Adiponectin as a Potential Biomarker for Pregnancy Disorders
Abstract
:1. Introduction
2. Clinical Biomarkers for Pregnancy Outcomes
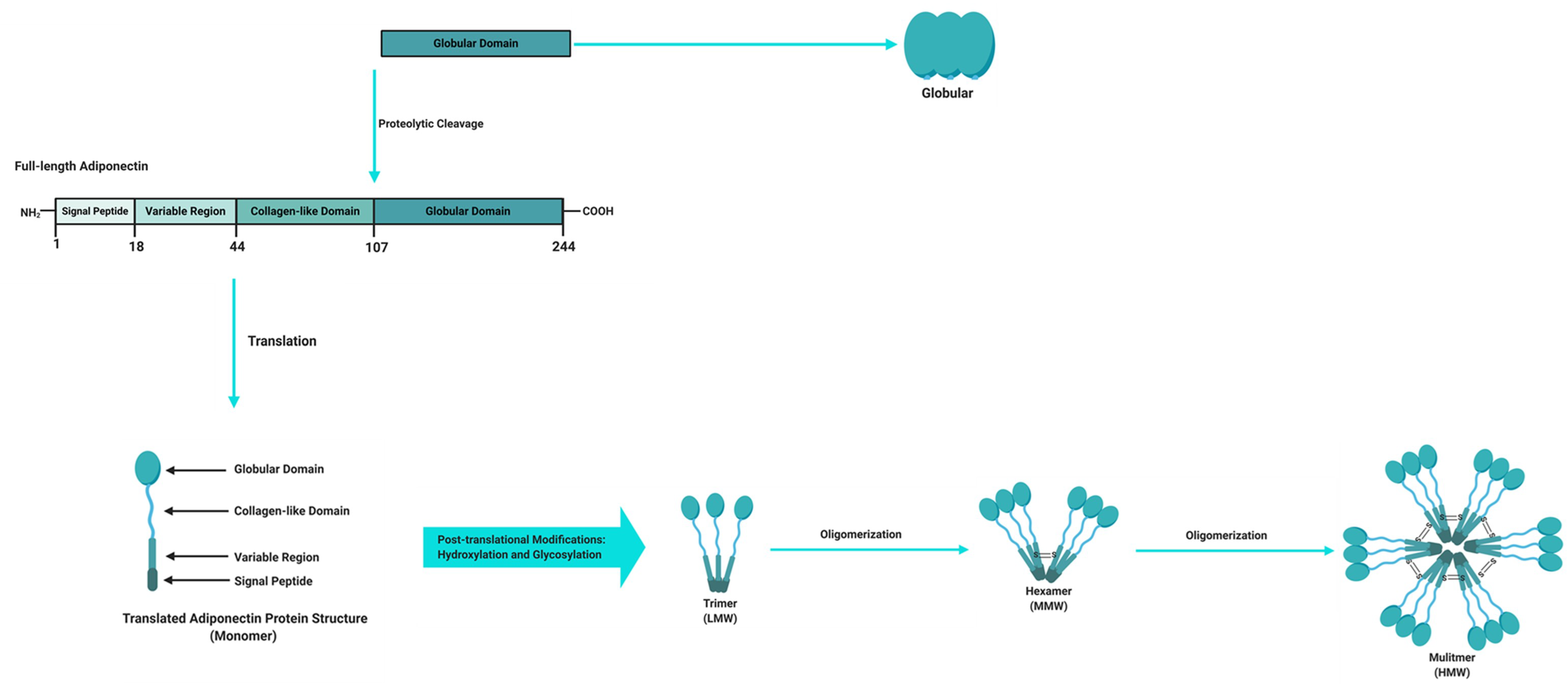
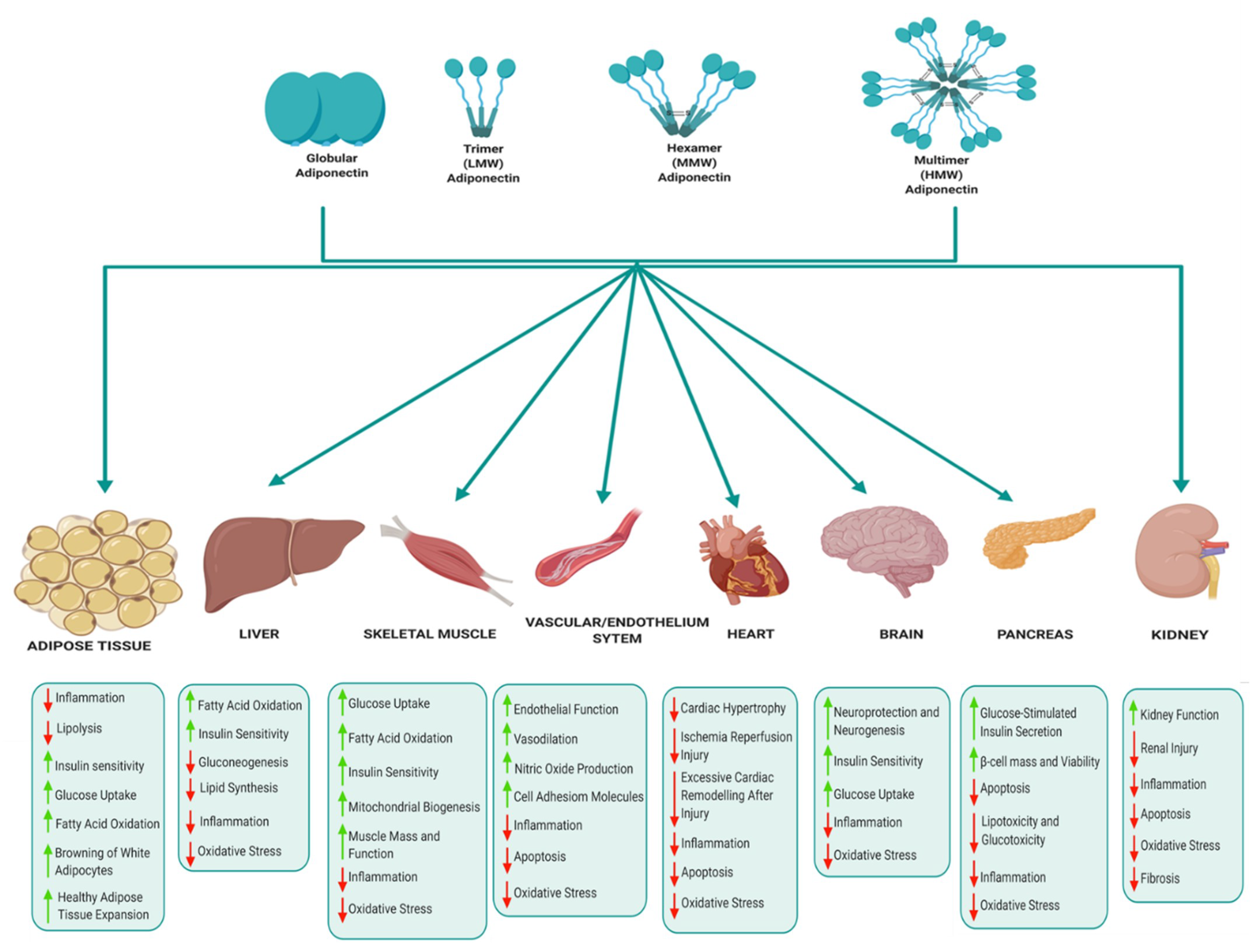
3. Adiponectin
4. Adiponectin in Pregnancy
5. Adiponectin in Common Pregnancy Disorders
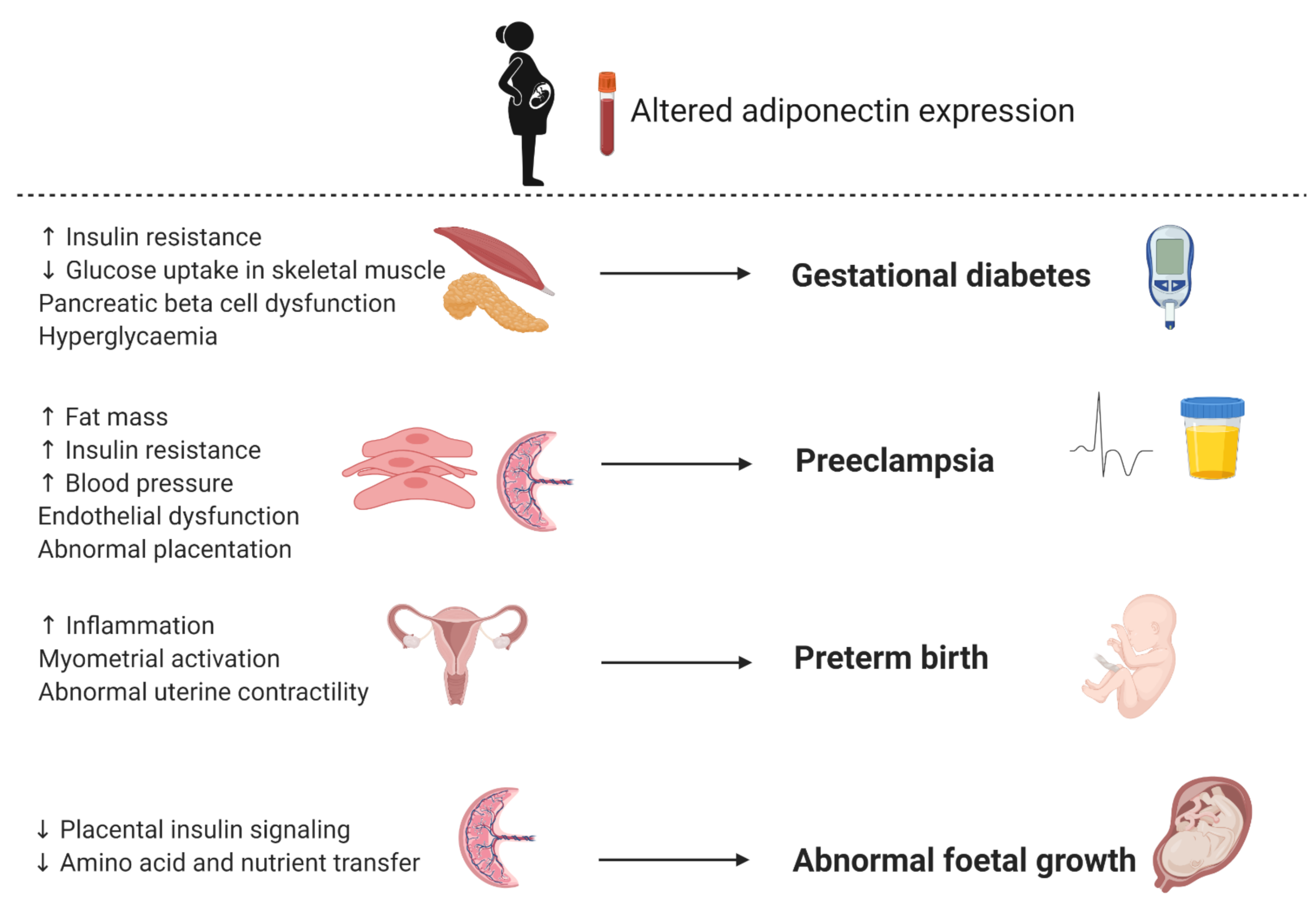
5.1. Gestational Diabetes
5.2. Preeclampsia
5.3. Preterm Birth
5.4. Foetal Growth
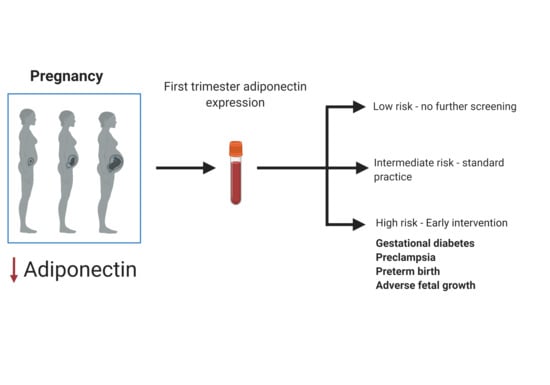
6. Clinical Implications of Adiponectin Screening
7. Conclusion and Future Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological Changes in Pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; DS, J.M. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30 (Suppl. 2), S112–S119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, H.D.; Chang, A.M.; Callaway, L.K.; Cowley, D.M.; Dyer, A.R.; Radaelli, T.; Farrell, K.A.; Huston-Presley, L.; Amini, S.B.; Kirwan, J.P.; et al. Hormonal and Metabolic Factors Associated With Variations in Insulin Sensitivity in Human Pregnancy. Diabetes Care 2010, 33, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nien, J.K.; Mazaki-Tovi, S.; Romero, R.; Kusanovic, J.P.; Erez, O.; Gotsch, F.; Pineles, B.L.; Friel, L.A.; Espinoza, J.; Goncalves, L.; et al. Resistin: A Hormone Which Induces Insulin Resistance Is Increased in Normal Pregnancy. J. Perinat. Med. 2007, 35, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavalza-Gómez, A.B.; Anaya-Prado, R.; Rincón-Sánchez, A.R.; Mora-Martínez, J.M. Adipokines and Insulin Resistance during Pregnancy. Diabetes Res. Clin. Pract. 2008, 80, 8–15. [Google Scholar] [CrossRef]
- Dias, S.; Pheiffer, C.; Abrahams, Y.; Rheeder, P.; Adam, S. Molecular Biomarkers for Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 2926. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Joy Kaitu’u-Lino, T.; Walker, S.P.; MacDonald, T.M. Blood-Based Biomarkers in the Maternal Circulation Associated with Fetal Growth Restriction. Prenat. Diagn. 2019, 39, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Poon, L.C.; McIntyre, H.D.; Hyett, J.A.; da Fonseca, E.B.; Hod, M. The First-Trimester of Pregnancy—A Window of Opportunity for Prediction and Prevention of Pregnancy Complications and Future Life. Diabetes Res. Clin. Pract. 2018, 145, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Kapur, A. Links between Maternal Health and NCDs. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, T.A.; Wright, J. Twin Epidemics of Covid-19 and Non-Communicable Disease. BMJ 2020, 369, m2618. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Pheiffer, C.; Rheeder, P.; Adam, S. Screening and Diagnosis of Gestational Diabetes Mellitus in South Africa: What We Know so Far. S. Afr. Med. J. 2019, 109, 457–462. