The Effect of Fusarium verticillioides Fumonisins on Fatty Acids, Sphingolipids, and Oxylipins in Maize Germlings
Abstract
:1. Introduction
2. Results
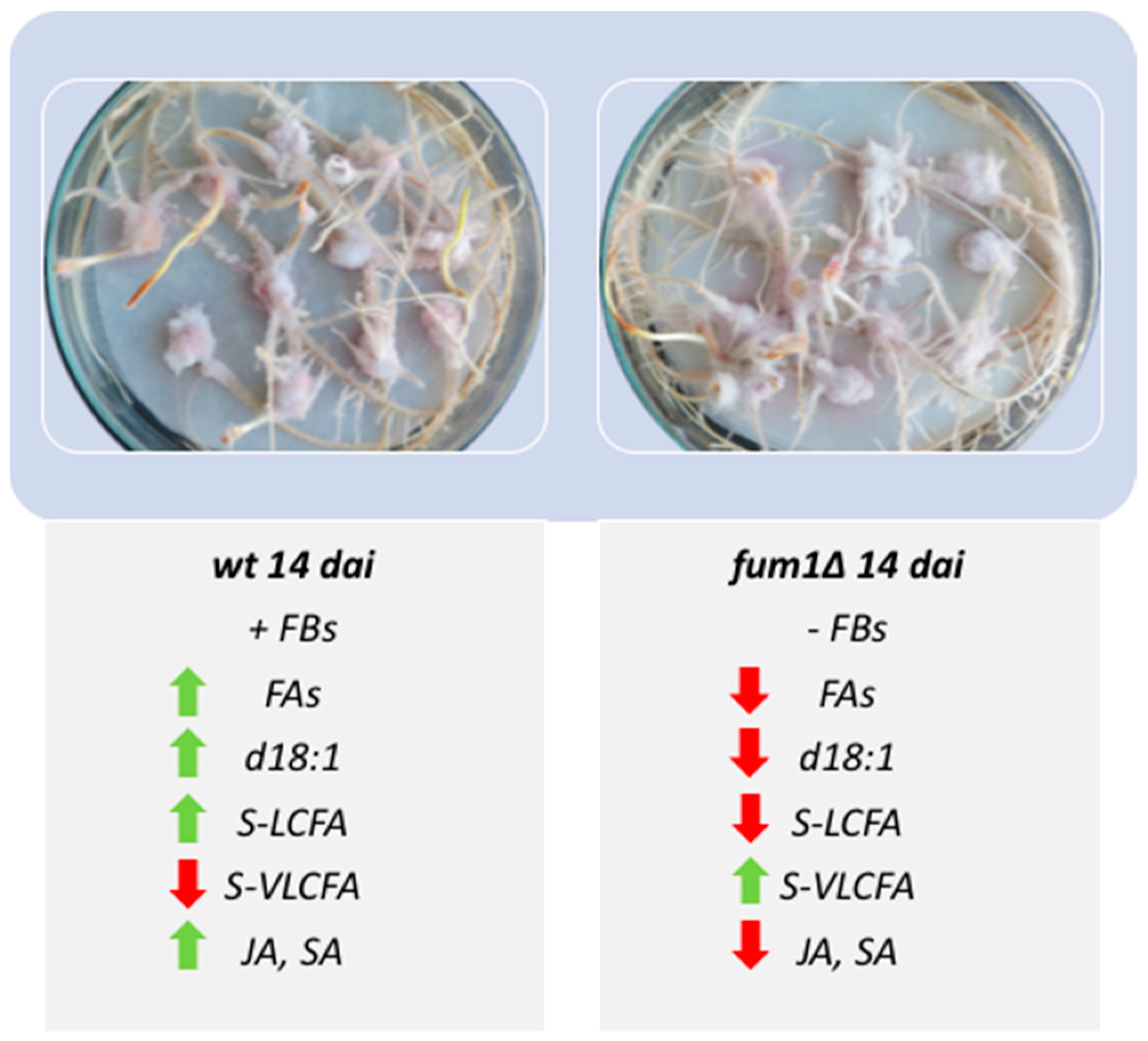
2.1. Infection of Maize Kernel with F. verticillioides Wild-Type and Fumonisin Non-Producing Mutant fum1∆
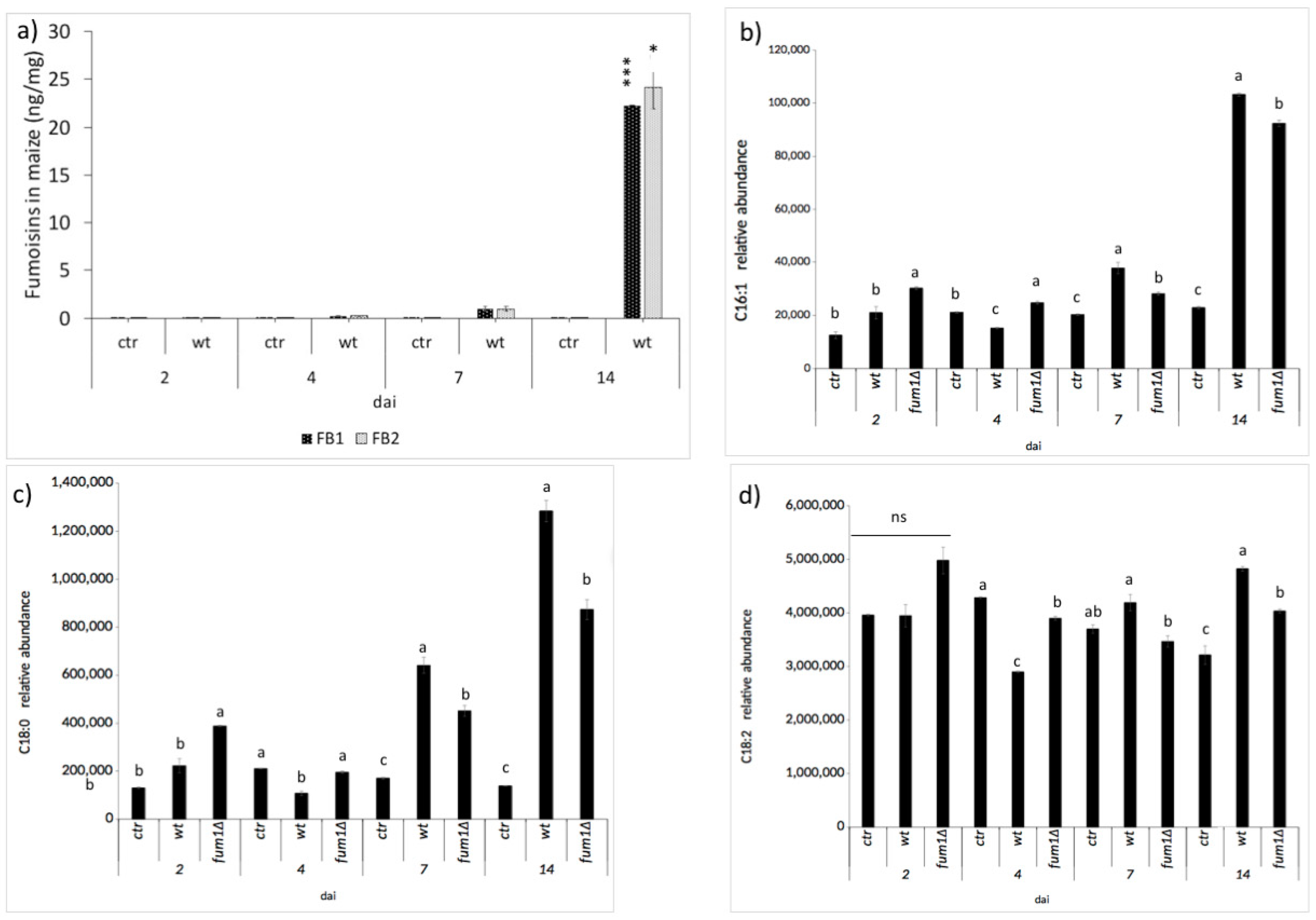
2.2. Fumonisin Contamination and Lipid Compounds
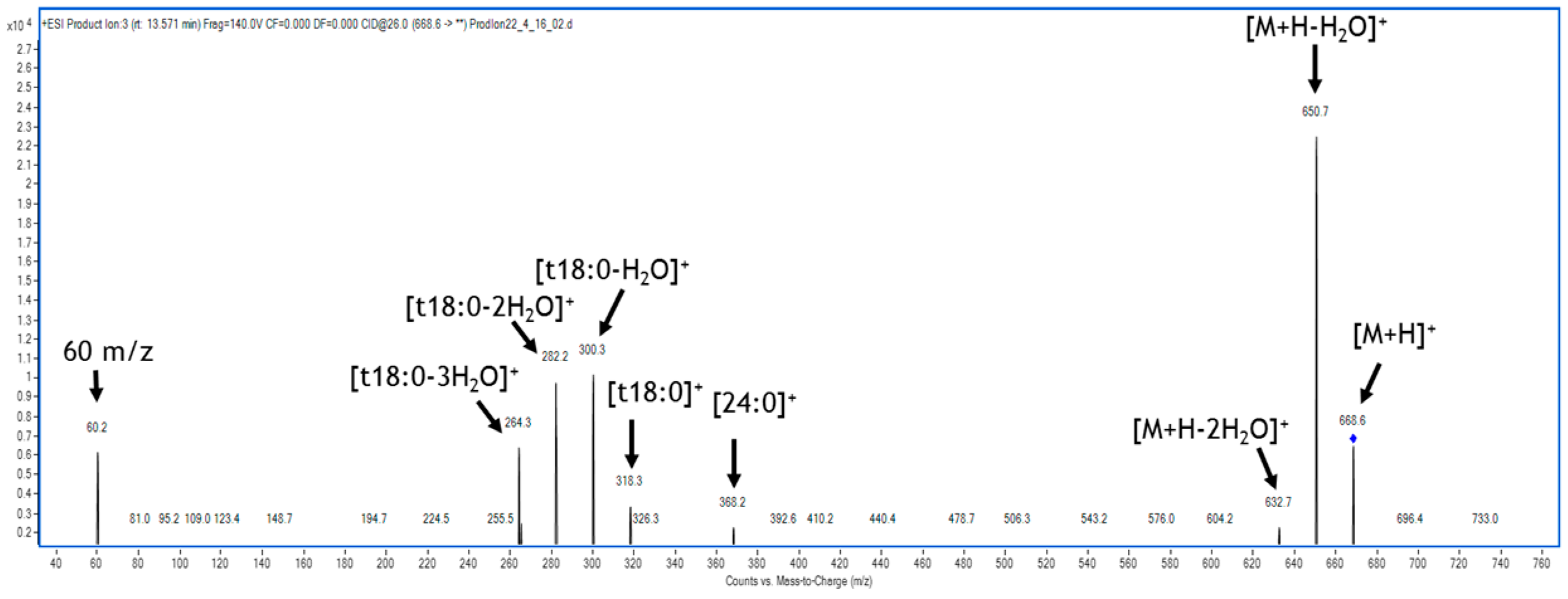
2.3. Maize and F. verticillioides Sphingolipidome Characterization
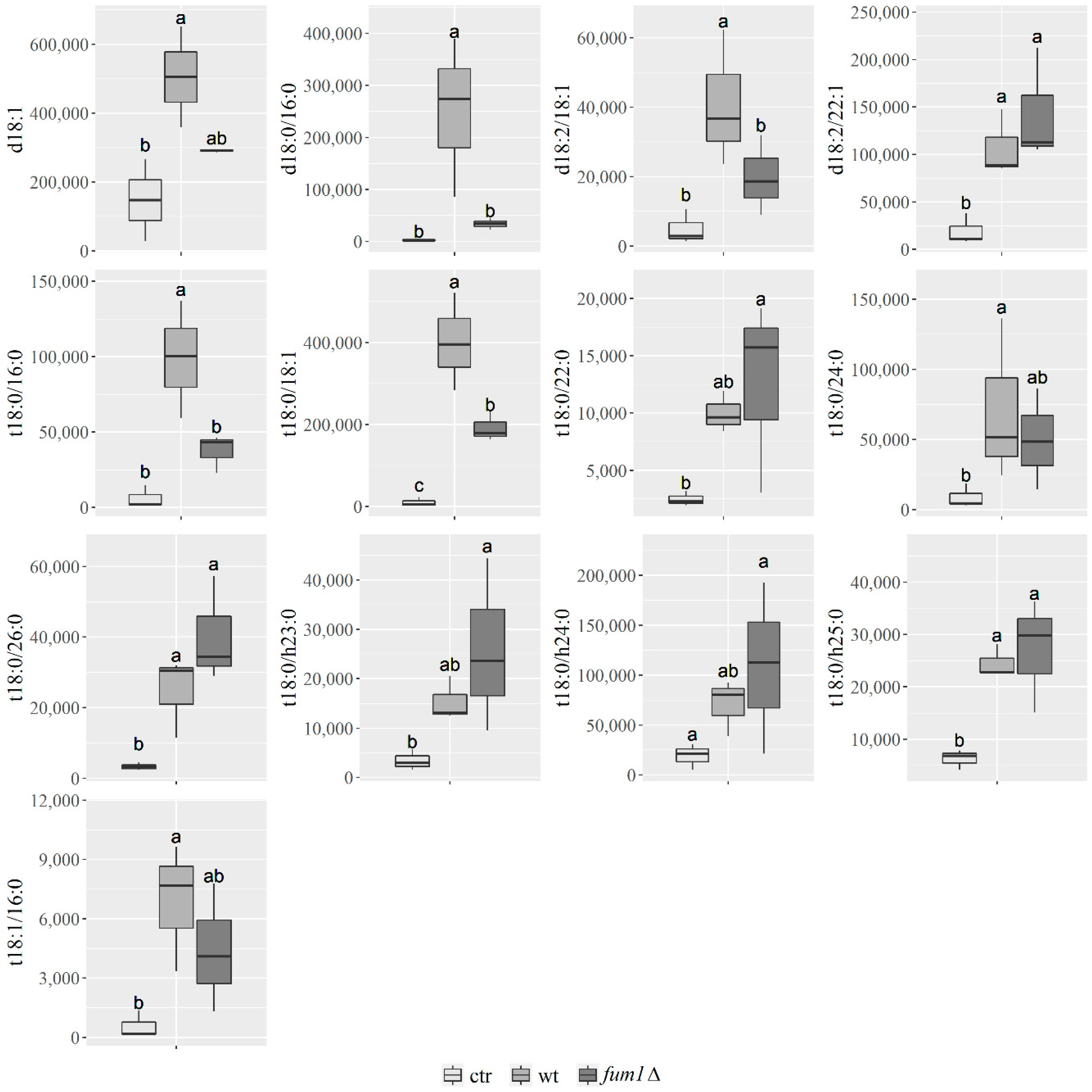
2.4. Sphingolipidome Characterization in Maize Kernels Infected with F. verticillioides Wild-Type and Fumonisin Non-Producing (fum1∆) Mutant Strain
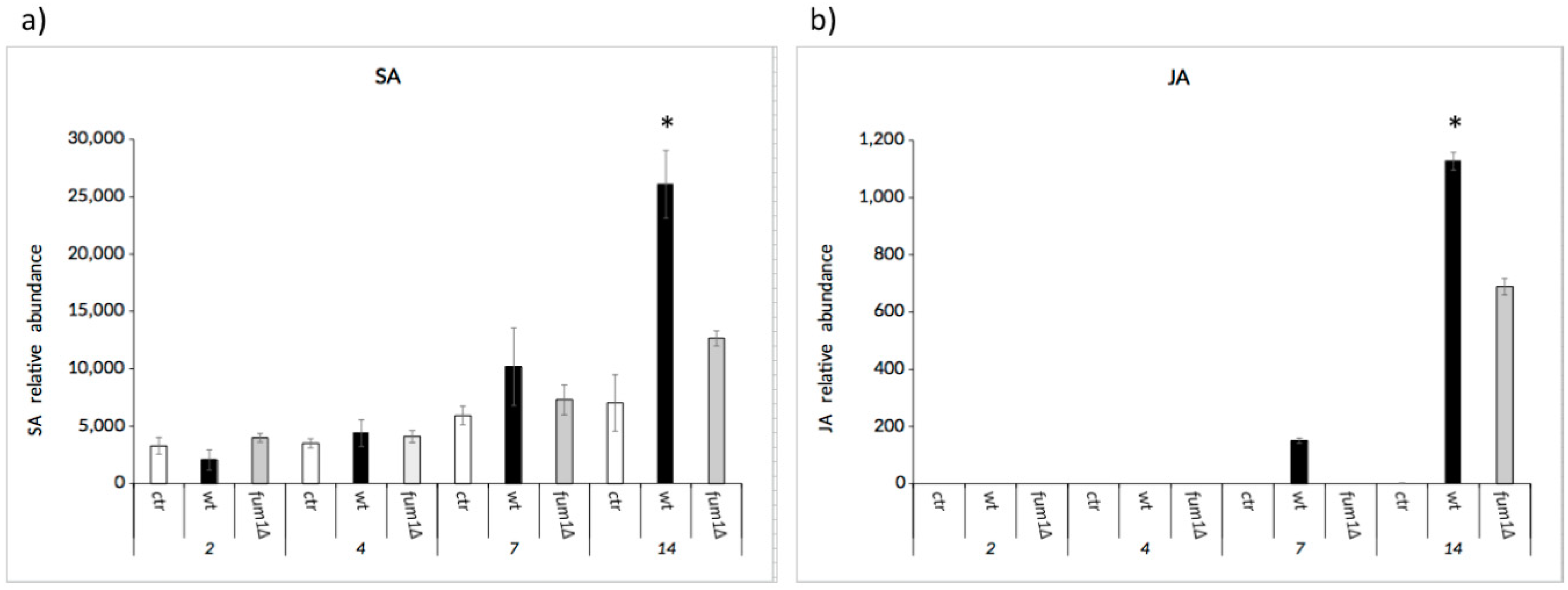
2.5. The Balance between the Salicylic and Jasmonic Acid
2.6. Exposure of Maize Protoplasts to FB1
3. Discussion
4. Materials and Methods
4.1. Fusarium verticillioides Strains and Culture Conditions
4.2. Kernel Infection Assay
4.3. HPLC–MS/MS Analysis
4.3.1. Sphingolipids, Oxylipin, and Fatty Acid Extraction and Analysis
4.3.2. Fumonisin Extraction and Analysis
4.3.3. SA and JA Extraction and Analysis
4.4. Nucleic Acid Manipulation: DNA for qPCR Analysis
4.5. Vybrant Assay for PCD Detection
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CerS | Ceramide Synthase |
| CH3COOH | Acetic Acid |
| DAI | Day after inoculation |
| DOAJ | Directory of open access journals |
| FA | Fatty Acid |
| FB | Fumonisin |
| Fum1∆ | FB non-producing mutant |
| JA | Jasmonic Acid |
| LCB | Long Chain Base |
| LCFA | Long Chain Fatty Acid |
| LOH1 | Ceramide Synthase 1 |
| LOH2 | Ceramide Synthase 2 |
| LOH3 | Ceramide Synthase 3 |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MRM | Multiple Reaction Monitoring |
| NAA | 1-naphthaleneacetic acid |
| PCD | Plant Cell Death |
| PUFA | Polyunsaturated Fatty Acid |
| SA | Salicylic Acid |
| SIM | Single Ion Monitoring |
| VLCFA | Very Long Chain Fatty Acid |
References
