The Paradigm Change of IL-33 in Vascular Biology
Abstract
:1. Interleukin (IL)-33 Biology, Structure and Signaling
2. IL-33 as a Nuclear Factor
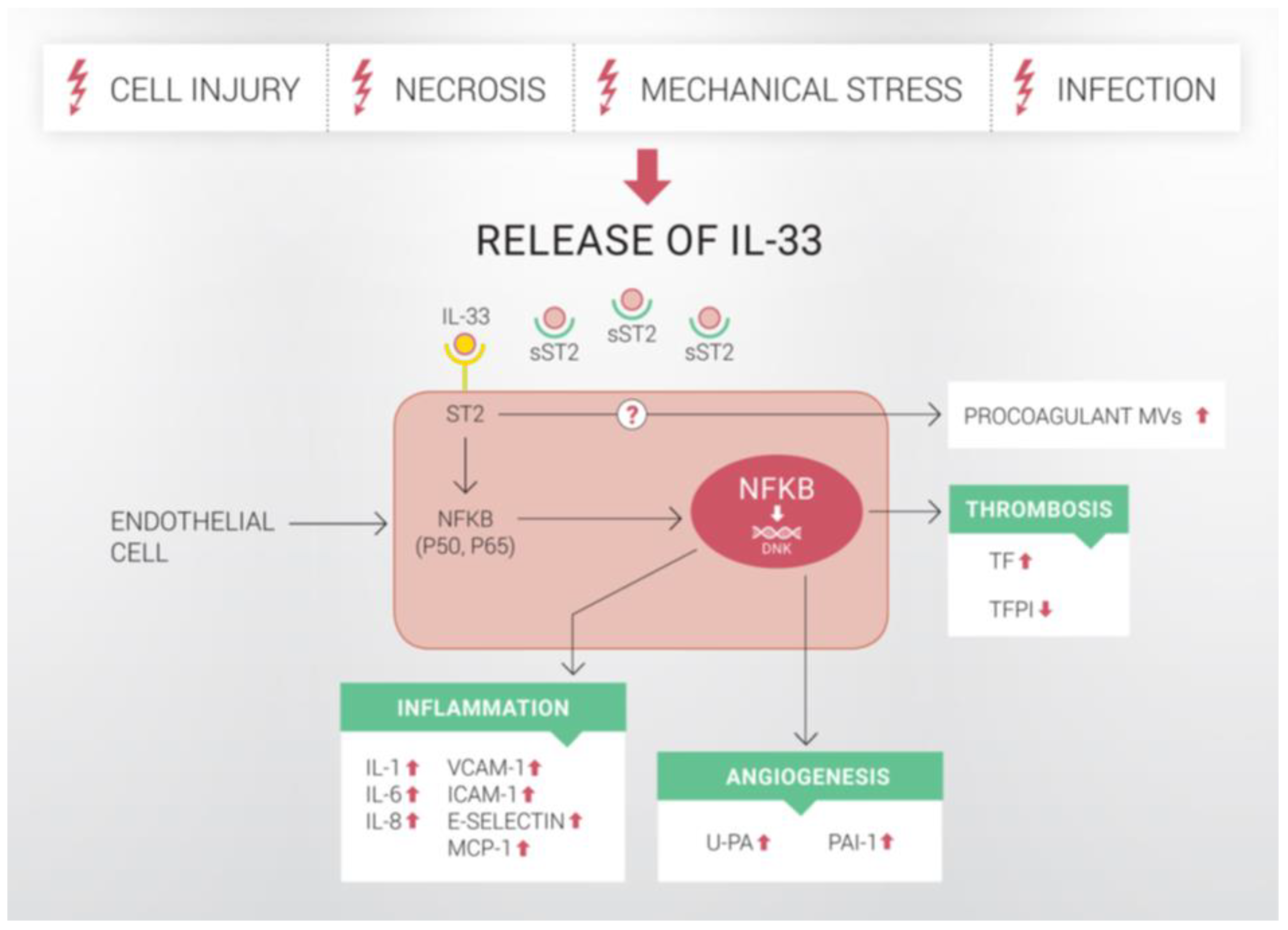
3. Cellular Sources and Expression under Pathological Conditions and Mechanisms of IL-33 Release
4. Regulators of IL-33 Expression in Cells of the Vasculature and Monocytes/Macrophages
5. IL-33 in Vascular Pathologies: Protective or Harmful?
5.1. In Vitro Effects of IL-33 on Cells of the Vascular Wall and Blood-Born Cells
5.1.1. Effects of IL-33 on ECs
5.1.2. Effects of IL-33 in Monocytes/Macrophages
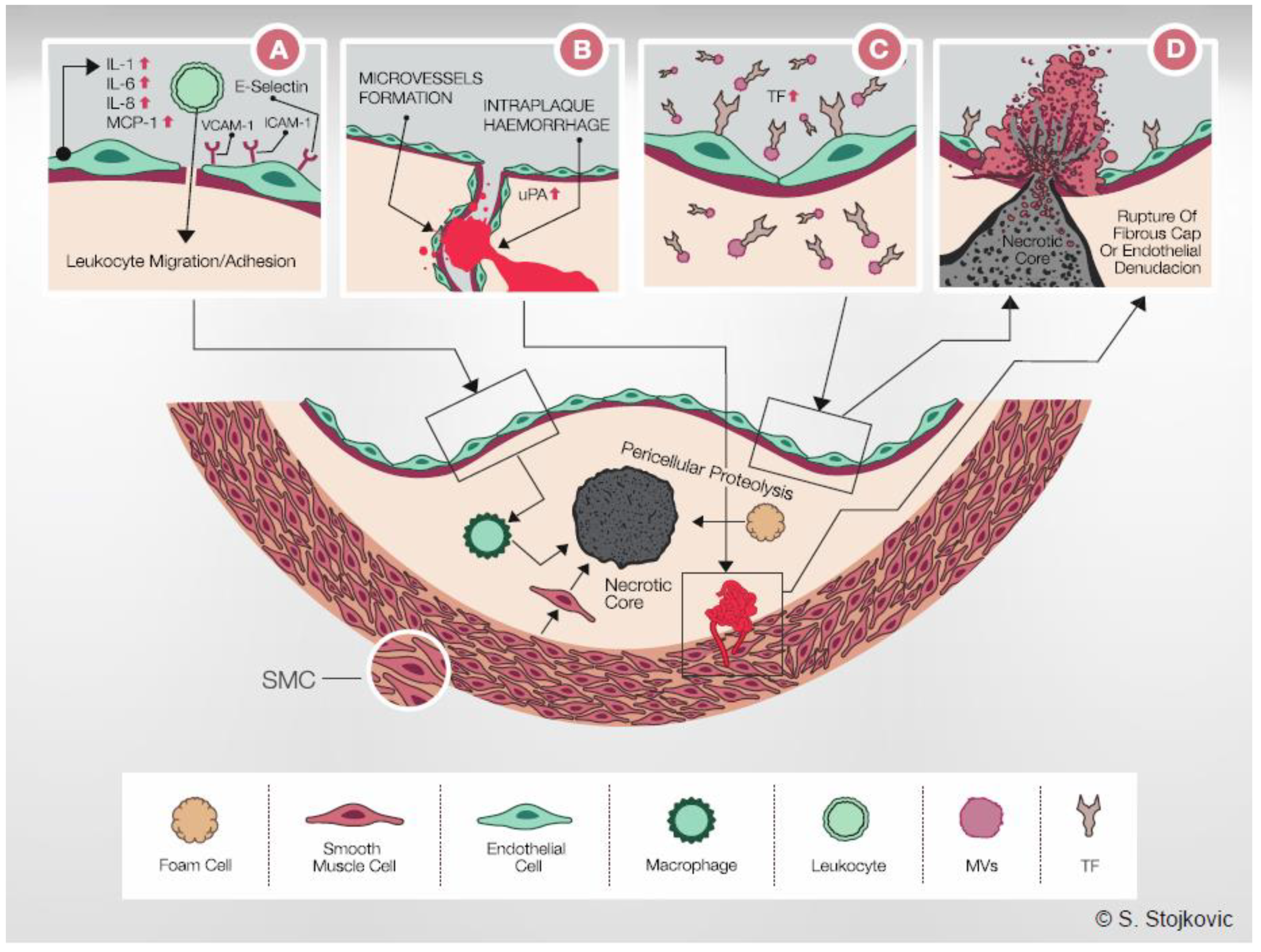
5.2. In Vivo Effects of IL-33 in Models of Vascular Diseases
6. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| ApoE | apolipoprotein E |
| ATP | adenosine triphosphate |
| BPIFB4 | BPI fold containing family B, member 4 |
| BRG1 | brahma-related gene 1 |
| BTK | Bruton’s tyrosine kinase |
| cAMP | cyclic adenosine monophosphate |
| CBM | chromatin-binding motif |
| CREB | cAMP response element-binding protein |
| ECs | endothelial cells |
| eNOS | endothelial nitric oxide synthase |
| Erk | extracellular signal-regulated kinase |
| FoxO1 | forkhead box O1 |
| Gαq/11 | Gα protein q/11 |
| GM-CSF | granulocyte macrophage-colony stimulating factor |
| GSK3 | glycogen sythase kinase 3 |
| HASMCs | human aortic SMCs |
| HCASMCs | human coronary artery SMCs |
| HIF | hypoxia-inducible factor |
| HEVECs | high endothelial venules endothelial cells |
| HSVECs | human saphenous vein ECs |
| HSVSMCs | human saphenous vein SMCs |
| HTH | Helix-Turn-Helix |
| HUVECs | human umbilical vein endothelial cells |
| ICAM | intercellular adhesion molecule-1 |
| Ig | immunoglobulin |
| IκBα | inhibitor of κBα |
| IL | interleukin |
| IL1RAcP | IL-1R accessory protein |
| IL1RL1 | IL-1-receptor-like 1 |
| Inpp5d | Inositol polyphosphate-5-phosphatase D |
| IRAK | IL-1R-associated kinase |
| IRF1 | Interferon Regulatory Factor 1 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinases |
| kDA | kilodalton |
| LDL | low density lipoprotein |
| LMCD1 | LIM and cysteine-rich domains protein 1 |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinases |
| miRs | microRNAs |
| MCP-1 | monocyte chemoattractant protein |
| MLKL | mixed lineage kinase domain-like |
| MMP | matrix metalloproteinase |
| MVs | microvesicles |
| MyD88 | myeloid differentiation factor 88 |
| MYPT1 | myosin phosphatase target subunit 1 |
| NAD | nicotinamide adenine dinucleotide |
| NF | nuclear factor |
| NFATc1 | NF of activated T cells |
| NF-HEV | NF from human high endothelial venules |
| NF-kB | nuclear factor-kappa B |
| oxLDL | oxidized low-density lipoprotein |
| PAI-1 | plasminogen activator inhibitor type-1 |
| Par1 | protease-activated receptor 1 |
| PBMC | peripheral blood mononuclear cells |
| PI3K | phosphoinositide-3-kinase |
| PLCβ3 | phospholipase Cβ3 |
| PR3 | proteinase 3 |
| PTPN12 | Protein Tyrosine Phosphatase Non-Receptor Type 12 |
| RIPK | receptor-interacting protein kinase |
| SAA | serum amyloid A |
| SIGIRR | single Ig IL-1R-related molecule |
| siRNA | silencing RNA |
| SMC | smooth muscle cell |
| sST2 | soluble ST2 |
| ST2 | suppression of tumorigenicity 2 |
| ST2L | membrane-bound ST2 |
| SYK | spleen tyrosine kinase |
| TAC | transverse aortic constriction |
| TF | tissue factor |
| TFPI | tissue factor pathway inhibitor |
| Th | T helper |
| TIMP | tissue inhibitor of metalloproteinases |
| TIR | toll/IL-1 receptor |
| TLR2 | toll-like receptor 2 |
| TMED1 | transmembrane emp24 domain-containing protein 1 |
| TNF | tumor necrosis factor |