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as Novel Biomarkers and Regulators of the Metabolic Syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef] [Green Version]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ Is a Novel Adipose-Specific Gene Dysregulated in Obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. CDNA Cloning and Expression of a Novel Adipose Specific Collagen-like Factor, ApM1 (AdiPose Most Abundant Gene Transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Nakano, Y.; Tobe, T.; Choi-Miura, N.-H.; Mazda, T.; Tomita, M. Isolation and Characterization of GBP28, a Novel Gelatin-Binding Protein Purified from Human Plasma. J. Biochem. 1996, 120, 803–812. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [Green Version]
- Naimo, G.D.; Gelsomino, L.; Catalano, S.; Mauro, L.; Andò, S. Interfering Role of ERα on Adiponectin Action in Breast Cancer. Front. Endocrinol. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruebis, J.; Tsao, T.-S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.S.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic Cleavage Product of 30-KDa Adipocyte Complement-Related Protein Increases Fatty Acid Oxidation in Muscle and Causes Weight Loss in Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired Multimerization of Human Adiponectin Mutants Associated with Diabetes. Molecular Structure and Multimer Formation of Adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magkos, F.; Sidossis, L.S. Recent Advances in the Measurement of Adiponectin Isoform Distribution. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Arita, Y.; Yamagata, K.; Matsukawa, Y.; Okutomi, K.; Horie, M.; Shimomura, I.; Hotta, K.; Kuriyama, H.; Kihara, S.; et al. Genomic Structure and Mutations in Adipose-Specific Gene, Adiponectin. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin—A Key Adipokine in the Metabolic Syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef]
- Tsao, T.-S.; Murrey, H.; Hug, C.; Lee, D.; Lodish, H. Oligomerization State-Dependent Activation of NF-Kappa B Signaling Pathway by Adipocyte Complement-Related Protein of 30 KDa (Acrp30). J. Biol. Chem. 2002, 277, 29359–29362. [Google Scholar] [CrossRef] [Green Version]
- Pajvani, U.; Hawkins, M.; Combs, T.; Rajala, M.; Doebber, T.; Berger, J.; Wagner, J.; Wu, M.; Knopps, A.; Xiang, A.; et al. Complex Distribution, Not Absolute Amount of Adiponectin, Correlates with Thiazolidinedione-Mediated Improvement in Insulin Sensitivity. J. Biol. Chem. 2004, 279, 12152–12162. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the Past Two Decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, M.M.; Austrheim-Smith, I.T.; Stanhope, K.L.; Van Loan, M.D.; Ali, M.R.; Wolfe, B.M.; Havel, P.J. Circulating Concentrations of High-Molecular-Weight Adiponectin Are Increased Following Roux-En-Y Gastric Bypass Surgery. Diabetologia 2006, 49, 2552–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and Its Receptors Are Expressed in Bone-Forming Cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Yoda-Murakami, M.; Taniguchi, M.; Takahashi, K.; Kawamata, S.; Saito, K.; Choi-Miura, N.