Synthesis of Novel Pyrido[1,2-c]pyrimidine Derivatives with 6-Fluoro-3-(4-piperidynyl)-1,2-benzisoxazole Moiety as Potential SSRI and 5-HT1A Receptor Ligands
Abstract
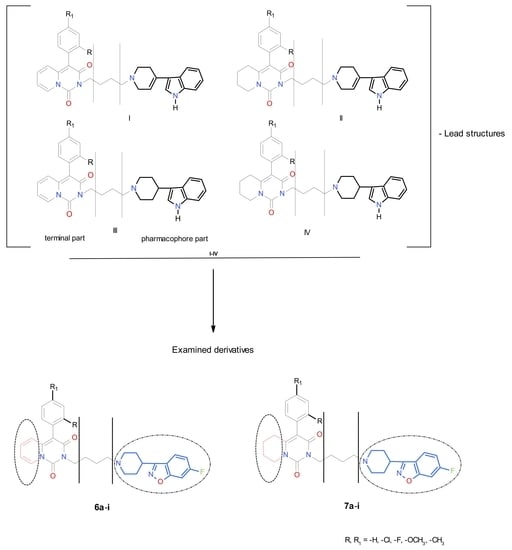
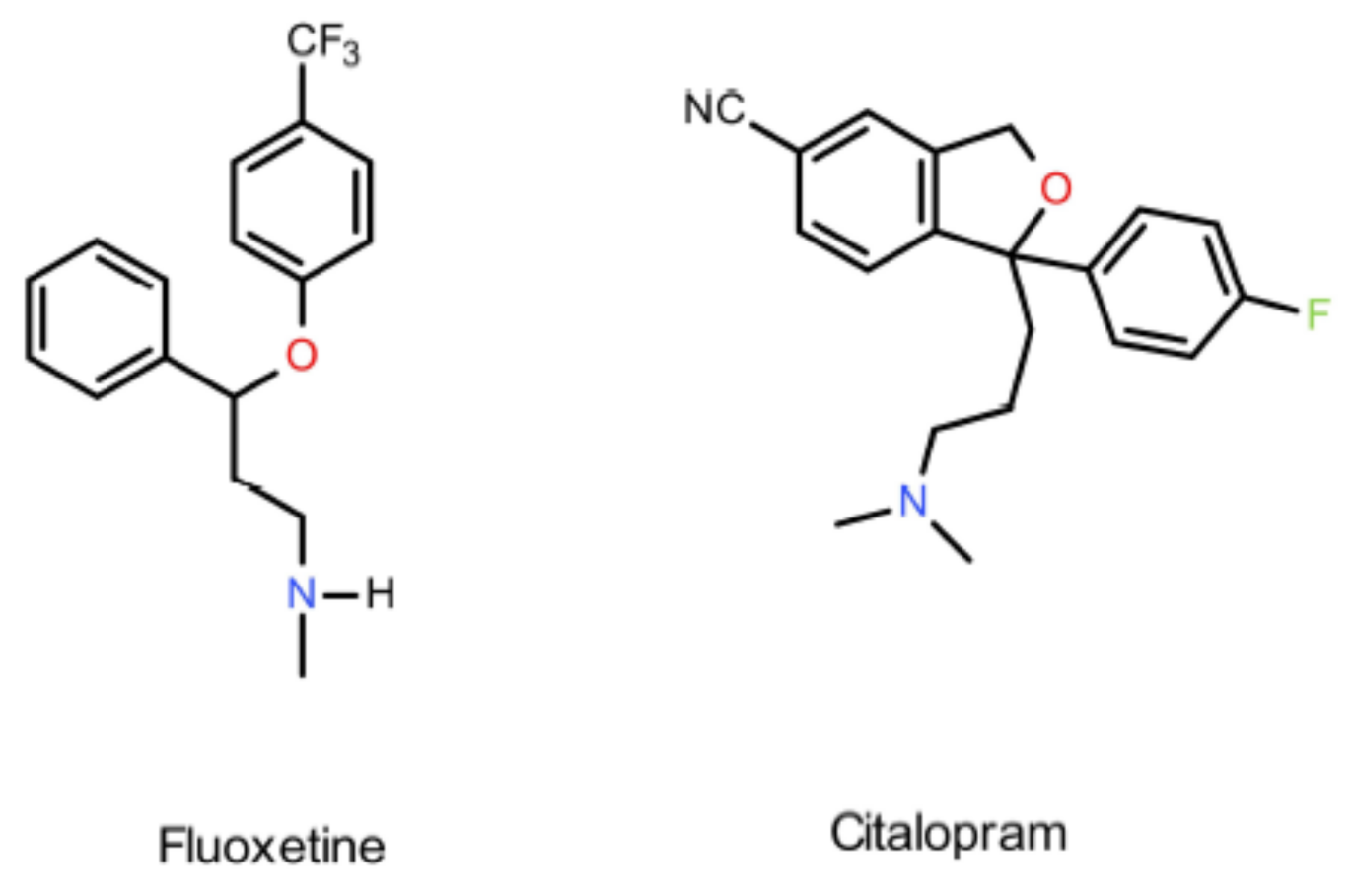
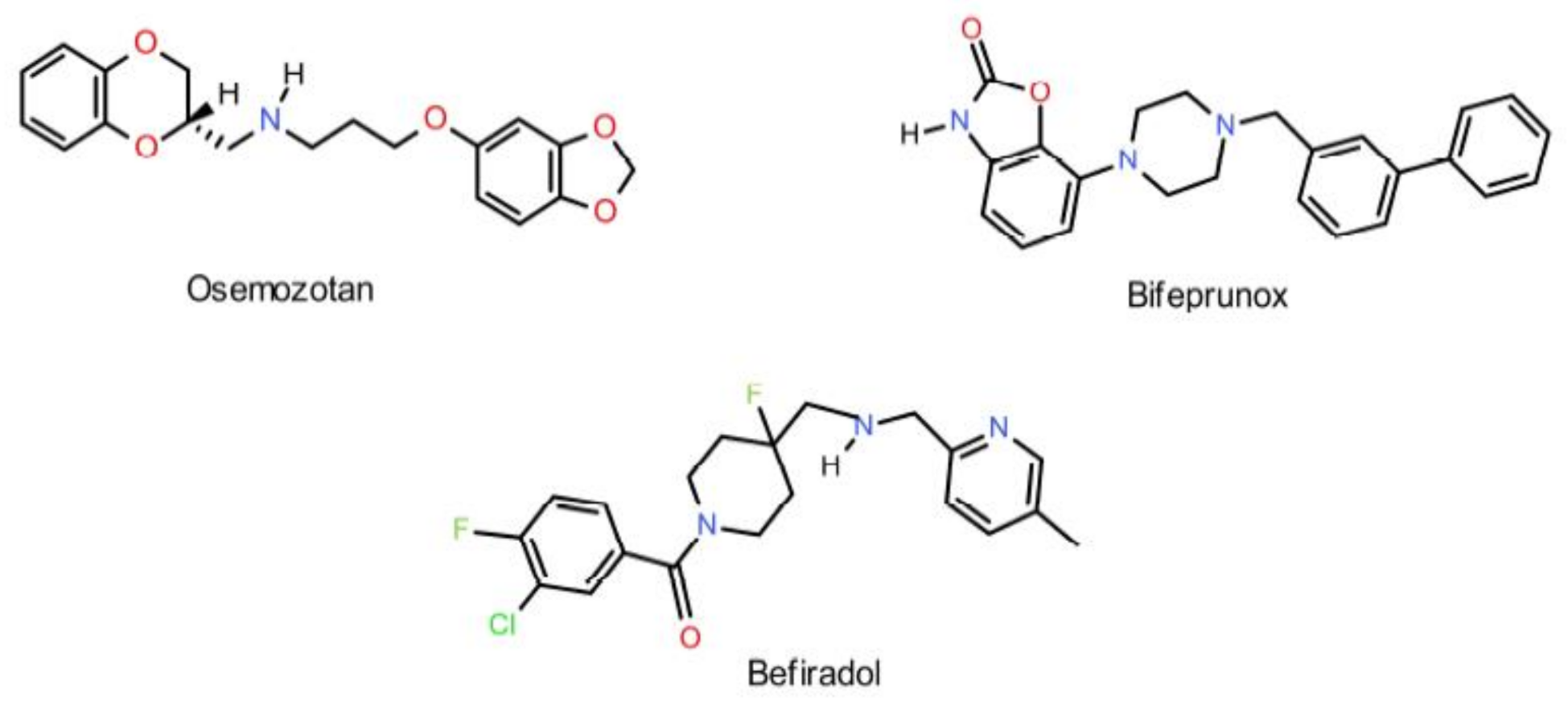
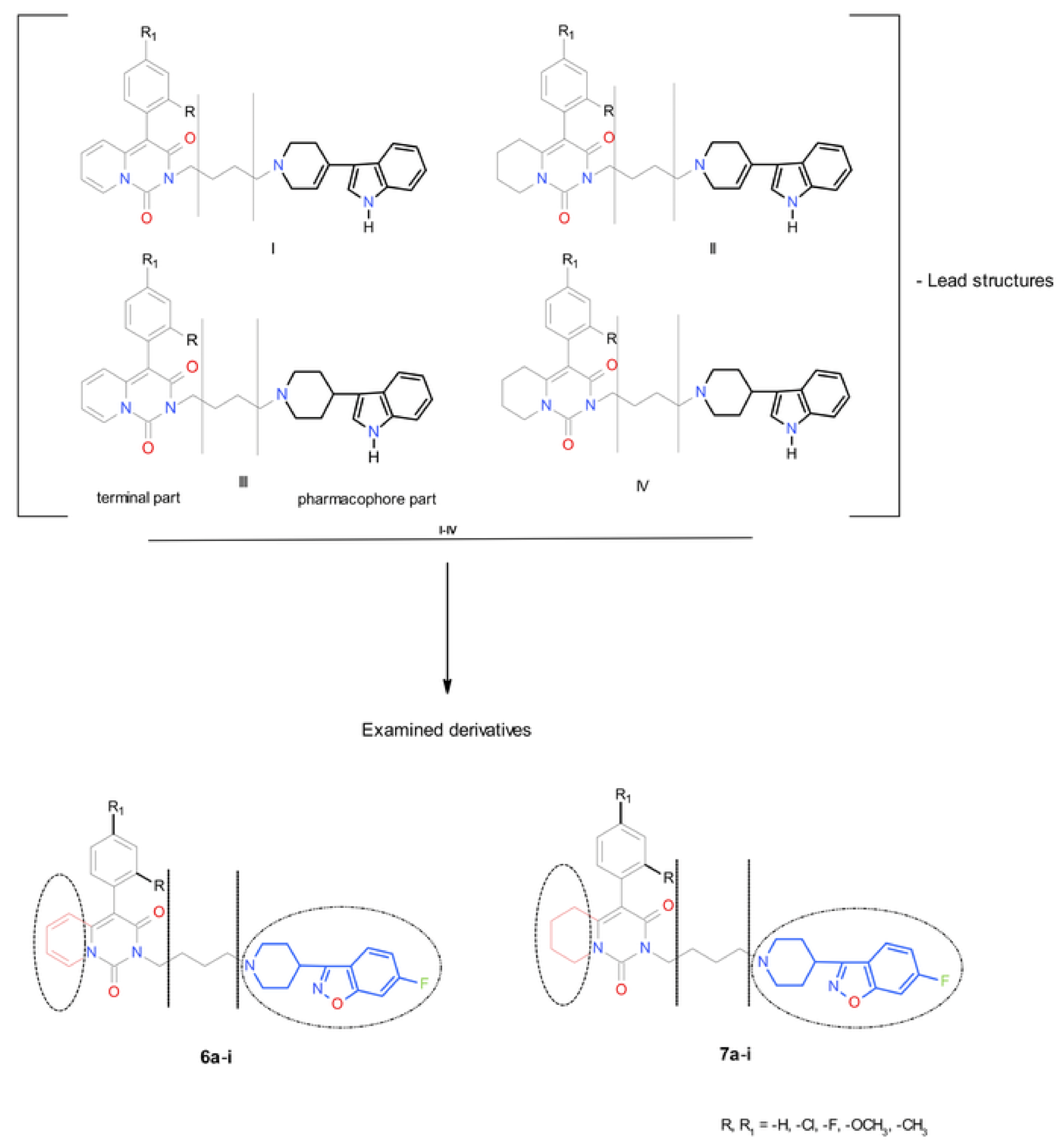
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Radioligand Binding Assay for 5-HT1A and SERT
2.2.2. In Vivo Studies
2.2.3. Metabolic Stability Evaluation
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Compounds
3.2.1. Procedure for the Synthesis of 2-(4-Bromobutyl)-4-aryl-pyrido[1,2-c]pyrimidine-1,3-diones (4a–i) and 2-(4-bromobutyl)-4-aryl-5,6,7,8-tetrahydropyrido[1,2-c]pyrimidine-1,3-diones (5a–i)
3.2.2. General Procedure for the Synthesis of Derivatives of 4-Aryl-2H-pyrido[1,2-c]pyrimidine-1,3-dione (6a–i) and 4-aryl-5,6,7,8-tetrahydropyrido[1,2-c]pyrimidine-1,3-dione (7a–i)
4-Phenyl-2-{4-[4-(6-fluoro-1,2-benzoxazol-3-yl)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6a
4-(2-Methylphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6b
4-(2-Methoxphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6c
4-(2-Chlorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6d
4-(2-Fluorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6e
4-(4-Methylphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6f
4-(4-Methoxyphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6g
4-(4-Chlorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6h
4-(4-Fluorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-pyrido[1,2-c]pyrimidine-1,3-dione 6i
4-Phenyl-2-{4-[4-(6-fluoro-1,2-benzoxazol-3-yl)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7a
4-(2-Methylphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7b
4-(2-Methoxphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7c
4-(2-Chlorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7d
4-(2-Fluorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7e
4-(4-Methylphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7f
4-(4-Methoxyphenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7g