| t-PA | tissue-type plasminogen activator |
| TRAF6 | TNF-α receptor-associated factor 6 |
| u-PA | urokinase-type plasminogen activator |
| uPAR | u-PA receptor |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
References
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [Green Version]
- Bertheloot, D.; Latz, E. HMGB1, IL-1alpha, IL-33 and S100 proteins: Dual-function alarmins. Cell. Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Altara, R.; Ghali, R.; Mallat, Z.; Cataliotti, A.; Booz, G.W.; Zouein, F.A. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc. Res. 2018, 114, 1578–1594. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.F.; Wu, M.X.; Lin, Y.Q.; Xie, S.L.; Huang, T.C.; Liu, P.M.; Nie, R.Q.; Meng, Q.Q.; Luo, N.S.; Chen, Y.X.; et al. IL-33 promotes IL-10 production in macrophages: A role for IL-33 in macrophage foam cell formation. Exp. Mol. Med. 2017, 49, e388. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hammel, M.; He, Y.; Tainer, J.A.; Jeng, U.S.; Zhang, L.; Wang, S.; Wang, X. Structural insights into the interaction of IL-33 with its receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 14918–14923. [Google Scholar] [CrossRef] [Green Version]
- Rivers-Auty, J.; Daniels, M.J.D.; Colliver, I.; Robertson, D.L.; Brough, D. Redefining the ancestral origins of the interleukin-1 superfamily. Nat. Commun. 2018, 9, 1156. [Google Scholar] [CrossRef]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Lingel, A.; Weiss, T.M.; Niebuhr, M.; Pan, B.; Appleton, B.A.; Wiesmann, C.; Bazan, J.F.; Fairbrother, W.J. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors-insight into heterotrimeric IL-1 signaling complexes. Structure 2009, 17, 1398–1410. [Google Scholar] [CrossRef] [Green Version]
- Klemenz, R.; Hoffmann, S.; Werenskiold, A.K. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA 1989, 86, 5708–5712. [Google Scholar] [CrossRef] [Green Version]
- Chackerian, A.A.; Oldham, E.R.; Murphy, E.E.; Schmitz, J.; Pflanz, S.; Kastelein, R.A. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 2007, 179, 2551–2555. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Takagi, T.; Tsukamoto, T.; Tetsuka, T.; Tominaga, S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993, 318, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.M.; Subbannayya, Y.; Rex, D.A.B.; Raju, R.; Chatterjee, O.; Advani, J.; Radhakrishnan, A.; Keshava Prasad, T.S.; Wani, M.R.; Pandey, A. A network map of IL-33 signaling pathway. J. Cell Commun. Signal. 2018, 12, 615–624. [Google Scholar] [CrossRef]
- Connolly, D.J.; O’Neill, L.A.; McGettrick, A.F. The GOLD domain-containing protein TMED1 is involved in interleukin-33 signaling. J. Biol. Chem. 2013, 288, 5616–5623. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.M.; Nirujogi, R.S.; Rojas, P.L.; Patil, A.H.; Manda, S.S.; Subbannayya, Y.; Roa, J.C.; Chatterjee, A.; Prasad, T.S.; Pandey, A. Quantitative phosphoproteomic analysis of IL-33-mediated signaling. Proteomics 2015, 15, 532–544. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.S.; Choi, H.J.; Min, J.K.; Pyun, B.J.; Maeng, Y.S.; Park, H.; Kim, J.; Kim, Y.M.; Kwon, Y.G. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 2009, 114, 3117–3126. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Z. The enigmatic processing and secretion of interleukin-33. Cell. Mol. Immunol. 2010, 7, 260–262. [Google Scholar] [CrossRef] [Green Version]
- Bulek, K.; Swaidani, S.; Qin, J.; Lu, Y.; Gulen, M.F.; Herjan, T.; Min, B.; Kastelein, R.A.; Aronica, M.; Kosz-Vnenchak, M.; et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J. Immunol. 2009, 182, 2601–2609. [Google Scholar] [CrossRef] [Green Version]