-H.; Tomita, M. Change in Expression of GBP28/Adiponectin in Carbon Tetrachloride-Administrated Mouse Liver. Biochem. Biophys. Res. Commun. 2001, 285, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Delaigle, A.M.; Jonas, J.-C.; Bauche, I.B.; Cornu, O.; Brichard, S.M. Induction of Adiponectin in Skeletal Muscle by Inflammatory Cytokines: In Vivo and in Vitro Studies. Endocrinology 2004, 145, 5589–5597. [Google Scholar] [CrossRef] [Green Version]
- Caminos, J.; Nogueiras, R.; Gallego, R.; Bravo, S.; Tovar, S.; García-Caballero, T.; Casanueva, F.; Dieguez, C. Expression and Regulation of Adiponectin and Receptor in Human and Rat Placenta. J. Clin. Endocrinol. Metab. 2005, 90, 4276–4286. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of Adiponectin Receptors That Mediate Antidiabetic Metabolic Effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Fukuda, S.; Kita, S.; Obata, Y.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; Nishizawa, H.; Funahashi, T.; Takagi, J.; et al. The Unique Prodomain of T-Cadherin Plays a Key Role in Adiponectin Binding with the Essential Extracellular Cadherin Repeats 1 and 2. J. Biol. Chem. 2017, 292, 7840–7849. [Google Scholar] [CrossRef] [Green Version]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The Effect of Adiponectin in the Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Potential Role of Polyphenols in the Modulation of Adiponectin Signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef]
- Leth, H.; Andersen, K.K.; Frystyk, J.; Tarnow, L.; Rossing, P.; Parving, H.-H.; Flyvbjerg, A. Elevated Levels of High-Molecular-Weight Adiponectin in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 3186–3191. [Google Scholar] [CrossRef] [Green Version]
- Menzaghi, C.; Trischitta, V. The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes 2018, 67, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.; Sifuentes, A.; Holland, W. Regulation of Glucose and Lipid Homeostasis by Adiponectin: Effects on Hepatocytes, Pancreatic β Cells and Adipocytes. Best practice & research. Clin. Endocrinol. Metab. 2014, 28, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sweeney, G. Adiponectin Action in Skeletal Muscle. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 33–41. [Google Scholar] [CrossRef]
- Krause, M.; Milne, K.; Hawke, T. Adiponectin—Consideration for Its Role in Skeletal Muscle Health. Int. J. Mol. Sci. 2019, 20, 1528. [Google Scholar] [CrossRef] [Green Version]