4-(4-Chlorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7h
4-(4-Fluorophenyl)-2-{4-[4-(6-fluoro-1,2-benzoxazol)-1-piperidyl]butyl}-5,6,7,8-tetrahydro-pyrido[1,2-c]pyrimidine-1,3-dione 7i
3.3. Biological Tests
3.3.1. In Vitro Tests
5-HT1A Binding Assay
SERT Binding Assay
5-HT2A, 5-HT6, 5-HT7 and D2 Binding Assay
3.3.2. In Vivo Tests
Body Temperature in Mice
Forced Swim Test in Mice
Metabolic Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akimova, E.; Lanzenberger, R.; Kasper, S. The Serotonin-1A Receptor in Anxiety Disorders. Biol. Psychiatry 2009, 66, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Artigas, F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013, 137, 119–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breier, A. Serotonin, schizophrenia and antipsychotic drug action. Schizophr. Res. 1995, 14, 187–202. [Google Scholar] [CrossRef]
- Pacher, P.; Kohegyi, E.; Kecskemeti, V.; Furst, S. Current Trends in the Development of New Antidepressants. Curr. Med. Chem. 2001, 8, 89–100. [Google Scholar] [CrossRef]
- Doucet, M.V.; Harkin, A.; Dev, K.K. The PSD-95/nNOS complex: New drugs for depression? Pharmacol. Ther. 2012, 133, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.; Celada, P.; Martin-Ruiz, R.; Diaz-Mataix, L.; Mourelle, M.; Delgadillo, J.; Hervas, I.; Artigas, F. Modulation of serotonergic function in rat brain by VN2222, a serotonin reuptake inhibitor and 5-HT1A receptor agonist. Neuropsychopharmacology 2003, 28, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Artigas, F.; Adell, A.; Celada, P. Pindolol Augmentation of Antidepressant Response. Curr. Drug Targets 2006, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Blier, P. The pharmacology of putative early-onset antidepressant strategies. Eur. Neuropsychopharmacol. 2003, 13, 57–66. [Google Scholar] [CrossRef]
- Spinks, D.; Spinks, G. Serotonin Reuptake Inhibition: An Update on Current Research Strategies. Curr. Med. Chem. 2002, 9, 799–810. [Google Scholar] [CrossRef]