- Divon, H.H.; Fluhr, R. Nutrition acquisition strategies during fungal infection of plants. FEMS Microbiol. Lett. 2007, 266, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Oliver, R.P.; Ipcho, S.V.S. Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol. Plant Pathol. 2004, 5, 347–352. [Google Scholar] [CrossRef]
- Maiorano, A.; Reyneri, A.; Sacco, D.; Magni, A.; Ramponi, C. A dynamic risk assessment model (FUMAgrain) of fumonisin synthesis by Fusarium verticillioides in maize grain in Italy. Crop Prot. 2009, 28, 243–256. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0470276460. [Google Scholar]
- Rai, M.; Agarkar, G. Plant–fungal interactions: What triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016, 42, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [CrossRef]
- Abbas, H.K.; Tanaka, T.; Duke, S.O.; Porter, J.K.; Wray, E.M.; Hodges, L.; Sessions, A.E.; Wang, E.; Merrill, A.H., Jr.; Riley, R.T. Fumonisin-and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol. 1994, 106, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, D.G.; Bostock, R.M.; Wang, H. Sphingosine-related mycotoxins in plant and animal diseases. Can. J. Bot. 1995, 73 (Suppl. 1), 459–467. [Google Scholar] [CrossRef]
- Yoo, H.S.; Norred, W.P.; Showker, J.; Riley, R.T. Elevated sphingoid bases and complex sphingolipid depletion as contributing factors in fumonisin-induced cytotoxicity. Toxicol. Appl. Pharmacol. 1996, 138, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Plattner, R.D. Fumonisin B1-nonproducing strains of Fusarium verticillioides cause maize (Zea mays) ear infection and ear rot. J. Agric. Food Chem. 2000, 48, 5773–5780. [Google Scholar] [CrossRef]
- Williams, L.D.; Glenn, A.E.; Zimeri, A.M.; Bacon, C.W.; Smith, M.A.; Riley, R.T. Fumonisin disruption of ceramide biosynthesis in maize roots and the effects on plant development and Fusarium verticillioides-induced seedling disease. J. Agric. Food Chem. 2007, 55, 2937–2946. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Van Hove, F.; Waalwijk, C.; Logrieco, A.; Munaut, F.; Moretti, A. Gibberella musae (Fusarium musae) sp. nov., a recently discovered species from banana is sister to F. verticillioides. Mycologia 2011, 103, 570–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.; Hou, Y.; Wang, L.; Huang, S. Effects of disruption of five FUM Genes on Fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef] [Green Version]
- Futerman, A.H. Inhibition of sphingolipid synthesis: Effects on glycosphingolipid- GPI-anchored protein microdomains. Trends Cell Biol. 1995, 5, 377–380. [Google Scholar] [CrossRef]
- Spiegel, S.; Merrill, A.H. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996, 10, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Beccaccioli, M.; Reverberi, M.; Scala, V. Fungal lipids: Biosynthesis and signalling during plant-pathogen interaction. Front. Biosci.-Landmark 2019, 24, 172–185. [Google Scholar]
- Merrill, A.H., Jr.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef]
- Wolpert, T.J.; Dunkle, L.D.; Ciuffetti, L.M. Host-selective toxins and avirulence determinants: What’s in a name? Annu. Rev. Phytopathol. 2002, 40, 251–285. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Lohmar, J.M.; Busman, M.; Brown, D.W.; Naumann, T.A.; Divon, H.H.; Uhlig, S.; Proctor, R.H. Identification and distribution of gene clusters required for synthesis of sphingolipid metabolism inhibitors in diverse species of the filamentous fungus Fusarium. BMC Genomics 2020, 21, 1–24. [Google Scholar]
- Shi, L.; Bielawski, J.; Mu, J.; Dong, H.; Teng, C.; Zhang, J.; Yang, X.; Tomishige, N.; Hanada, K.; Hannun, Y.A.; et al. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res. 2007, 17, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Stone, J.M.; Heard, J.E.; Kovtun, Y.; Yorgey, P.; Sheen, J.; Ausubel, F.M. Fumonisin B1–induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 2000, 12, 1823–1835. [Google Scholar] [PubMed] [Green Version]