- Nanayakkara, G.; Kariharan, T.; Wang, L.; Zhong, J.; Amin, R. The Cardio-Protective Signaling and Mechanisms of Adiponectin. Am. J. Cardiovasc. Dis. 2012, 2, 253–266. [Google Scholar]
- Kadowaki, T.; Yamauchi, T.; Kubota, N. The Physiological and Pathophysiological Role of Adiponectin and Adiponectin Receptors in the Peripheral Tissues and CNS. FEBS Lett. 2008, 582, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin Receptor Signalling in the Brain. Br. J. Pharmacol. 2012, 165, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Bloemer, J.; Pinky, P.D.; Govindarajulu, M.; Hong, H.; Judd, R.; Amin, R.H.; Moore, T.; Dhanasekaran, M.; Reed, M.N.; Suppiramaniam, V. Role of Adiponectin in Central Nervous System Disorders. Neural Plast. 2018, 2018, 4593530. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Barua, S.; Jeong, Y.J.; Lee, J.E. Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6419. [Google Scholar] [CrossRef]
- Lee, Y.; Magkos, F.; Mantzoros, C.S.; Kang, E.S. Effects of Leptin and Adiponectin on Pancreatic β-Cell Function. Metabolism 2011, 60, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Kiortsis, D.N. The Role of Adiponectin in Renal Physiology and Development of Albuminuria. J. Endocrinol. 2014, 221, R49–R61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aye, I.L.M.H.; Powell, T.L.; Jansson, T. Review: Adiponectin—The Missing Link between Maternal Adiposity, Placental Transport and Fetal Growth? Placenta 2013, 34, S40–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, P.M.; Hoegh, M.; Minium, J.; Huston-Presley, L.; Bernard, S.; Kalhan, S.; Hauguel-De Mouzon, S. Adiponectin in Human Pregnancy: Implications for Regulation of Glucose and Lipid Metabolism. Diabetologia 2006, 49, 1677–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazaki-Tovi, S.; Kanety, H.; Pariente, C.; Hemi, R.; Wiser, A.; Schiff, E.; Sivan, E. Maternal Serum Adiponectin Levels during Human Pregnancy. J. Perinatol. 2007, 27, 77–81. [Google Scholar] [CrossRef]
- Retnakaran, A.; Retnakaran, R. Adiponectin in Pregnancy: Implications for Health and Disease. Curr. Med. Chem. 2012, 19, 5444–5450. [Google Scholar] [CrossRef]
- Nien, J.K.; Mazaki-Tovi, S.; Romero, R.; Erez, O.; Kusanovic, J.P.; Gotsch, F.; Pineles, B.L.; Gomez, R.; Edwin, S.; Mazor, M.; et al. Plasma Adiponectin Concentrations in Non-Pregnant, Normal and Overweight Pregnant Women. J. Perinat. Med. 2007, 35, 522–531. [Google Scholar] [CrossRef]
- Jara, A.; Dreher, M.; Porter, K.; Christian, L.M. The Association of Maternal Obesity and Race with Serum Adipokines in Pregnancy and Postpartum: Implications for Gestational Weight Gain and Infant Birth Weight. Brain Behav. Immun. Health 2020, 3, 100053. [Google Scholar] [CrossRef]
- Suwaki, N.; Masuyama, H.; Nakatsukasa, H.; Masumoto, A.; Sumida, Y.; Takamoto, N.; Hiramatrsu, Y. Hypoadiponectinemia and Circulating Angiogenic Factors in Overweight Patients Complicated with Pre-Eclampsia. Am. J. Obstet. Gynecol. 2006, 195, 1687–1692. [Google Scholar] [CrossRef]
