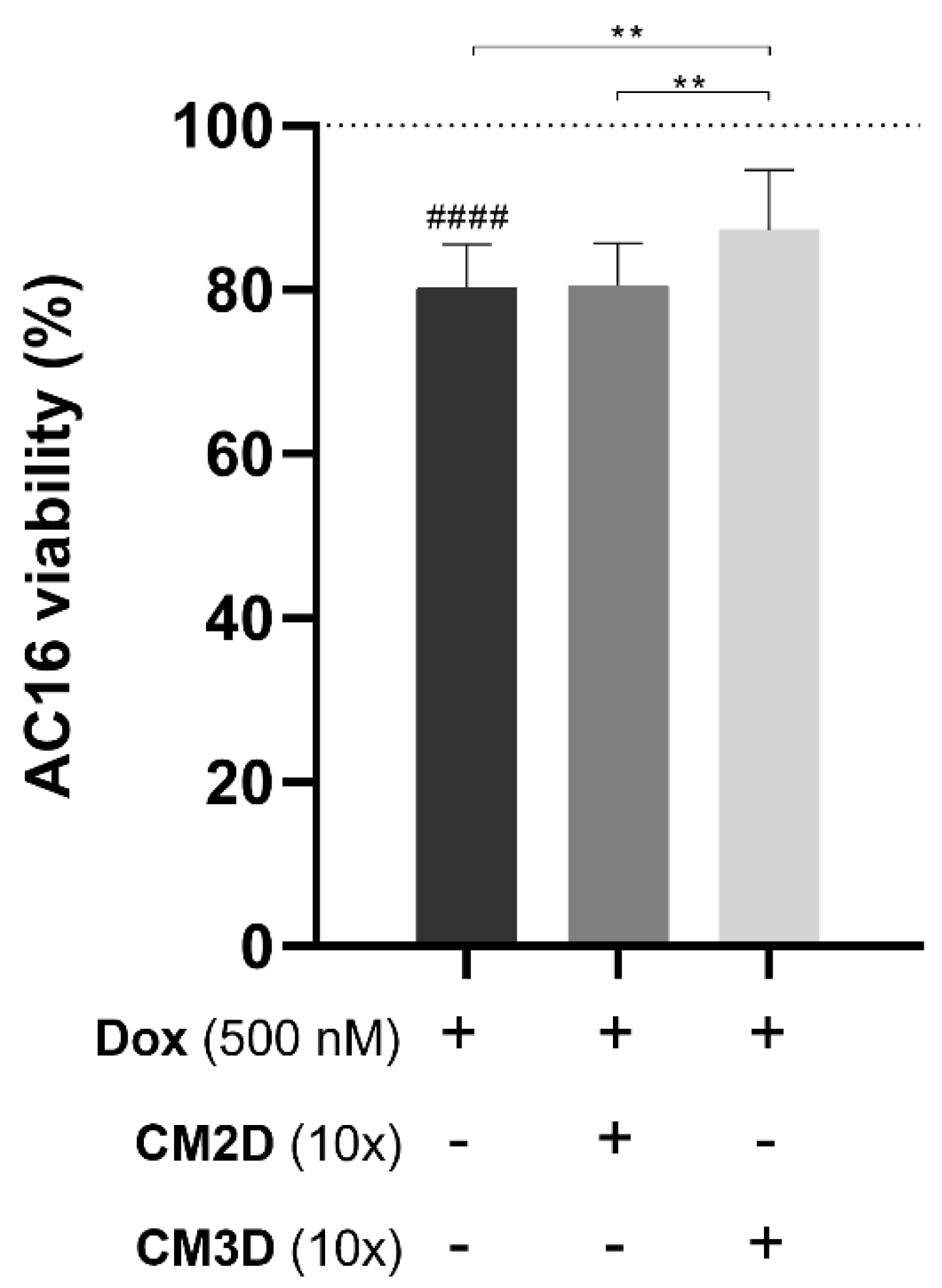
2.4. The Cytotoxic Effect of Doxorubicin on Cardiomyocytes Is Ameliorated by the MSC Secretome from 3D Cultures
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. MSC Isolation
4.3. Conditioned Media Production from MSC Cultures
4.4. LC-MS/MS Analysis
4.5. MS Data Analysis
4.6. Ingenuity Pathway Analysis (IPA)
4.7. In Vitro Cell Viability Assay
4.8. Immunoblotting Analysis
4.9. In Vitro Scratch Assay
4.10. In Vitro Chemoinvasion Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | adipose tissue |
| BM | bone marrow |
| CM | conditioned medium (secretome) |
| CM2D | secretome/conditioned medium from 2D cultures of mesenchymal stem cells |
| CM3D | secretome/conditioned medium from 3D cultures of mesenchymal stem cells |
| Dox | doxorubicin |
| iMSCs | induced pluripotent stem cell-derived MSCs |
| IPA | ingenuity pathway analysis |
| MDR | multidrug resistance |
| MSCs | mesenchymal stem/stromal cells |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| ROS | reactive oxidative species |
| TAFs | tumor-associated fibroblasts |
| TNBC | triple negative invasive breast cancer |
| UC | umbilical cord |
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E. Metastatic breast cancer: The treatment challenge. Clin. Breast Cancer 2008, 8, 224–233. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.F.; Sousa, E.; de Lourdes Bastos, M.; Marisa Costa, V. The Role of the Metabolism of Anticancer Drugs in Their Induced-Cardiotoxicity. Curr. Drug Metab. 2015, 17, 75–90. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
- Hrynchak, I.; Sousa, E.; Pinto, M.; Costa, V.M. The importance of drug metabolites synthesis: The case-study of cardiotoxic anticancer drugs. Drug Metab. Rev. 2017, 49, 158–196. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Abdo Salem, A.M.; Saad, A.; Afifi, A.M.; Afify, A.Y.; Afify, H.; Salem, H.S.E.; Ghanem, E.; Abdel-Daim, M.M. Mesenchymal stem cell therapy for doxorubicin-induced cardiomyopathy: Potential mechanisms, governing factors, and implications of the heart stem cell debate. Front. Pharmacol. 2019, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Leonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559–84571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, Q.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, H.; Huang, C.; Lei, Y. Cancer drug resistance: Redox resetting renders a way. Oncotarget 2016, 7, 42740–42761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.M.; Bárcia, R.N.; Simões, S.I.; Gaspar, M.M.; Calado, S.; Água-Doce, A.; Almeida, S.C.; Almeida, J.; Filipe, M.; Teixeira, M.; et al. The role of human umbilical cord tissue-derived mesenchymal stromal cells (UCX®) in the treatment of inflammatory arthritis. J. Transl. Med. 2013, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, D.S.; Mosqueira, D.; Sousa, L.M.; Teixeira, M.; Filipe, M.; Resende, T.P.; Araújo, A.F.; Valente, M.; Almeida, J.; Martins, J.P.; et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, J.P.; Camões, S.P.; Gaspar, M.M.; Rodrigues, J.S.; Carvalheiro, M.; Bárcia, R.N.; Cruz, P.; Cruz, H.; Simões, S.; Santos, J.M. The secretome derived from 3D-cultured umbilical cord tissue MSCs counteracts manifestations typifying rheumatoid arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Khan, M.; Adil, S.E.R.; Olson, A.L. The role of mesenchymal stem cells in oncology and regenerative medicine. Futur. Oncol. 2017, 13, 821–831. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Xu, W.; Bian, Z.; Fan, Q.; Li, G.; Tang, T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009, 281, 32–41. [Google Scholar] [CrossRef]