- Onda, H.; Kasuya, H.; Takakura, K.; Hori, T.; Imaizumi, T.; Takeuchi, T.; Inoue, I.; Takeda, J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 1999, 19, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Baekkevold, E.S.; Roussigne, M.; Yamanaka, T.; Johansen, F.E.; Jahnsen, F.L.; Amalric, F.; Brandtzaeg, P.; Erard, M.; Haraldsen, G.; Girard, J.P. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 2003, 163, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Roussel, L.; Erard, M.; Cayrol, C.; Girard, J.P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008, 9, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Moussion, C.; Ortega, N.; Girard, J.P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef] [Green Version]
- Kuchler, A.M.; Pollheimer, J.; Balogh, J.; Sponheim, J.; Manley, L.; Sorensen, D.R.; De Angelis, P.M.; Scott, H.; Haraldsen, G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am. J. Pathol. 2008, 173, 1229–1242. [Google Scholar] [CrossRef] [Green Version]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef] [Green Version]
- Sundlisaeter, E.; Edelmann, R.J.; Hol, J.; Sponheim, J.; Kuchler, A.M.; Weiss, M.; Udalova, I.A.; Midwood, K.S.; Kasprzycka, M.; Haraldsen, G. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am. J. Pathol. 2012, 181, 1099–1111. [Google Scholar] [CrossRef]
- Ali, S.; Mohs, A.; Thomas, M.; Klare, J.; Ross, R.; Schmitz, M.L.; Martin, M.U. The dual function cytokine IL-33 interacts with the transcription factor NF-kappaB to dampen NF-kappaB-stimulated gene transcription. J. Immunol. 2011, 187, 1609–1616. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.S.; Park, J.A.; Kim, J.; Rho, S.S.; Park, H.; Kim, Y.M.; Kwon, Y.G. Nuclear IL-33 is a transcriptional regulator of NF-kappaB p65 and induces endothelial cell activation. Biochem. Biophys. Res. Commun. 2012, 421, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Perros, F.; Caramori, G.; Meng, C.; Dormuller, P.; Chou, P.C.; Church, C.; Papi, A.; Casolari, P.; Welsh, D.; et al. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem. Biophys. Res. Commun. 2014, 451, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Tao, L.; Chen, C.; Song, H.; Li, Z.; Gao, Y.; Nie, J.; Piccioni, M.; Shi, G.; Li, B. The Deubiquitinase USP17 Regulates the Stability and Nuclear Function of IL-33. Int. J. Mol. Sci. 2015, 16, 27956–27966. [Google Scholar] [CrossRef] [Green Version]
- Clerman, A.; Noor, Z.; Fishelevich, R.; Lockatell, V.; Hampton, B.S.; Shah, N.G.; Salcedo, M.V.; Todd, N.W.; Atamas, S.P.; Luzina, I.G. The full-length interleukin-33 (FLIL33)-importin-5 interaction does not regulate nuclear localization of FLIL33 but controls its intracellular degradation. J. Biol. Chem. 2017, 292, 21653–21661. [Google Scholar] [CrossRef] [Green Version]
- Travers, J.; Rochman, M.; Miracle, C.E.; Habel, J.E.; Brusilovsky, M.; Caldwell, J.M.; Rymer, J.K.; Rothenberg, M.E. Chromatin regulates IL-33 release and extracellular cytokine activity. Nat. Commun. 2018, 9, 3244. [Google Scholar] [CrossRef] [PubMed]
- Gautier, V.; Cayrol, C.; Farache, D.; Roga, S.; Monsarrat, B.; Burlet-Schiltz, O.; Gonzalez de Peredo, A.; Girard, J.P. Extracellular IL-33 cytokine, but not endogenous nuclear IL-33, regulates protein expression in endothelial cells. Sci. Rep. 2016, 6, 34255. [Google Scholar] [CrossRef]
- Demyanets, S.; Konya, V.; Kastl, S.P.; Kaun, C.; Rauscher, S.; Niessner, A.; Pentz, R.; Pfaffenberger, S.; Rychli, K.; Lemberger, C.E.; et al. Interleukin-33 induces expression of adhesion molecules and inflammatory activation in human endothelial cells and in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2080–2089. [Google Scholar] [CrossRef] [Green Version]