- Nishimura, M.T.; Stein, M.; Hou, B.H.; Vogel, J.P.; Edwards, H.; Somerville, S.C. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 2003, 301, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, D.; Sánchez-Nieto, S.; Plasencia, J. Fumonisin B1, a toxin produced by Fusarium verticillioides, modulates maize β-1, 3-glucanase activities involved in defense response. Planta 2012, 235, 965–978. [Google Scholar] [CrossRef]
- Liang, H.; Yao, N.; Song, J.T.; Luo, S.; Lu, H.; Greenberg, J.T. Ceramides modulate programmed cell death in plants. Genes Dev. 2003, 17, 2636–2641. [Google Scholar] [CrossRef] [Green Version]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and plant defense/disease: The “death” connection and beyond. Front. Plant Sci. 2012, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Markham, J.E.; Molino, D.; Gissot, L.; Bellec, Y.; Hématy, K.; Marion, J.; Belcram, K.; Palauqui, J.-C.; Satiat-JeuneMaître, B.; Faure, J.-D. Sphingolipids containing very-long-chain fatty acids define a secretory pathway for specific polar plasma membrane protein targeting in Arabidopsis. Plant Cell 2011, 23, 2362–2378. [Google Scholar] [CrossRef] [Green Version]
- De Zutter, N.; Audenaert, K.; Ameye, M.; De Boevre, M.; De Saeger, S.; Haesaert, G.; Smagghe, G. The plant response induced in wheat ears by a combined attack of Sitobion avenae aphids and Fusarium graminearum boosts fungal infection and deoxynivalenol production. Mol. Plant Pathol. 2017, 18, 98–109. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Liu, L.; Sonbol, F.-M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Walley, J.W.; Kliebenstein, D.J.; Bostock, R.M.; Dehesh, K. Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 2013, 16, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trépanier, M.; Bécard, G.; Moutoglis, P.; Willemot, C.; Gagné, S.; Avis, T.J.; Rioux, J.-A. Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl. Environ. Microbiol. 2005, 71, 5341–5347. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Chin, C.-K. Modification of fatty acids in eggplant affects its resistance to Verticillium dahliae. Physiol. Mol. Plant Pathol. 2000, 56, 217–225. [Google Scholar] [CrossRef]