- Ott, R.; Stupin, J.H.; Melchior, K.; Schellong, K.; Ziska, T.; Dudenhausen, J.W.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Alterations of Adiponectin Gene Expression and DNA Methylation in Adipose Tissues and Blood Cells Are Associated with Gestational Diabetes and Neonatal Outcome. Clin. Epigenetics 2018, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Naruse, K.; Yamasaki, M.; Umekage, H.; Sado, T.; Sakamoto, Y.; Morikawa, H. Peripheral Blood Concentrations of Adiponectin, an Adipocyte-Specific Plasma Protein, in Normal Pregnancy and Preeclampsia. J. Reprod. Immunol. 2005, 65, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, C.; Willmer, T.; Dias, S.; Abrahams, Y.; Louw, J.; Goedecke, J.H. Ethnic and Adipose Depot Specific Associations between DNA Methylation and Metabolic Risk. Front. Genet. 2020, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Rosario, F.J.; Powell, T.L.; Jansson, T. Adiponectin Supplementation in Pregnant Mice Prevents the Adverse Effects of Maternal Obesity on Placental Function and Fetal Growth. Proc. Natl. Acad. Sci. USA 2015, 112, 12858–12863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, M.E.; Rosario, F.J.; Wesolowski, S.R.; Powell, T.L.; Jansson, T. Normalizing Adiponectin Levels in Obese Pregnant Mice Prevents Adverse Metabolic Outcomes in Offspring. FASEB J. 2019, 33, 2899–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, O.R.; Rosario, F.J.; Powell, T.L.; Jansson, T. Normalisation of Circulating Adiponectin Levels in Obese Pregnant Mice Prevents Cardiac Dysfunction in Adult Offspring. Int. J. Obes. 2020, 44, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Zuo, M.; Liao, G.; Zhang, W.; Xu, D.; Lu, J.; Tang, M.; Yan, Y.; Hong, C.; Wang, Y. Effects of Exogenous Adiponectin Supplementation in Early Pregnant PCOS Mice on the Metabolic Syndrome of Adult Female Offspring. J. Ovarian Res. 2020. [Google Scholar] [CrossRef]
- Santangelo, C.; Zicari, A.; Mandosi, E.; Scazzocchio, B.; Mari, E.; Morano, S.; Masella, R. Could Gestational Diabetes Mellitus be Managed through Dietary Bioactive Compounds? Current Knowledge and Future Perspectives. Br. J. Nutr. 2016, 115, 1129–1144. [Google Scholar] [CrossRef]
- Jack, B.U.; Malherbe, C.J.; Mamushi, M.; Muller, C.J.F.; Joubert, E.; Louw, J.; Pheiffer, C. Adipose Tissue as a Possible Therapeutic Target for Polyphenols: A Case for Cyclopia Extracts as Anti-Obesity Nutraceuticals. Biomed. Pharmacother 2019, 120, 109439. [Google Scholar] [CrossRef]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 3), S173–S211. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed. 2017. Available online: http://diabetesatlas.org/resources/2017-atlas.html (accessed on 10 June 2020).