- Zeyda, M.; Wernly, B.; Demyanets, S.; Kaun, C.; Hammerle, M.; Hantusch, B.; Schranz, M.; Neuhofer, A.; Itariu, B.K.; Keck, M.; et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int. J. Obes. 2013, 37, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef]
- Govatati, S.; Pichavaram, P.; Janjanam, J.; Zhang, B.; Singh, N.K.; Mani, A.M.; Traylor, J.G., Jr.; Orr, A.W.; Rao, G.N. NFATc1-E2F1-LMCD1-Mediated IL-33 Expression by Thrombin Is Required for Injury-Induced Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, M.; Ljujic, B.; Babic, S.; Maravic-Stojkovic, V.; Mitrovic, S.; Arsenijevic, N.; Radak, D.; Pejnovic, N.; Lukic, M.L. IL-33/IL-33R in various types of carotid artery atherosclerotic lesions. Cytokine 2019, 120, 242–250. [Google Scholar] [CrossRef]
- Marzullo, A.; Ambrosi, F.; Inchingolo, M.; Manca, F.; Devito, F.; Angiletta, D.; Zito, A.; Scicchitano, P.; Ciccone, M.M. ST2L Transmembrane Receptor Expression: An Immunochemical Study on Endarterectomy Samples. PLoS ONE 2016, 11, e0156315. [Google Scholar] [CrossRef]
- Li, J.; Xia, N.; Wen, S.; Li, D.; Lu, Y.; Gu, M.; Tang, T.; Jiao, J.; Lv, B.; Nie, S.; et al. IL (Interleukin)-33 Suppresses Abdominal Aortic Aneurysm by Enhancing Regulatory T-Cell Expansion and Activity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 446–458. [Google Scholar] [CrossRef]
- Caporali, A.; Meloni, M.; Miller, A.M.; Vierlinger, K.; Cardinali, A.; Spinetti, G.; Nailor, A.; Faglia, E.; Losa, S.; Gotti, A.; et al. Soluble ST2 is regulated by p75 neurotrophin receptor and predicts mortality in diabetic patients with critical limb ischemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e149–e160. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Cao, H.; Wei, Y.; Li, H.H. Alteration of the IL-33-sST2 pathway in hypertensive patients and a mouse model. Hypertens. Res. 2019, 42, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Y.; Hong, J.; Gannon, J.; Kakkar, R.; Lee, R.T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl. Acad. Sci. USA 2015, 112, 7249–7254. [Google Scholar] [CrossRef] [Green Version]
- Mildner, M.; Storka, A.; Lichtenauer, M.; Mlitz, V.; Ghannadan, M.; Hoetzenecker, K.; Nickl, S.; Dome, B.; Tschachler, E.; Ankersmit, H.J. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc. Res. 2010, 87, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Demyanets, S.; Kaun, C.; Pentz, R.; Krychtiuk, K.A.; Rauscher, S.; Pfaffenberger, S.; Zuckermann, A.; Aliabadi, A.; Groger, M.; Maurer, G.; et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J. Mol. Cell. Cardiol. 2013, 60, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartunek, J.; Delrue, L.; Van Durme, F.; Muller, O.; Casselman, F.; De Wiest, B.; Croes, R.; Verstreken, S.; Goethals, M.; de Raedt, H.; et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J. Am. Coll. Cardiol. 2008, 52, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Takeda, T.; Unno, H.; Morita, H.; Futamura, K.; Emi-Sugie, M.; Arae, K.; Shoda, T.; Okada, N.; Igarashi, A.; Inoue, E.; et al. Platelets constitutively express IL-33 protein and modulate eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2016, 138, 1395–1403.e6. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zhao, J.; Schrott, V.; Zhang, Y.; Gladwin, M.; Bullock, G.; Zhao, Y. Red Blood Cells Store and Release Interleukin-33. J. Investig. Med. 2015, 63, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Cayrol, C.; Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef] [Green Version]