- Trivanović, D.; Krstić, J.; Jauković, A.; Bugarski, D.; Santibanez, J.F. Mesenchymal stromal cell engagement in cancer cell epithelial to mesenchymal transition. Dev. Dyn. 2018, 247, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Wu, W.; Xi, X.; Yu, R. Adipose-Derived Stem Cells Promote Proliferation and Invasion in Cervical Cancer by Targeting the HGF/c-MET Pathway. Cancer Manag. Res. 2020, 12, 11823–11832. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, X.; Shang, C.; Ying, Q.; Zhou, X.; Zhu, R.; Lu, H.; Hao, X.; Dong, Q.; Jiang, Z. Bone Marrow Mesenchymal Stem Cells-Derived Extracellular Vesicles Promote Proliferation, Invasion and Migration of Osteosarcoma Cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/β-Catenin Axis. OncoTargets Ther. 2021, 14, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Ayuzawa, R.; Doi, C.; Rachakatla, R.S.; Pyle, M.M.; Maurya, D.K.; Troyer, D.; Tamura, M. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009, 280, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Fong, C.; Chak, L.; Biswas, A.; Tan, J.; Gauthaman, K. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, K.; Yee, F.C.; Cheyyatraivendran, S.; Biswas, A.; Choolani, M.; Bongso, A. Human umbilical cord wharton’s jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J. Cell. Biochem. 2012, 113, 2027–2039. [Google Scholar] [CrossRef]
- Akimoto, K.; Kimura, K.; Nagano, M.; Takano, S.; To’a Salazar, G.; Yamashita, T.; Ohneda, O. Umbilical Cord Blood-Derived Mesenchymal Stem Cells Inhibit, But Adipose Tissue-Derived Mesenchymal Stem Cells Promote, Glioblastoma Multiforme Proliferation. Stem Cells Dev. 2013, 22, 1370–1386. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.R.; Imhoff, F.M.; Baird, S.K. Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and -2. Mol. Carcinog. 2015, 54, 1214–1219. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, C.; Chen, X.; Tao, C.; Cheng, H.; Lu, X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol. Lett. 2018, 15, 8536–8544. [Google Scholar] [CrossRef]
- Mirabdollahi, M.; Sadeghi-Aliabadi, H.; Javanmard, S.H. Human Wharton’s jelly mesenchymal stem cells-derived secretome could inhibit breast cancer growth in vitro and in vivo. Iran. J. Basic Med. Sci. 2020, 23, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Gan, S.U.; Ngo, K.S.; Gauthaman, K.; Biswas, A.; Choolani, M.; Bongso, A.; Fong, C.Y. Human umbilical cord Wharton’s jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Jeong, J.-H. Superiority of three-dimensional stem cell clusters over monolayer culture: An archetype to biological application. Macromol. Res. 2016, 24, 1037–1046. [Google Scholar] [CrossRef]
- Davidson, M.; Nesti, C.; Palenzuela, L.; Walker, W.; Hernandez, E.; Protas, L.; Hirano, M.; Isaac, N. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 39, 133–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; Liao, S.; Wang, W.; Wang, J.; Li, X.; Ding, Y.; Liang, Y.; Gao, F.; Yang, M.; et al. Potent Paracrine Effects of human induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells Attenuate Doxorubicin-induced Cardiomyopathy. Sci. Rep. 2015, 5, 11235. [Google Scholar] [CrossRef]