- Pacher, P.; Kecskemeti, V.; Pal Pacher, V.K. Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Curr. Med. Chem. 2004, 11, 925–943. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Ohno, Y. Therapeutic Role of 5-HT1A Receptors in The Treatment of Schizophrenia and Parkinson’s Disease. CNS Neurosci. Ther. 2011, 17, 58–65. [Google Scholar] [CrossRef]
- Newman-Tancredi, A. Biased agonism at serotonin 5-HT 1A receptors: Preferential postsynaptic activity for improved therapy of CNS disorders. Neuropsychiatry 2011, 1, 149–164. [Google Scholar] [CrossRef]
- Adell, A.; Castro, E.; Celada, P.; Bortolozzi, A.; Pazos, A.; Artigas, F. Strategies for producing faster acting antidepressants. Drug Discov. Today 2005, 10, 578–585. [Google Scholar] [CrossRef]
- Carr, G.V.; Lucki, I. The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology 2011, 213, 265–287. [Google Scholar] [CrossRef] [Green Version]
- Lacivita, E.; Leopoldo, M.; Berardi, F.; Perrone, R. 5-HT1A receptor, an old target for new therapeutic agents. Curr. Top. Med. Chem. 2008, 8, 1024–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artigas, F.; Bramwell, S.R.; Grahame-Smith, D.G.; Probst, A.; Palacios, J.M. 5-HT and antidepressants: New views from microdialysis studies. Trends Pharmacol. Sci. 1993, 14, 262. [Google Scholar] [CrossRef]
- Blier, P.; Ward, N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry 2003, 53, 193–203. [Google Scholar] [CrossRef]
- Blier, P.; De Montigny, C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: Electrophysiological studies in the rat brain. Synapse 1987, 1, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Rickels, K.; Athanasiou, M.; Reed, C. Vilazodone, a novel, dual-acting antidepressant: Current status, future promise and potential for individualized treatment of depression. Per. Med. 2009, 6, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Howland, R.H. Vilazodone: Another novel atypical antidepressant drug. J. Psychosoc. Nurs. Ment. Health Serv. 2011, 49, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Vilazodone: In major depressive disorder. CNS Drugs 2011, 25, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Bang-Andersen, B.; Ruhland, T.; Jørgensen, M.; Smith, G.; Frederiksen, K.; Jensen, K.G.; Zhong, H.; Nielsen, S.M.; Hogg, S.; Mørk, A.; et al. Discovery of 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): A Novel Multimodal Compound for the Treatment of Major Depressive Disorder. J. Med. Chem. 2011, 54, 3206–3221. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Chodkowski, A.; Izbicki, Ł.; Król, M.; Kleps, J.; Turło, J.; Nowak, G.; Stachowicz, K.; Dybała, M.; Siwek, A. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity, Part 1. Eur. J. Med. Chem. 2009, 44, 1710–1717. [Google Scholar] [CrossRef]
- Herold, F.; Izbicki, Ł.; Chodkowski, A.; Dawidowski, M.; Król, M.; Kleps, J.; Turło, J.; Wolska, I.; Nowak, G.; Stachowicz, K. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity: Part 2. Eur. J. Med. Chem. 2009, 44, 4702–4715. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Chodkowski, A.; Izbicki, Ł.; Turło, J.; Dawidowski, M.; Kleps, J.; Nowak, G.; Stachowicz, K.; Dybała, M.; Siwek, A.; et al. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity. Part 3. Eur. J. Med. Chem. 2011, 46, 142–149. [Google Scholar] [CrossRef]
- Chodkowski, A.; Wróbel, M.Z.; Turło, J.; Kleps, J.; Siwek, A.; Nowak, G.; Belka, M.; Bączek, T.; Mazurek, A.P.; Herold, F. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity. Part 4. Eur. J. Med. Chem. 2015, 90, 21–32. [Google Scholar] [CrossRef]
- Gomolka, A.; Ciesielska, A.; Wróbel, M.Z.; Chodkowski, A.; Kleps, J.; Dawidowski, M.; Siwek, A.; Wolak, M.; Stachowicz, K.; Slawinska, A.; et al. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity. Part 5. Eur. J. Med. Chem. 2015, 98, 221–236. [Google Scholar] [CrossRef]
- Wróbel, M.Z.; Chodkowski, A.; Herold, F.; Gomółka, A.; Kleps, J.; Mazurek, A.P.; Pluciński, F.; Mazurek, A.; Nowak, G.; Siwek, A.; et al. Synthesis and biological evaluation of novel pyrrolidine-2,5-dione derivatives as potential antidepressant agents. Part 1. Eur. J. Med. Chem. 2013, 63, 484–500. [Google Scholar] [CrossRef]
- Martin, K.F.; Heal, D.J. 8-OH-DPAT-Induced Hypothermia in Rodents. A Specific Model of 5-HTlA Autoreceptor Function. In Serotonin: Molecular Biology, Receptors and Functional Effects; Birkhäuser Basel: Basel, Switzerland, 1991; pp. 483–490. ISBN 9783034872614. [Google Scholar]