- Kachroo, A.; Shanklin, J.; Whittle, E.; Lapchyk, L.; Hildebrand, D.; Kachroo, P. The Arabidopsis stearoyl-acyl carrier protein-desaturase family and the contribution of leaf isoforms to oleic acid synthesis. Plant Mol. Biol. 2007, 63, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, E.; Höppner, F.; Ellner, F.; Weinert, J. Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res. 2017, 33, 167–182. [Google Scholar] [CrossRef]
- Scala, V.; Giorni, P.; Cirlini, M.; Ludovici, M.; Visentin, I.; Cardinale, F.; Fabbri, A.A.; Fanelli, C.; Reverberi, M.; Battilani, P.; et al. LDS1-produced oxylipins are negative regulators of growth, conidiation and fumonisin synthesis in the fungal maize pathogen Fusarium verticillioides. Front. Microbiol. 2014, 5, 669. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Battilani, P. Role of maize hybrids and their chemical composition in Fusarium infection and fumonisin production. J. Agric. Food Chem. 2012, 60, 3800–3808. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Kolomiets, M.V. The lipid language of plant-fungal interactions. Fungal Genet. Biol. 2011, 48, 4–14. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Giorni, P.; Cirlini, M.; Reverberi, M.; Gregori, R.; Ludovici, M.; Camera, E.; Fanelli, C.; Battilani, P.; Scala, V. Maize lipids play a pivotal role in the fumonisin accumulation. World Mycotoxin J. 2015, 8, 87–97. [Google Scholar] [CrossRef]
- Gao, X.; Kolomiets, M. V Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 2009, 28, 79–88. [Google Scholar] [CrossRef]