- Luthi, A.U.; Cullen, S.P.; McNeela, E.A.; Duriez, P.J.; Afonina, I.S.; Sheridan, C.; Brumatti, G.; Taylor, R.C.; Kersse, K.; Vandenabeele, P.; et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 2009, 31, 84–98. [Google Scholar] [CrossRef]
- Talabot-Ayer, D.; Lamacchia, C.; Gabay, C.; Palmer, G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J. Biol. Chem. 2009, 284, 19420–19426. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Oboki, K.; Kajiwara, N.; Morii, E.; Aozasa, K.; Flavell, R.A.; Okumura, K.; Saito, H.; Nakae, S. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 2009, 183, 7890–7897. [Google Scholar] [CrossRef] [Green Version]
- Lefrancais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Kang, T.; Hong, J.; Lee, S.; Choi, J.; Jhun, H.; Kwak, A.; Hong, K.; Kim, E.; Jo, S.; et al. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J. Biol. Chem. 2012, 287, 8205–8213. [Google Scholar] [CrossRef] [Green Version]
- Clancy, D.M.; Sullivan, G.P.; Moran, H.B.T.; Henry, C.M.; Reeves, E.P.; McElvaney, N.G.; Lavelle, E.C.; Martin, S.J. Extracellular Neutrophil Proteases Are Efficient Regulators of IL-1, IL-33, and IL-36 Cytokine Activity but Poor Effectors of Microbial Killing. Cell Rep. 2018, 22, 2937–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carta, S.; Lavieri, R.; Rubartelli, A. Different Members of the IL-1 Family Come Out in Different Ways: DAMPs vs. Cytokines? Front. Immunol. 2013, 4, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 2011, 186, 4375–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, R.; Hei, H.; Dobner, S.; Lee, R.T. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J. Biol. Chem. 2012, 287, 6941–6948. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wei, J.; Ren, L.; Zhang, J.; Yang, M.; Jing, L.; Wang, J.; Sun, Z.; Zhou, X. Endosulfan inducing apoptosis and necroptosis through activation RIPK signaling pathway in human umbilical vascular endothelial cells. Environ. Sci. Pollut. Res. Int. 2017, 24, 215–225. [Google Scholar] [CrossRef]
- Duez, C.; Gross, B.; Marquillies, P.; Ledroit, V.; Ryffel, B.; Glineur, C. Regulation of IL (Interleukin)-33 Production in Endothelial Cells via Kinase Activation and Fas/CD95 Upregulation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2619–2631. [Google Scholar] [CrossRef]
- Liu, L.; Mao, L.; Wu, X.; Wu, T.; Liu, W.; Yang, Y.; Zhang, T.; Xu, Y. BRG1 regulates endothelial-derived IL-33 to promote ischemia-reperfusion induced renal injury and fibrosis in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2551–2561. [Google Scholar] [CrossRef]
- Stav-Noraas, T.E.; Edelmann, R.J.; Poulsen, L.C.; Sundnes, O.; Phung, D.; Kuchler, A.M.; Muller, F.; Kamen, A.A.; Haraldsen, G.; Kaarbo, M.; et al. Endothelial IL-33 Expression Is Augmented by Adenoviral Activation of the DNA Damage Machinery. J. Immunol. 2017, 198, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Govatati, S.; Pichavaram, P.; Janjanam, J.; Guo, L.; Virmani, R.; Rao, G.N. Myristoylation of LMCD1 Leads to Its Species-Specific Derepression of E2F1 and NFATc1 in the Modulation of CDC6 and IL-33 Expression During Development of Vascular Lesions. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1256–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorzelak-Pabis, P.; Chalubinski, M.; Wojdan, K.; Luczak, E.; Borowiec, M.; Broncel, M. IL-22 modulates inflammatory properties of human primary aortic smooth muscle cells. Adv. Clin. Exp. Med. 2017, 26, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puca, A.A.; Carrizzo, A.; Spinelli, C.; Damato, A.; Ambrosio, M.; Villa, F.; Ferrario, A.; Maciag, A.; Fornai, F.; Lenzi, P.; et al. Single systemic transfer of a human gene associated with exceptional longevity halts the progression of atherosclerosis and inflammation in ApoE knockout mice through a CXCR4-mediated mechanism. Eur. Heart J. 2020, 41, 2487–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Zhu, Z.; Cheng, N.; Yan, Q.; Ye, R.D. Serum amyloid A induces interleukin-33 expression through an IRF7-dependent pathway. Eur. J. Immunol. 2014, 44, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Foks, A.C.; van Puijvelde, G.H.; Bot, I.; ter Borg, M.N.; Habets, K.L.; Johnson, J.L.; Yagita, H.; van Berkel, T.J.; Kuiper, J. Interruption of the OX40-OX40 ligand pathway in LDL receptor-deficient mice causes regression of atherosclerosis. J. Immunol. 2013, 191, 4573–4580. [Google Scholar] [CrossRef] [Green Version]
- Nile, C.J.; Barksby, E.; Jitprasertwong, P.; Preshaw, P.M.; Taylor, J.J. Expression and regulation of interleukin-33 in human monocytes. Immunology 2010, 130, 172–180. [Google Scholar] [CrossRef]
- Sato, S.; Yanagawa, Y.; Hiraide, S.; Iizuka, K. Cyclic AMP signaling enhances lipopolysaccharide sensitivity and interleukin-33 production in RAW264.7 macrophages. Microbiol. Immunol. 2016, 60, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Gilliam, E.A.; Button, J.; Li, L. Dynamic modulation of innate immune response by varying dosages of lipopolysaccharide (LPS) in human monocytic cells. J. Biol. Chem. 2014, 289, 21584–21590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.K.; Palma, M.; Buechel, B.; Moore, J.; Davra, V.; Chu, N.; Millman, A.; Hallab, N.J.; Kanneganti, T.D.; Birge, R.B.; et al. Sterile particle-induced inflammation is mediated by macrophages releasing IL-33 through a Bruton’s tyrosine kinase-dependent pathway. Nat. Mater. 2019, 18, 289–297. [Google Scholar] [CrossRef]
- De Falco, G.; Terlizzi, M.; Sirignano, M.; Commodo, M.; D’Anna, A.; Aquino, R.P.; Pinto, A.; Sorrentino, R. Human peripheral blood mononuclear cells (PBMCs) from smokers release higher levels of IL-1-like cytokines after exposure to combustion-generated ultrafine particles. Sci. Rep. 2017, 7, 43016. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.; Hayakawa, M.; Ozaki, H.; Takezako, N.; Obata, H.; Ibaraki, N.; Tsuru, T.; Tominaga, S.; Yanagisawa, K. ST2 gene expression is proliferation-dependent and its ligand, IL-33, induces inflammatory reaction in endothelial cells. Mol. Cell. Biochem. 2010, 335, 75–81. [Google Scholar] [CrossRef]
- Yamamoto, M.; Umebashi, K.; Tokito, A.; Imamura, J.; Jougasaki, M. Interleukin-33 induces growth-regulated oncogene-alpha expression and secretion in human umbilical vein endothelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R272–R279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanari, E.; Stojkovic, S.; Kaun, C.; Lemberger, C.E.; de Martin, R.; Rauscher, S.; Groger, M.; Maurer, G.; Neumayer, C.; Huk, I.; et al. Interleukin-33 stimulates GM-CSF and M-CSF production by human endothelial cells. Thromb. Haemost. 2016, 116, 317–327. [Google Scholar] [CrossRef]
- Umebashi, K.; Tokito, A.; Yamamoto, M.; Jougasaki, M. Interleukin-33 induces interleukin-8 expression via JNK/c-Jun/AP-1 pathway in human umbilical vein endothelial cells. PLoS ONE 2018, 13, e0191659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollheimer, J.; Bodin, J.; Sundnes, O.; Edelmann, R.J.; Skanland, S.S.; Sponheim, J.; Brox, M.J.; Sundlisaeter, E.; Loos, T.; Vatn, M.; et al. Interleukin-33 drives a proinflammatory endothelial activation that selectively targets nonquiescent cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e47–e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Zhang, M.; Liang, X.; Jia, X.; Jia, J.; Zhao, M.; Fan, Y. Interleukin-33 promotes inflammation-induced lymphangiogenesis via ST2/TRAF6-mediated Akt/eNOS/NO signalling pathway. Sci. Rep. 2017, 7, 10602. [Google Scholar] [CrossRef] [Green Version]