- ACD/ChemSketch 2017; Version 2017.1.2; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2017.
- Di Giovanni, G.; Esposito, E.; Di Matteo, V. Role of Serotonin in Central Dopamine Dysfunction. CNS Neurosci. Ther. 2010, 16, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Etievant, A.; Bétry, C.; Haddjeri, N. Partial Dopamine D2/Serotonin 5-HT1A Receptor Agonists as New Therapeutic Agents. Open Neuropsychopharmacol. J. 2010, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sakaue, M.; Somboonthum, P.; Nishihara, B.; Koyama, Y.; Hashimoto, H.; Baba, A.; Matsuda, T. Postsynaptic 5-hydroxytryptamine 1A receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br. J. Pharmacol. 2000, 129, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Fountoulakis, K.N.; Kelsoe, J.R.; Akiskal, H. Receptor targets for antidepressant therapy in bipolar disorder: An overview. J. Affect. Disord. 2012, 138, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol. Ther. 2006, 110, 135–370. [Google Scholar] [CrossRef]
- Ślifirski, G.; Król, M.; Kleps, J.; Podsadni, P.; Belka, M.; Bączek, T.; Siwek, A.; Stachowicz, K.; Szewczyk, B.; Nowak, G.; et al. Synthesis of new 5,6,7,8-tetrahydropyrido[1,2-c]pyrimidine derivatives with rigidized tryptamine moiety as potential SSRI and 5-HT1A receptor ligands. Eur. J. Med. Chem. 2019, 180, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Chang, Y.; Pi, W.; Ang, W.; Liu, Y.; Li, C.; Zheng, J.; Xiong, L.; Yang, T.; Luo, Y. Synthesis and evaluation of amide side-chain modified Agomelatine analogues as potential antidepressant-like agents. Bioorg. Med. Chem. Lett. 2014, 24, 1672–1676. [Google Scholar] [CrossRef]
- Goodwin, G.M.; De Souza, R.J.; Green, A.R. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(DI-n-propylamino)tetralin (8-OH-DPAT). Neuropharmacology 1985, 24, 1187–1194. [Google Scholar] [CrossRef]
- Forster, E.A.; Cliffe, I.A.; Bill, D.J.; Dover, G.M.; Jones, D.; Reilly, Y.; Fletcher, A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur. J. Pharmacol. 1995, 281, 81–88. [Google Scholar] [CrossRef]