- Cronjé, M.J.; Weir, I.E.; Bornman, L. Salicylic acid-mediated potentiation of Hsp70 induction correlates with reduced apoptosis in tobacco protoplasts. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 61, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Jardine, D.J.; Leslie, J.F. Aggressiveness to mature maize plants of Fusarium strains differing in ability to produce fumonisin. Plant Dis. 1999, 83, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, A.E.; Munkvold, G.P.; Plattner, R.D.; Proctor, R.H. FUM1—A gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Mol. Plant Microbe Interact. 2002, 15, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Ludovici, M.; Ialongo, C.; Reverberi, M.; Beccaccioli, M.; Scarpari, M.; Scala, V. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis of Fusarium verticillioides and maize kernels. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.L.; Theumer, M.G.; Mary, V.S.; Rubinstein, H.R. Fumonisins: Probable role as effectors in the complex interaction of susceptible and resistant maize hybrids and Fusarium verticillioides. J. Agric. Food Chem. 2012, 60, 5667–5675. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.; Brands, M.; Wewer, V.; Duan, Y.; Hölzl, G.; Dörmann, P. Lipids in plant–microbe interactions. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 1379–1395. [Google Scholar] [CrossRef]
- Zitomer, N.C.; Glenn, A.E.; Bacon, C.W.; Riley, R.T. A single extraction method for the analysis by liquid chromatography/tandem mass spectrometry of fumonisins and biomarkers of disrupted sphingolipid metabolism in tissues of maize seedlings. Anal. Bioanal. Chem. 2008, 391, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, L.V.; Napier, J.A.; Molino, D.; Faure, J.D. Plant sphingolipids: Their importance in cellular organization and adaption. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 1329–1335. [Google Scholar] [CrossRef]
- Stagnati, L.; Rahjoo, V.; Samayoa, L.F.; Holland, J.B.; Borrelli, V.M.G.; Busconi, M.; Lanubile, A.; Marocco, A. A Genome-wide association study to understand the effect of Fusarium verticillioides infection on seedlings of a maize diversity panel. G3 Genes, Genomes, Genet. 2020, 10, 1685–1696. [Google Scholar] [CrossRef] [Green Version]