- Hendijani, F.; Javanmard, S.H.; Rafiee, L.; Sadeghi-Aliabadi, H. Effect of human Wharton’s jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res. Pharm. Sci. 2015, 10, 134–142. [Google Scholar] [PubMed]
- Scotland, P.B.; Heath, J.L.; Conway, A.E.; Porter, N.B.; Armstrong, M.B.; Walker, J.A.; Klebig, M.L.; Lavau, C.P.; Wechsler, D.S. The PICALM Protein Plays a Key Role in Iron Homeostasis and Cell Proliferation. PLoS ONE 2012, 7, e44252. [Google Scholar] [CrossRef] [Green Version]
- McDonald, P.C.; Fielding, A.B.; Dedhar, S. Integrin-linked kinase—Essential roles in physiology and cancer biology. J. Cell Sci. 2008, 121, 3121–3132. [Google Scholar] [CrossRef] [Green Version]
- Conroy, H.; Mawhinney, L.; Donnelly, S.C. Inflammation and cancer: Macrophage migration inhibitory factor (MIF)-the potential missing link. QJM 2010, 103, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liang, J.; Zhang, Z.; Lou, H.; Zhao, L.; Xu, Y.; Ou, R. MicroRNA-329-3p targets MAPK1 to suppress cell proliferation, migration and invasion in cervical cancer. Oncol. Rep. 2017, 37, 2743–2750. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.-M.; Sun, D.-N.; Jiao, Y.-L.; Wang, P.; Zhang, J.; Wang, M.; Ma, J.; Sun, M.; Gu, B.-L.; Chen, P.; et al. Macrophage migration inhibitory factor promotes tumor aggressiveness of esophageal squamous cell carcinoma via activation of Akt and inactivation of GSK3β. Cancer Lett. 2018, 412, 289–296. [Google Scholar] [CrossRef]
- Fan, C.; Tu, C.; Qi, P.; Guo, C.; Xiang, B.; Zhou, M.; Li, X.; Wu, X.; Li, X.; Li, G.; et al. GPC6 promotes cell proliferation, migration, and invasion in nasopharyngeal carcinoma. J. Cancer 2019, 10, 3926–3932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, F.; Lin, X.; Chang, C.; Feng, X.H. Nuclear Export of Smad2 and Smad3 by RanBP3 Facilitates Termination of TGF-β Signaling. Dev. Cell 2009, 16, 345–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nian, H.; Ma, B. Calpain–calpastatin system and cancer progression. Biol. Rev. 2021, 96, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Nohata, N.; Hanazawa, T.; Kikkawa, N.; Yamamoto, N.; Yoshino, H.; Itesako, T.; Enokida, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br. J. Cancer 2013, 109, 2636–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ma, H.; Wang, Y.; Du, X.; Yao, J. Cystatin SN affects cell proliferation by regulating the ERα/PI3K/AKT/ERα loopback pathway in breast cancer. OncoTargets Ther. 2019, 12, 11359–11369. [Google Scholar] [CrossRef] [Green Version]
- Nitulescu, G.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.; Grădinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [Green Version]
- Modjtahedi, N.; Giordanetto, F.; Madeo, F.; Kroemer, G. Apoptosis-inducing factor: Vital and lethal. Trends Cell Biol. 2006, 16, 264–272. [Google Scholar] [CrossRef]
- Lüersen, K.; Stegehake, D.; Daniel, J.; Drescher, M.; Ajonina, I.; Ajonina, C.; Hertel, P.; Woltersdorf, C.; Liebau, E. The Glutathione Reductase GSR-1 Determines Stress Tolerance and Longevity in Caenorhabditis elegans. PLoS ONE 2013, 8, e60731. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.P.C. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef]