- Dias, S.; Adam, S.; Rheeder, P.; Pheiffer, C. Prevalence of and Risk Factors for Gestational Diabetes Mellitus in South Africa. S. Afr. Med. J. 2019, 109, 463–467. [Google Scholar] [CrossRef]
- Moran, P.S.; Wuytack, F.; Turner, M.; Normand, C.; Brown, S.; Begley, C.; Daly, D. Economic Burden of Maternal Morbidity—A Systematic Review of Cost-of-Illness Studies. PLoS ONE 2020, 15, e0227377. [Google Scholar] [CrossRef] [PubMed]
- Kolu, P.; Raitanen, J.; Rissanen, P.; Luoto, R. Health Care Costs Associated with Gestational Diabetes Mellitus among High-Risk Women—Results from a Randomised Trial. BMC Pregnancy Childbirth 2012, 12, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühl, C. Insulin Secretion and Insulin Resistance in Pregnancy and GDM. Implications for Diagnosis and Management. Diabetes 1991, 40 (Suppl. 2), 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; O’Sullivan, M.J.; Skyler, J.S. Insulin Action during Pregnancy. Studies with the Euglycemic Clamp Technique. Diabetes 1985, 34, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal Changes in Glucose Metabolism during Pregnancy in Obese Women with Normal Glucose Tolerance and Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Siddeek, B.; Boubred, F.; Benahmed, M.; Simeoni, U. The Offspring of the Diabetic Mother--Short- and Long-Term Implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 256–269. [Google Scholar] [CrossRef]
- Ratner, R.E. Prevention of Type 2 Diabetes in Women With Previous Gestational Diabetes. Diabetes Care 2007, 30, S242. [Google Scholar] [CrossRef] [Green Version]
- Matta-Coelho, C.; Monteiro, A.M.; Fernandes, V.; Pereira, M.L.; Souto, S.B. Portuguese Diabetes and Pregnancy Study Group Universal vs. Risk-Factor-Based Screening for Gestational Diabetes—An Analysis from a 5-Year Portuguese Cohort. Endocrine 2019, 63, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Adam, S.; Rheeder, P. Screening for Gestational Diabetes Mellitus in a South African Population: Prevalence, Comparison of Diagnostic Criteria and the Role of Risk Factors. S. Afr. Med. J. 2017, 107, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal Circulating Concentrations of Tumor Necrosis Factor-Alpha, Leptin, and Adiponectin in Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Sci. World J. 2014, 2014, 926932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, M.; Battista, M.-C.; Doyon, M.; Ménard, J.; Ardilouze, J.-L.; Perron, P.; Hivert, M.-F. Lower Adiponectin Levels at First Trimester of Pregnancy Are Associated with Increased Insulin Resistance and Higher Risk of Developing Gestational Diabetes Mellitus. Diabetes Care 2013, 36, 1577–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thagaard, I.N.; Krebs, L.; Holm, J.-C.; Lange, T.; Larsen, T.; Christiansen, M. Adiponectin and Leptin as First Trimester Markers for Gestational Diabetes Mellitus: A Cohort Study. Clin. Chem. Lab. Med. 2017, 55, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, S.; Sassarini, J.; Kelsey, T.W.; Lindsay, R.S.; Sattar, N.; Nelson, S.M. Accuracy of Circulating Adiponectin for Predicting Gestational Diabetes: A Systematic Review and Meta-Analysis. Diabetologia 2016, 59, 692–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, T.; Gebhardt, C.; Scholz, M.; Schleinitz, D.; Blüher, M.; Stumvoll, M.; Kovacs, P.; Fasshauer, M.; Tönjes, A. Adipocytokines Are Not Associated with Gestational Diabetes Mellitus but with Pregnancy Status. Cytokine 2020, 131, 155088. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-Eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Fondjo, L.A.; Owiredu, W.K.; Czika, A.; Nelson, W.; Lamptey, J.; Wang, Y.-X.; Ding, Y.-B. The Role of Adiponectin in Placentation and Preeclampsia. Cell Biochem. Funct. 2020, 38, 106–117. [Google Scholar] [CrossRef]
- MacKay, A.P.; Berg, C.J.; Atrash, H.K. Pregnancy-Related Mortality from Preeclampsia and Eclampsia. Obstet. Gynecol. 2001, 97, 533–538. [Google Scholar] [CrossRef]
- Newman, M.G.; Robichaux, A.G.; Stedman, C.M.; Jaekle, R.K.; Fontenot, M.T.; Dotson, T.; Lewis, D.F. Perinatal Outcomes in Preeclampsia that Is Complicated by Massive Proteinuria. Am. J. Obstet. Gynecol. 2003, 188, 264–268. [Google Scholar] [CrossRef]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J. Clin. Med. 2019, 8, 1625. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, E.; Duval, F.; Vialard, F.; Dieudonné, M.-N. The Roles of Leptin and Adiponectin at the Fetal-Maternal Interface in Humans. Horm. Mol. Biol. Clin. Investig. 2015, 24, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Tian, Y.; Li, X. Adiponectin Participates in Preeclampsia by Regulating the Biological Function of Placental Trophoblasts through P38 MAPK-STAT5 Pathway. Iran. J. Public Health 2018, 47, 1838–1844. [Google Scholar] [PubMed]
- Ramsay, J.E.; Jamieson, N.; Greer, I.A.; Sattar, N. Paradoxical Elevation in Adiponectin Concentrations in Women with Preeclampsia. Hypertension 2003, 42, 891–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Yang, X.; Wu, Y.; Wang, H.; Huang, H.; Dong, M. Serum Adiponectin, Leptin and Soluble Leptin Receptor in Pre-Eclampsia. Int. J. Gynecol. Obstet. 2006, 95, 121–126. [Google Scholar] [CrossRef]
- Takemura, Y.; Osuga, Y.; Koga, K.; Tajima, T.; Hirota, Y.; Hirata, T.; Morimoto, C.; Harada, M.; Yano, T.; Taketani, Y. Selective Increase in High Molecular Weight Adiponectin Concentration in Serum of Women with Preeclampsia. J. Reprod. Immunol. 2007, 73, 60–65. [Google Scholar] [CrossRef]