- Stagnati, L.; Lanubile, A.; Samayoa, L.F.; Bragalanti, M.; Giorni, P.; Busconi, M.; Holland, J.B.; Marocco, A. A genome wide association study reveals markers and genes associated with resistance to Fusarium verticillioides infection of seedlings in a maize diversity panel. G3 Genes, Genomes, Genet. 2019, 9, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, D.; Du Fall, L.A.; Livk, A.; Mathesius, U.; Lipscombe, R.J.; Oliver, R.P.; Friesen, T.L.; Solomon, P.S. A functional genomics approach to dissect the mode of action of the Stagonospora nodorum effector protein SnToxA in wheat. Mol. Plant Pathol. 2012, 13, 467–482. [Google Scholar] [CrossRef]
- Proctor, R.H.; Desjardins, A.E.; Plattner, R.D.; Hohn, T.M. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genet. Biol. 1999, 27, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Duan, J.; Aida, K.; Tsuduki, T.; Hirata, T. Identification of glucosylceramides containing sphingatrienine in maize and rice using ion trap mass spectrometry. Lipids 2010, 45, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Wu, Y.; Wang, S.; Guo, Q.; Lu, M.; Wu, C.; Chen, Y. Simultaneous determination of 16 mycotoxins in cereals using an Agilent Triple Quadrupole LC/MS system and e-Method. Agil. Technol. 2017, 2017, 1–10. [Google Scholar]
- Antiga, L.; La Starza, S.R.; Miccoli, C.; D’Angeli, S.; Scala, V.; Zaccaria, M.; Shu, X.; Obrian, G.; Beccaccioli, M.; Payne, G.A. Aspergillus flavus Exploits maize kernels using an “Orphan” secondary metabolite cluster. Int. J. Mol. Sci. 2020, 21, 8213. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.; Grottoli, A.; Cigliano, R.A.; Anzar, I.; Beccaccioli, M.; Fanelli, C.; Dall’Asta, C.; Battilani, P.; Reverberi, M.; Sanseverino, W. Careful with that axe, gene, genome perturbation after a PEG-mediated protoplast transformation in Fusarium verticillioides. Toxins 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- D’Angeli, S.; Altamura, M.M. Osmotin induces cold protection in olive trees by affecting programmed cell death and cytoskeleton organization. Planta 2007, 225, 1147–1163. [Google Scholar] [CrossRef]