- Joshi, M.B.; Philippova, M.; Ivanov, D.; Allenspach, R.; Erne, P.; Resink, T.J. T-cadherin protects endothelial cells from oxidative stress-induced apoptosis. FASEB J. 2005, 19, 1737–1739. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.S.; Kasahara, D.I.; Verbout, N.G.; Fedulov, A.V.; Zhu, M.; Si, H.; Wurmbrand, A.P.; Hug, C.; Ranscht, B.; Shore, S.A. Role of the Adiponectin binding protein, T-cadherin (Cdh13), in allergic airways responses in mice. PLoS ONE 2012, 7, e41088. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, H.S. The anti-inflammatory role of tissue inhibitor of metalloproteinase-2 in lipopolysaccharide-stimulated microglia. J. Neuroinflamm. 2014, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Qin, Z.; Li, R.; Wang, S.; Wang, W.; Tang, M.; Zhang, W. The role of ANXA5 in DBP-induced oxidative stress through ERK/Nrf2 pathway. Environ. Toxicol. Pharmacol. 2019, 72, 103236. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Fernández-Varo, G.; Melgar-Lesmes, P.; Marfà, S.; Reichenbach, V.; Morales-Ruiz, M.; Jiménez, W. Factors Involved in Extracellular Matrix Turnover in Human Derived Cardiomyocytes. Cell. Physiol. Biochem. 2013, 32, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Abd El Kader, T.; Kubota, S.; Janune, D.; Nishida, T.; Hattori, T.; Aoyama, E.; Perbal, B.; Kuboki, T.; Takigawa, M. Anti-fibrotic effect of CCN3 accompanied by altered gene expression profile of the CCN family. J. Cell Commun. Signal. 2013, 7, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ni, B.; Mao, Z.; Xi, Y.; Chu, X.; Zhang, R.; Ma, X.; You, H. NOV/CCN3 induces cartilage protection by inhibiting PI3K/AKT/mTOR pathway. J. Cell. Mol. Med. 2019, 23, 7525–7534. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, Y.; Wan, J.; Zhang, P.; Pei, F. COX6B1 relieves hypoxia/reoxygenation injury of neonatal rat cardiomyocytes by regulating mitochondrial function. Biotechnol. Lett. 2019, 41, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Mofid, A.; Newman, N.S.; Paul, P.J.; Abbasi, C.; Matkar, P.N.; Rudenko, D.; Kuliszewski, M.A.; Chen, H.H.; Afrasiabi, K.; Tsoporis, J.N.; et al. Cardiac overexpression of S100A6 attenuates cardiomyocyte apoptosis and reduces infarct size after myocardial ischemia-reperfusion. J. Am. Heart Assoc. 2017, 6, e004738. [Google Scholar] [CrossRef] [Green Version]
- Echtermeyer, F.; Harendza, T.; Hubrich, S.; Lorenz, A.; Herzog, C.; Mueller, M.; Schmitz, M.; Grund, A.; Larmann, J.; Stypmann, J.; et al. Syndecan-4 signalling inhibits apoptosis and controls NFAT activity during myocardial damage and remodelling. Cardiovasc. Res. 2011, 92, 123–131. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Lamura, L.; Gallo, M.; Maffia, V.; Normanno, N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J. Cell Biochem. 2012, 113, 3363–3370. [Google Scholar] [CrossRef]
- Chen, L.L.; Gao, G.X.; Shen, F.X.; Chen, X.; Gong, X.H.; Wu, W.J. SDC4 gene silencing favors human papillary thyroid carcinoma cell apoptosis and inhibits epithelial mesenchymal transition via wnt/β-catenin pathway. Mol. Cells 2018, 41, 853–867. [Google Scholar] [CrossRef]