- D’Anna, R.; Baviera, G.; Corrado, F.; Giordano, D.; De Vivo, A.; Nicocia, G.; Di Benedetto, A. Adiponectin and Insulin Resistance in Early- and Late-Onset Pre-Eclampsia. BJOG 2006, 113, 1264–1269. [Google Scholar] [CrossRef]
- Thagaard, I.N.; Hedley, P.L.; Holm, J.-C.; Lange, T.; Larsen, T.; Krebs, L.; Christiansen, M. Leptin and Adiponectin as Markers for Preeclampsia in Obese Pregnant Women, a Cohort Study. Pregnancy Hypertens. 2019, 15, 78–83. [Google Scholar] [CrossRef]
- Hendler, I.; Blackwell, S.C.; Mehta, S.H.; Whitty, J.E.; Russell, E.; Sorokin, Y.; Cotton, D.B. The Levels of Leptin, Adiponectin, and Resistin in Normal Weight, Overweight, and Obese Pregnant Women with and without Preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 979–983. [Google Scholar] [CrossRef]
- Walani, S.R. Global Burden of Preterm Birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef]
- Heinonen, K.; Eriksson, J.G.; Lahti, J.; Kajantie, E.; Pesonen, A.-K.; Tuovinen, S.; Osmond, C.; Raikkonen, K. Late Preterm Birth and Neurocognitive Performance in Late Adulthood: A Birth Cohort Study. Pediatrics 2015, 135, e818–e825. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.S.; Magalhães, L.C.; Alves, C.R.L.; Moreira, R.S.; Magalhães, L.C.; Alves, C.R.L. Effect of Preterm Birth on Motor Development, Behavior, and School Performance of School-Age Children: A Systematic Review. J. Pediatr. 2014, 90, 119–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.B.; Green, N.S.; Steiner, C.A.; Meikle, S.; Howse, J.L.; Poschman, K.; Dias, T.; Potetz, L.; Davidoff, M.J.; Damus, K.; et al. Cost of Hospitalization for Preterm and Low Birth Weight Infants in the United States. Pediatrics 2007, 120, e1–e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrou, S.; Abangma, G.; Johnson, S.; Wolke, D.; Marlow, N. Costs and Health Utilities Associated with Extremely Preterm Birth: Evidence from the EPICure Study. Value Health 2009, 12, 1124–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmanan, A.; Agni, M.; Lieu, T.; Fleegler, E.; Kipke, M.; Friedlich, P.S.; McCormick, M.C.; Belfort, M.B. The Impact of Preterm Birth <37 Weeks on Parents and Families: A Cross-Sectional Study in the 2 Years after Discharge from the Neonatal Intensive Care Unit. Health Qual. Life Outcomes 2017, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of Preterm Birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The Biological Basis and Prevention of Preterm Birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bittar, R.E.; da Fonseca, E.B.; de Carvalho, M.H.B.; Martinelli, S.; Zugaib, M. Predicting Preterm Delivery in Asymptomatic Patients with Prior Preterm Delivery by Measurement of Cervical Length and Phosphorylated Insulin-like Growth Factor-Binding Protein-1. Ultrasound Obstet. Gynecol. 2007, 29, 562–567. [Google Scholar] [CrossRef]
- Wennerholm, U.; Holm, B.; Mattsby-Baltzer, I.; Nielsen, T.; Platz-Christensen, J.; Sundell, G.; Hosseini, N.; Hagberg, H. Fetal Fibronectin, Endotoxin, Bacterial Vaginosis and Cervical Length as Predictors of Preterm Birth and Neonatal Morbidity in Twin Pregnancies. BJOG Int. J. Obstet. Gynaecol. 1997, 104, 1398–1404. [Google Scholar] [CrossRef]
- Jung, E.Y.; Park, J.W.; Ryu, A.; Lee, S.Y.; Cho, S.; Park, K.H. Prediction of Impending Preterm Delivery Based on Sonographic Cervical Length and Different Cytokine Levels in Cervicovaginal Fluid in Preterm Labor. J. Obstet. Gynaecol. Res. 2016, 42, 158–165. [Google Scholar] [CrossRef]
- Latorre Uriza, C.; Velosa-Porras, J.; Roa, N.S.; Quiñones Lara, S.M.; Silva, J.; Ruiz, A.J.; Escobar Arregoces, F.M. Periodontal Disease, Inflammatory Cytokines, and PGE2 in Pregnant Patients at Risk of Preterm Delivery: A Pilot Study. Available online: https://www.hindawi.com/journals/idog/2018/7027683/ (accessed on 3 December 2020).
- Garshasbi, A.; Ghazanfari, T.; Faghih Zadeh, S. Beta-Human Chorionic Gonadotropin in Cervicovaginal Secretions and Preterm Delivery. Int. J. Gynecol. Obstet. 2004, 86, 358–364. [Google Scholar] [CrossRef]
- Tagore, S.; Kwek, K. Comparative Analysis of Insulin-like Growth Factor Binding Protein-1 (IGFBP-1), Placental Alpha-Microglobulin-1 (PAMG-1) and Nitrazine Test to Diagnose Premature Rupture of Membranes in Pregnancy. J. Perinat. Med. 2010, 38, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Suff, N.; Story, L.; Shennan, A. The Prediction of Preterm Delivery: What Is New? Semin. Fetal Neonatal Med. 2019, 24, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, R.; Torloni, M.R.; Voltolini, C.; Torricelli, M.; Merialdi, M.; Betrán, A.P.; Widmer, M.; Allen, T.; Davydova, I.; Khodjaeva, Z.; et al. Biomarkers of Spontaneous Preterm Birth: An Overview of The Literature in the Last Four Decades. Reprod. Sci. 2011, 18, 1046–1070. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Papageorghiou, A.T.; Kennedy, S.H.; Villar, J. Novel Biomarkers for the Prediction of the Spontaneous Preterm Birth Phenotype: A Systematic Review and Meta-Analysis. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Khanam, R.; Vinayachandran, V.; Baqui, A.H.; London, S.J.; Biswal, S. Epigenetic Biomarkers and Preterm Birth. Environ. Epigenetics 2020, 6, dvaa005. [Google Scholar] [CrossRef]
- Oskovi Kaplan, Z.A.; Ozgu-Erdinc, A.S. Prediction of Preterm Birth: Maternal Characteristics, Ultrasound Markers, and Biomarkers: An Updated Overview. Available online: https://www.hindawi.com/journals/jp/2018/8367571/ (accessed on 1 December 2020).