- Yap, C.; Xue, Y.; Chen, Y. Application of Support Vector Machines to In Silico Prediction of Cytochrome P450 Enzyme Substrates and Inhibitors. Curr. Top. Med. Chem. 2006, 6, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Ulenberg, S.; Belka, M.; Georgiev, P.; Król, M.; Herold, F.; Bączek, T. In Vitro approach for identification of a leading cytochrome P450 isoenzyme responsible for biotransformation of novel arylpiperazine drug candidates and their inhibition potency towards CYP3A4. Acta Pol. Pharm. Drug Res. 2020, 77, 69–76. [Google Scholar] [CrossRef]
- Ślifirski, G.; Król, M.; Kleps, J.; Ulenberg, S.; Belka, M.; Bączek, T.; Siwek, A.; Stachowicz, K.; Szewczyk, B.; Nowak, G.; et al. Synthesis of novel pyrido[1,2-c]pyrimidine derivatives with rigidized tryptamine moiety as potential SSRI and 5-HT1A receptor ligands. Eur. J. Med. Chem. 2019, 166, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Kos, T.; Marciniec, K.; Satała, G.; Canale, V.; Kamiński, K.; Hołuj, M.; Lenda, T.; Koralewski, R.; Bednarski, M.; et al. Novel multi-target azinesulfonamides of cyclic amine derivatives as potential antipsychotics with pro-social and pro-cognitive effects. Eur. J. Med. Chem. 2018, 145, 790–804. [Google Scholar] [CrossRef]
- Yung-Chi, C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]



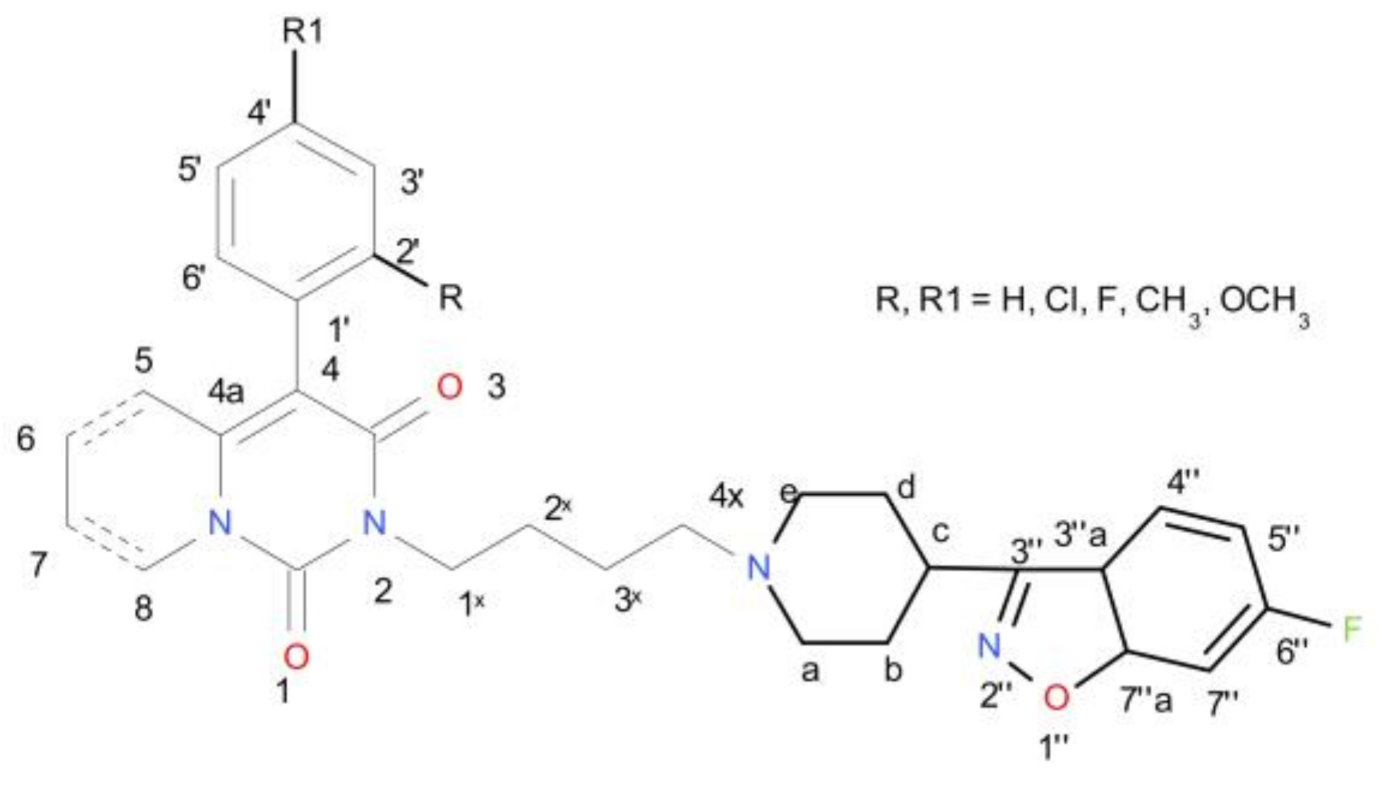
| Ki [nM] | |||||
|---|---|---|---|---|---|
| Compound | R | R1 | 5-HT1A | SERT | cLogP |
| 6a | –H | –H | 23.0 ± 1.0 | 32.0 ± 3.6 | 4.32 |
| 6b | –CH3 | –H | 30.0 ± 3.5 | >5000 | 4.81 |
| 6c | –OCH3 | –H | 7.0 ± 1.0 | >5000 | 4.30 |
| 6d | –Cl | –H | 11.0 ± 1.4 | 373.0 ± 32.0 | 4.98 |
| 6e | –F | –H | 15.0 ± 0.6 | >1000 | 4.52 |
| 6f | –H | –CH3 | 74.0 ± 4.0 | 772.0 ± 36.0 | 4.81 |
| 6g | –H | –OCH3 | 10.0 ± 1.1 | 730.0 ± 79.0 | 4.98 |
| 6h | –H | –Cl | 21.0 ± 1.5 | 310.0 ± 1.0 | 4.98 |
| 6i | –H | –F | 17.0 ± 2.0 | 342.0 ± 28.8 | 4.55 |
| 7a | –H | –H | 27.0 ± 2.0 | 520.0 ± 58.8 | 5.02 |
| 7b | –CH3 | –H | 35.0 ± 3.5 | >1000 | 5.51 |
| 7c | –OCH3 | –H | 62.0 ± 6.0 | 878.0 ± 88.0 | 5.00 |