- Dobson, J.R.; Taipaleenmäki, H.; Hu, Y.J.; Hong, D.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Pratap, J. Hsa-mir-30c promotes the invasive phenotype of metastatic breast cancer cells by targeting NOV/CCN3. Cancer Cell Int. 2014, 14, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juurikka, K.; Butler, G.S.; Salo, T.; Nyberg, P.; Åström, P. The role of MMP8 in cancer: A systematic review. Int. J. Mol. Sci. 2019, 20, 4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Peng, C.; Lu, X.; Guo, M.; Yang, T.; Zhou, J.; Hai, Y. PDCD5 inhibits osteosarcoma cell metastasis via targeting tgf-β1/smad signaling pathway and is associated with good prognosis. Am. J. Transl. Res. 2019, 11, 1116–1128. [Google Scholar]
- Menyhárt, O.; Harami-Papp, H.; Sukumar, S.; Schäfer, R.; Magnani, L.; de Barrios, O.; Győrffy, B. Guidelines for the selection of functional assays to evaluate the hallmarks of cancer. Biochim. Biophys. Acta-Rev. Cancer 2016, 1866, 300–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.S.; Flórido, A.; Saraiva, N.; Cerqueira, S.; Ramalhete, S.; Cipriano, M.; Cabral, M.F.; Miranda, J.P.; Castro, M.; Costa, J.; et al. Role of the Copper(II) Complex Cu[15]pyN5 in Intracellular ROS and Breast Cancer Cell Motility and Invasion. Chem. Biol. Drug Des. 2015, 86, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Soares, R.; Coelho, M.; Martins, J.P.; Basto, V.; Cruz, P.; Cruz, H. Optimised and Defined Method for Isolation and Preservation of Percursor Cells from Human Umbilical Cord. 2009. INPI PAT20081000083882; PCT/IB2008/054067; WO200904. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009044379 (accessed on 8 March 2021).
- Martins, J.P.; Santos, J.M.; Almeida, J.M.; Filipe, M.A.; de Almeida, M.V.T.; Almeida, S.C.P.; Água-Doce, A.; Varela, A.; Gilljam, M.; Stellan, B.; et al. Towards an advanced therapy medicinal product based on mesenchymal stromal cells isolated from the umbilical cord tissue: Quality and safety data. Stem Cell Res. Ther. 2014, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, J.; Bakheit, M.A.; Liu, Z.; Yin, H.; Mu, Y.; Guo, S.; Beyer, D.; Oliva, A.; Ahmed, J.S.; Seitzer, U. Development of a recombinant indirect ELISA for the diagnosis of Theileria sp. (China) infection in small ruminants. Parasitol. Res. 2006, 98, 561–567. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serras, A.S.; Camões, S.P.; Antunes, B.; Costa, V.M.; Dionísio, F.; Yazar, V.; Vitorino, R.; Remião, F.; Castro, M.; Oliveira, N.G.; et al. The Secretome of Human Neonatal Mesenchymal Stem Cells Modulates Doxorubicin-Induced Cytotoxicity: Impact in Non-Tumor Cells. Int. J. Mol. Sci. 2021, 22, 13072. https://doi.org/10.3390/ijms222313072
Serras AS, Camões SP, Antunes B, Costa VM, Dionísio F, Yazar V, Vitorino R, Remião F, Castro M, Oliveira NG, et al. The Secretome of Human Neonatal Mesenchymal Stem Cells Modulates Doxorubicin-Induced Cytotoxicity: Impact in Non-Tumor Cells. International Journal of Molecular Sciences. 2021; 22(23):13072. https://doi.org/10.3390/ijms222313072
Chicago/Turabian StyleSerras, Ana S., Sérgio P. Camões, Bernardo Antunes, Vera M. Costa, Flávio Dionísio, Volkan Yazar, Rui Vitorino, Fernando Remião, Matilde Castro, Nuno G. Oliveira, and et al. 2021. "The Secretome of Human Neonatal Mesenchymal Stem Cells Modulates Doxorubicin-Induced Cytotoxicity: Impact in Non-Tumor Cells" International Journal of Molecular Sciences 22, no. 23: 13072. https://doi.org/10.3390/ijms222313072