- Mazaki-Tovi, S.; Romero, R.; Vaisbuch, E.; Erez, O.; Mittal, P.; Chaiworapongsa, T.; Kim, S.K.; Pacora, P.; Yeo, L.; Gotsch, F.; et al. Dysregulation of Maternal Serum Adiponectin in Preterm Labor. J. Matern. Fetal Neonatal. Med. 2009, 22, 887–904. [Google Scholar] [CrossRef] [Green Version]
- Vyas, V.; Guerra, D.D.; Bok, R.; Powell, T.; Jansson, T.; Hurt, K.J. Adiponectin Links Maternal Metabolism to Uterine Contractility. FASEB J. 2019, 33, 14588–14601. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.C.; White, S.L.; Patel, N.; Seed, P.T.; Briley, A.L.; Sandall, J.; Welsh, P.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; et al. Prediction of Uncomplicated Pregnancies in Obese Women: A Prospective Multicentre Study. BMC Med. 2017, 15, 194. [Google Scholar] [CrossRef] [Green Version]
- Mierzyński, R.; Dłuski, D.; Nowakowski, Ł.; Poniedziałek-Czajkowska, E.; Leszczyńska-Gorzelak, B. Adiponectin and Omentin Levels as Predictive Biomarkers of Preterm Birth in Patients with Gestational Diabetes Mellitus. Biomed. Res. Int. 2018, 2018, 7154216. [Google Scholar] [CrossRef] [Green Version]
- Gicquel, C.; Le Bouc, Y. Hormonal Regulation of Fetal Growth. Horm. Res. 2006, 65 (Suppl. 3), 28–33. [Google Scholar] [CrossRef]
- Dessì, A.; Ottonello, G.; Fanos, V. Physiopathology of Intrauterine Growth Retardation: From Classic Data to Metabolomics. J. Matern. Fetal Neonatal. Med. 2012, 25, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.C.P.; Friis, C.M.; Godang, K.; Bollerslev, J.; Haugen, G.; Henriksen, T. Maternal Factors Associated with Fetal Growth and Birthweight Are Independent Determinants of Placental Weight and Exhibit Differential Effects by Fetal Sex. PLoS ONE 2014, 9, e87303. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Figueroa, H.; Truong, V.T.T.; Pedroza, C.; Chauhan, S.P. Large for Gestational Age Infants and Adverse Outcomes among Uncomplicated Pregnancies at Term. Am. J. Perinatol. 2017, 34, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.B.; Stockburger, J.; Tuuli, M.G.; Macones, G.A.; Odibo, A.O.; Trudell, A.S. Large-for-Gestational Age and Stillbirth: Is There a Role for Antenatal Testing? Ultrasound Obstet. Gynecol. 2019, 54, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Briana, D.D.; Malamitsi-Puchner, A. The Role of Adipocytokines in Fetal Growth. Ann. N. Y. Acad. Sci. 2010, 1205, 82–87. [Google Scholar] [CrossRef]
- Nicoletto, S.F.; Rinaldi, A. In the Womb’s Shadow. EMBO Rep. 2011, 12, 30–34. [Google Scholar] [CrossRef]
- Mazaki-Tovi, S.; Kanety, H.; Pariente, C.; Hemi, R.; Schiff, E.; Sivan, E. Cord Blood Adiponectin in Large-for-Gestational Age Newborns. Am. J. Obstet. Gynecol. 2005, 193, 1238–1242. [Google Scholar] [CrossRef]
- Lekva, T.; Roland, M.C.P.; Michelsen, A.E.; Friis, C.M.; Aukrust, P.; Bollerslev, J.; Henriksen, T.; Ueland, T. Large Reduction in Adiponectin During Pregnancy Is Associated With Large-for-Gestational-Age Newborns. J. Clin. Endocrinol. Metab. 2017, 102, 2552–2559. [Google Scholar] [CrossRef]
- Yalinbas, E.E.; Binay, C.; Simsek, E.; Aksit, M.A. The Role of Umbilical Cord Blood Concentration of IGF-I, IGF-II, Leptin, Adiponectin, Ghrelin, Resistin, and Visfatin in Fetal Growth. Am. J. Perinatol. 2019, 36, 600–608. [Google Scholar] [CrossRef]
- Martínez-Cordero, C.; Amador-Licona, N.; Guízar-Mendoza, J.M.; Hernández-Méndez, J.; Ruelas-Orozco, G. Body Fat at Birth and Cord Blood Levels of Insulin, Adiponectin, Leptin, and Insulin-like Growth Factor-I in Small-for-Gestational-Age Infants. Arch. Med. Res. 2006, 37, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Valdés, E.R.; Lattes, K.A.; Muñoz, H.S.; Barja, P.Y.; Papapietro, K.V. First-Trimester Adiponectin and Subsequent Development of Preeclampsia or Fetal Growth Restriction. Gynecol. Obstet. Invest. 2011, 72, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Naylor, S. Biomarkers: Current Perspectives and Future Prospects. Expert Rev. Mol. Diagn. 2003, 3, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. Biomarkers: Potential Uses and Limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O. Ethnic Differences in Maternal Adipokines during Normal Pregnancy. Int. J. Environ. Res. Public Health 2015, 13, 8. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pheiffer, C.; Dias, S.; Jack, B.; Malaza, N.; Adam, S. Adiponectin as a Potential Biomarker for Pregnancy Disorders. Int. J. Mol. Sci. 2021, 22, 1326. https://doi.org/10.3390/ijms22031326
Pheiffer C, Dias S, Jack B, Malaza N, Adam S. Adiponectin as a Potential Biomarker for Pregnancy Disorders. International Journal of Molecular Sciences. 2021; 22(3):1326. https://doi.org/10.3390/ijms22031326
Chicago/Turabian StylePheiffer, Carmen, Stephanie Dias, Babalwa Jack, Nompumelelo Malaza, and Sumaiya Adam. 2021. "Adiponectin as a Potential Biomarker for Pregnancy Disorders" International Journal of Molecular Sciences 22, no. 3: 1326. https://doi.org/10.3390/ijms22031326