| 7d | –Cl | –H | 71.0 ± 6.9 | 310.0 ± 34.5 | 5.68 |
| 7e | –F | –H | 36.0 ± 4.1 | >1000 | 5.22 |
| 7f | –H | –CH3 | 52.0 ± 2.5 | 1773.0 ± 180.0 | 5.90 |
| 7g | –H | –OCH3 | 5.0 ± 0.5 | 48.0 ± 2.4 | 5.00 |
| 7h | –H | –Cl | 25.0 ± 3.0 | 290.0 ± 22.7 | 5.68 |
| 7i | –H | –F | 9.5 ± 1.1 | 311.0 ± 35.0 | 5.22 |
| Reference compound | |||||
| Serotonin | 3.6 ± 0.4 | ||||
| Methiotepin | 4.8 ± 0.5 | ||||
| Imipramine | 17.0 ± 1.3 | ||||
| Ki [nM] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | 5-HT1A | SERT | 5-HT2A | 5-HT6 | 5-HT7 | D2 |
| 6a | –H | –H | 23.0 ± 1.0 | 32.0 ± 3.6 | 17 ± 3 | 376 ± 58 | 62 ± 5 | 7 ± 2 |
| 6d | –Cl | –H | 11.0 ± 1.4 | 373.0 ± 32.0 | 20 ± 4 | 709 ± 135 | 109 ± 14 | 9 ± 2 |
| 7g | –H | –OCH3 | 5.0 ± 0.5 | 48.0 ± 2.4 | 16 ± 2 | 400 ± 32 | 94 ± 11 | 10 ± 1 |
| 7i | –H | –F | 9.5 ± 1.1 | 311.0 ± 35.0 | 44 ± 6 | 740 ± 183 | 161 ± 27 | 17 ±2 |
| Reference Compound | ||||||||
| Olanzapine [37] | 4.6 ± 0.9 | 7 ± 1 | n.d. | n.d. | ||||
| Mianserin [37] | 2.8 ± 0.5 | n.d. | n.d. | n.d. | ||||
| Clozapine [37] | n.d. | n.d. | 18 ± 2 | n.d. | ||||
| Haloperidol [37] | n.d. | n.d. | n.d. | 4.5 ± 0.7 | ||||
| Apomorphine [37] | n.d. | n.d. | n.d. | 42 ± 6 | ||||
| Chlorpromazine [37] | n.d. | n.d. | n.d. | 1.8 ± 0.3 | ||||
| Treatment | Dose (mg/kg) | Δt ± SEM (°C) | |||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | ||
| Vehicle | - | −0.2 ± 0.2 | −0.0 ± 0.2 | −0.1 ± 0.1 | −0.0 ± 0.1 |
| 6a | 5 | −2.0 ± 0.2 b | −3.2 ± 0.5 b | −3.1 ± 0.5 b | −2.8 ± 0.5 b |
| 2.5 | −1.6 ± 0.2 b | −2.5 ± 0.3 b | −2.4 ± 0.3 b | −2.0 ± 0.2 b | |
| 1.25 | −1.7 ± 0.3 b | −1.7 ± 0.3 b | −1.6 ± 0.2 b | −1.6 ± 0.3 b | |
| 0.6 | −1.0 ± 0.2 b | −1.2 ± 0.1 b | −0.9 ± 0.1 a | −0.8 ± 0.2 a | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||
| Vehicle | - | −0.0 ± 0.2 | 0.0 ± 0.1 | −0.1 ± 0.1 | −0.1 ± 0.1 |
| 7g | 5 | −2.1 ± 0.1 b | −3.4 ± 0.3 b | −3.9 ± 0.5 b | −4.2 ± 0.8 b |
| 2.5 | −2.2 ± 0.2 b | −2.8 ± 0.3 b | −2.9 ± 0.3 b | −3.0 ± 0.4 b | |
| 1.25 | −1.5 ± 0.3 b | −1.7 ± 0.3 b | −1.3 ± 0.4 b | −1.0 ± 0.3 | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||