- Pepper, M.S. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1104–1117. [Google Scholar] [CrossRef] [Green Version]
- Stojkovic, S.; Kaun, C.; Heinz, M.; Krychtiuk, K.A.; Rauscher, S.; Lemberger, C.E.; de Martin, R.; Groger, M.; Petzelbauer, P.; Huk, I.; et al. Interleukin-33 induces urokinase in human endothelial cells-possible impact on angiogenesis. J. Thromb. Haemost. 2014, 12, 948–957. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Chen, S.; Tian, B.; Huang, K.; Corrigan, C.J.; Ying, S.; Wang, W.; Wang, C. IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF-1alpha and VEGF Expression in Vascular Endothelial Cells. EBioMedicine 2018, 33, 196–210. [Google Scholar] [CrossRef]
- Saxton, S.N.; Whitley, A.S.; Potter, R.J.; Withers, S.B.; Grencis, R.; Heagerty, A.M. Interleukin-33 rescues perivascular adipose tissue anticontractile function in obesity. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1387–H1397. [Google Scholar] [CrossRef]
- Stojkovic, S.; Kaun, C.; Basilio, J.; Rauscher, S.; Hell, L.; Krychtiuk, K.A.; Bonstingl, C.; de Martin, R.; Groger, M.; Ay, C.; et al. Tissue factor is induced by interleukin-33 in human endothelial cells: A new link between coagulation and inflammation. Sci. Rep. 2016, 6, 25171. [Google Scholar] [CrossRef] [Green Version]
- Van Hinsbergh, V.W. Endothelium-role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Pawlinski, R.; Mackman, N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb. Res. 2010, 125, S70–S73. [Google Scholar] [CrossRef] [Green Version]
- Leatham, E.W.; Bath, P.M.; Tooze, J.A.; Camm, A.J. Increased monocyte tissue factor expression in coronary disease. Br. Heart J. 1995, 73, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M.; Tedgui, A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Stojkovic, S.; Thulin, A.; Hell, L.; Thaler, B.; Rauscher, S.; Baumgartner, J.; Groger, M.; Ay, C.; Demyanets, S.; Neumayer, C.; et al. IL-33 stimulates the release of procoagulant microvesicles from human monocytes and differentially increases tissue factor in human monocyte subsets. Thromb. Haemost. 2017, 117, 1379–1390. [Google Scholar] [CrossRef]
- Joshi, A.D.; Oak, S.R.; Hartigan, A.J.; Finn, W.G.; Kunkel, S.L.; Duffy, K.E.; Das, A.; Hogaboam, C.M. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Oboki, K.; Morita, H.; Kajiwara, N.; Arae, K.; Tanaka, S.; Ikeda, M.; Iikura, M.; Akiyama, T.; Inoue, J.; et al. Paracrine IL-33 stimulation enhances lipopolysaccharide-mediated macrophage activation. PLoS ONE 2011, 6, e18404. [Google Scholar] [CrossRef]
- Yang, Z.; Grinchuk, V.; Urban, J.F., Jr.; Bohl, J.; Sun, R.; Notari, L.; Yan, S.; Ramalingam, T.; Keegan, A.D.; Wynn, T.A.; et al. Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS ONE 2013, 8, e59441. [Google Scholar] [CrossRef]