- Zeng, H.-Y.; Li, C.-Y.; Yao, N. Fumonisin B1: A tool for exploring the multiple functions of sphingolipids in plants. Front. Plant Sci. 2020, 11, 600458. [Google Scholar] [CrossRef] [PubMed]


| % Protoplasts | ||||||
|---|---|---|---|---|---|---|
| Treatment | Normal | Apoptotic | Necrotic | |||
| ctr | 41 | 31 | 29 | |||
| Maize Protoplasts + (FB1 + FB2) | 29 | *** | 36 | ** | 35 | * |
| Gene | Accession No. | t Annealing (°C) | Sequence |
|---|---|---|---|
| β-tubulin | NP_001105457 | 60 | Fw CTACCTCACGGCATCTGCTATGT Rev GTCACACACACTCGACTTCACG |
| β-tubulin | FVEG_04081 | 40 | Fw CTCTGCTCATTTCCAAGATCCGCG Rev GTAGTTGAGGTCACCGTAGGAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beccaccioli, M.; Salustri, M.; Scala, V.; Ludovici, M.; Cacciotti, A.; D’Angeli, S.; Brown, D.W.; Reverberi, M. The Effect of Fusarium verticillioides Fumonisins on Fatty Acids, Sphingolipids, and Oxylipins in Maize Germlings. Int. J. Mol. Sci. 2021, 22, 2435. https://doi.org/10.3390/ijms22052435
Beccaccioli M, Salustri M, Scala V, Ludovici M, Cacciotti A, D’Angeli S, Brown DW, Reverberi M. The Effect of Fusarium verticillioides Fumonisins on Fatty Acids, Sphingolipids, and Oxylipins in Maize Germlings. International Journal of Molecular Sciences. 2021; 22(5):2435. https://doi.org/10.3390/ijms22052435
Chicago/Turabian StyleBeccaccioli, Marzia, Manuel Salustri, Valeria Scala, Matteo Ludovici, Andrea Cacciotti, Simone D’Angeli, Daren W. Brown, and Massimo Reverberi. 2021. "The Effect of Fusarium verticillioides Fumonisins on Fatty Acids, Sphingolipids, and Oxylipins in Maize Germlings" International Journal of Molecular Sciences 22, no. 5: 2435. https://doi.org/10.3390/ijms22052435