| Vehicle | - | 0.2 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 |
| WAY-100635 | 0.1 | 0.1 ± 0.2 | 0.1 ± 0.2 | −0.1 ± 0.2 | −0.2 ± 0.1 |
| 8-OH-DPAT | 5 | −1.7 ± 0.2 b | −1.1 ± 0.2 b | −0.1 ± 0.1 | 0.3 ± 0.3 |
| p < 0.0001 | p < 0.005 | ns | ns | ||
| Treatment and Dose (mg/kg) | Δt ± SEM (°C) | |
|---|---|---|
| 30 min | 60 min | |
| Vehicle | 0.0 ± 0.1 | 0.0 ± 0.0 |
| Vehicle + 6a (0.6) | −1.0 ± 0.2 c | −1.2 ± 0.1 c |
| WAY-100635 + 6a | −0.5 ± 0.2 b,d | −0.4 ± 0.2 a,e |
| p < 0.0001 | p < 0.0001 | |
| Vehicle | −0.3 ± 0.1 | −0.2 ± 0.1 |
| Vehicle + 7g (1.25) | −1.5 ± 0.3 c | −1.7 ± 0.3 c |
| WAY-100635 + 7g | −1.2 ± 0.2 b | −0.7 ± 0.3 d |
| p < 0.0001 | p < 0.0005 | |
| Vehicle | 0.1 ± 0.1 | −0.0 ± 0.1 |
| WAY-100635 | 0.3 ± 0.3 | 0.2 ± 0.3 |
| ns | ns | |
| Compound | Average t1/2 [min] (n = 2) | SD [min] | RSD% |
|---|---|---|---|
| 6a | 3.61 | 0.43 | 11.18 |
| 6d | 3.20 | 0.09 | 2.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, M.; Ślifirski, G.; Kleps, J.; Ulenberg, S.; Belka, M.; Bączek, T.; Siwek, A.; Stachowicz, K.; Szewczyk, B.; Nowak, G.; et al. Synthesis of Novel Pyrido[1,2-c]pyrimidine Derivatives with 6-Fluoro-3-(4-piperidynyl)-1,2-benzisoxazole Moiety as Potential SSRI and 5-HT1A Receptor Ligands. Int. J. Mol. Sci. 2021, 22, 2329. https://doi.org/10.3390/ijms22052329
Król M, Ślifirski G, Kleps J, Ulenberg S, Belka M, Bączek T, Siwek A, Stachowicz K, Szewczyk B, Nowak G, et al. Synthesis of Novel Pyrido[1,2-c]pyrimidine Derivatives with 6-Fluoro-3-(4-piperidynyl)-1,2-benzisoxazole Moiety as Potential SSRI and 5-HT1A Receptor Ligands. International Journal of Molecular Sciences. 2021; 22(5):2329. https://doi.org/10.3390/ijms22052329
Chicago/Turabian StyleKról, Marek, Grzegorz Ślifirski, Jerzy Kleps, Szymon Ulenberg, Mariusz Belka, Tomasz Bączek, Agata Siwek, Katarzyna Stachowicz, Bernadeta Szewczyk, Gabriel Nowak, and et al. 2021. "Synthesis of Novel Pyrido[1,2-c]pyrimidine Derivatives with 6-Fluoro-3-(4-piperidynyl)-1,2-benzisoxazole Moiety as Potential SSRI and 5-HT1A Receptor Ligands" International Journal of Molecular Sciences 22, no. 5: 2329. https://doi.org/10.3390/ijms22052329