- McLaren, J.E.; Michael, D.R.; Salter, R.C.; Ashlin, T.G.; Calder, C.J.; Miller, A.M.; Liew, F.Y.; Ramji, D.P. IL-33 reduces macrophage foam cell formation. J. Immunol. 2010, 185, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Ashlin, T.G.; Buckley, M.L.; Salter, R.C.; Johnson, J.L.; Kwan, A.P.; Ramji, D.P. The anti-atherogenic cytokine interleukin-33 inhibits the expression of a disintegrin and metalloproteinase with thrombospondin motifs-1, -4 and -5 in human macrophages: Requirement of extracellular signal-regulated kinase, c-Jun N-terminal kinase and phosphoinositide 3-kinase signaling pathways. Int. J. Biochem. Cell Biol. 2014, 46, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, M.L.; Williams, J.O.; Chan, Y.H.; Laubertova, L.; Gallagher, H.; Moss, J.W.E.; Ramji, D.P. The interleukin-33-mediated inhibition of expression of two key genes implicated in atherosclerosis in human macrophages requires MAP kinase, phosphoinositide 3-kinase and nuclear factor-kappaB signaling pathways. Sci. Rep. 2019, 9, 11317. [Google Scholar] [CrossRef]
- Ahmed, A.; Koma, M.K. Interleukin-33 Triggers B1 Cell Expansion and Its Release of Monocyte/Macrophage Chemoattractants and Growth Factors. Scand. J. Immunol. 2015, 82, 118–124. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.C.; Phair, I.R.; Greger, C.; Pardali, K.; McGuire, V.A.; Clark, A.R.; Gaestel, M.; Arthur, J.S.C. IL-33 regulates cytokine production and neutrophil recruitment via the p38 MAPK-activated kinases MK2/3. Immunol. Cell Biol. 2019, 97, 54–71. [Google Scholar] [CrossRef]
- Padro, T.; Emeis, J.J.; Steins, M.; Schmid, K.W.; Kienast, J. Quantification of plasminogen activators and their inhibitors in the aortic vessel wall in relation to the presence and severity of atherosclerotic disease. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 893–902. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Demyanets, S.; Speidl, W.S.; Tentzeris, I.; Jarai, R.; Katsaros, K.M.; Farhan, S.; Krychtiuk, K.A.; Wonnerth, A.; Weiss, T.W.; Huber, K.; et al. Soluble ST2 and interleukin-33 levels in coronary artery disease: Relation to disease activity and adverse outcome. PLoS ONE 2014, 9, e95055. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, O.S.; Narayan, H.K.; Khan, S.Q.; Kelly, D.; Quinn, P.A.; Squire, I.B.; Davies, J.E.; Ng, L.L. Pre-discharge risk stratification in unselected STEMI: Is there a role for ST2 or its natural ligand IL-33 when compared with contemporary risk markers? Int. J. Cardiol. 2013, 167, 2182–2188. [Google Scholar] [CrossRef]
- Shen, J.; Shang, Q.; Wong, C.K.; Li, E.K.; Wang, S.; Li, R.J.; Lee, K.L.; Leung, Y.Y.; Ying, K.Y.; Yim, C.W.; et al. IL-33 and soluble ST2 levels as novel predictors for remission and progression of carotid plaque in early rheumatoid arthritis: A prospective study. Semin. Arthritis Rheum. 2015, 45, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Pickering, E.M.; Kopach, P.; Kang, P.H.; Lockatell, V.; Todd, N.W.; Papadimitriou, J.C.; McKenzie, A.N.; Atamas, S.P. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J. Immunol. 2012, 189, 403–410. [Google Scholar] [CrossRef]
- Martin, P.; Palmer, G.; Rodriguez, E.; Woldt, E.; Mean, I.; James, R.W.; Smith, D.E.; Kwak, B.R.; Gabay, C. Atherosclerosis severity is not affected by a deficiency in IL-33/ST2 signaling. Immun. Inflamm. Dis. 2015, 3, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demyanets, S.; Stojkovic, S.; Huber, K.; Wojta, J. The Paradigm Change of IL-33 in Vascular Biology. Int. J. Mol. Sci. 2021, 22, 13288. https://doi.org/10.3390/ijms222413288
Demyanets S, Stojkovic S, Huber K, Wojta J. The Paradigm Change of IL-33 in Vascular Biology. International Journal of Molecular Sciences. 2021; 22(24):13288. https://doi.org/10.3390/ijms222413288
Chicago/Turabian StyleDemyanets, Svitlana, Stefan Stojkovic, Kurt Huber, and Johann Wojta. 2021. "The Paradigm Change of IL-33 in Vascular Biology" International Journal of Molecular Sciences 22, no. 24: 13288. https://doi.org/10.3390/ijms222413288





