Diabetes and Cardiovascular Risk in Renal Transplant Patients
Abstract
:1. Introduction
2. Prediabetes and Post-Transplant Diabetes Mellitus (PTDM)—Diagnosis and Prevalence
3. Prediabetes and Post-Transplant Diabetes Mellitus (PTDM)—Risk Factors and Pathophysiology
4. Possible Biomarkers in PTDM
5. Cardiovascular Risk and Morbidity of Renal Transplant Patients
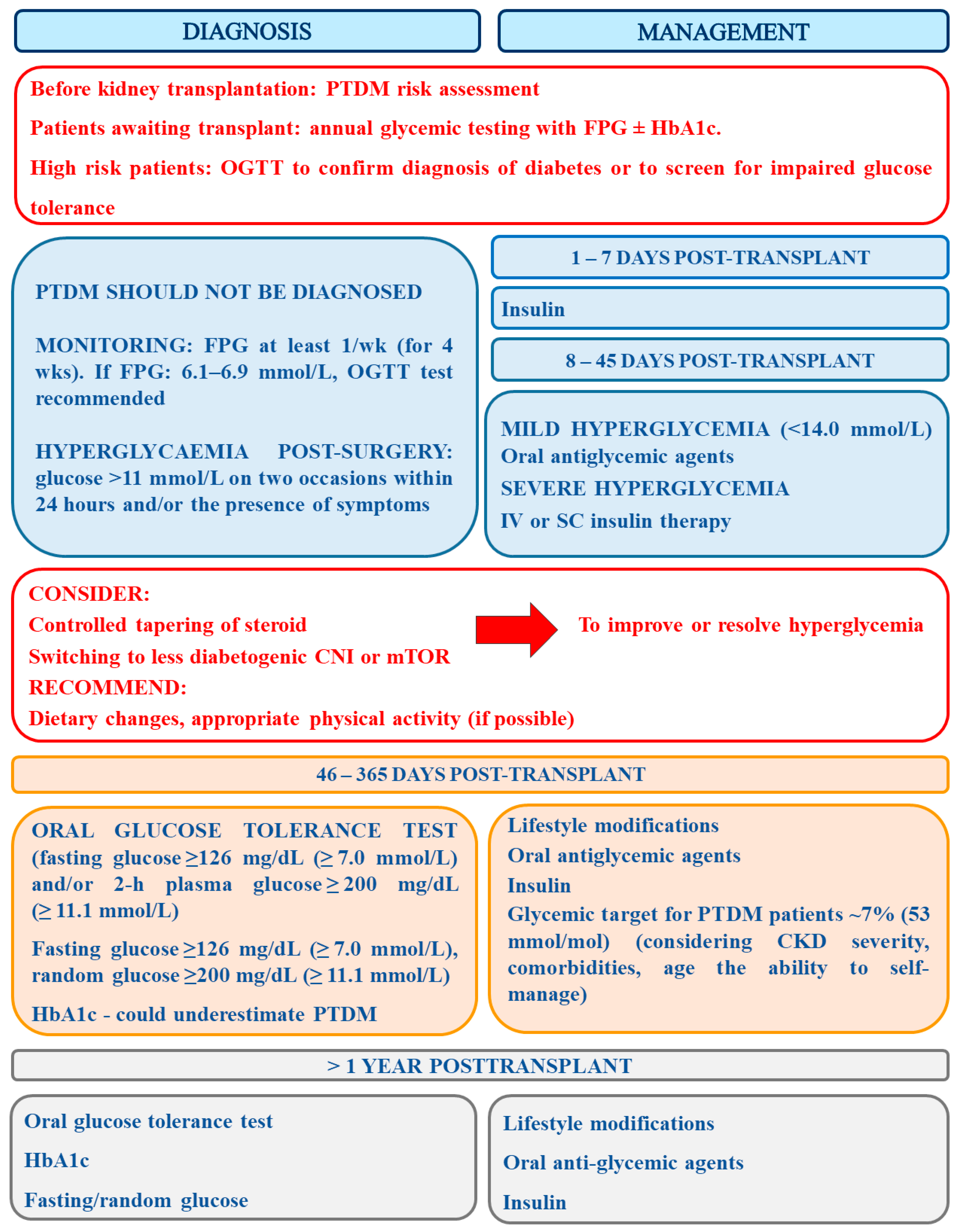
6. The Assessment of PTDM Risk and Recommendations Concerning Its Diagnosis and Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alnasrallah, B.; Pilmore, H.; Manley, P. Protocol for a pilot randomised controlled trial of metformin in pre-diabetes after kidney transplantation: The Transplantation and Diabetes (Transdiab) study. BMJ Open 2017, 7, e016813. [Google Scholar] [CrossRef] [Green Version]
- Port, F.K.; Wolfe, R.A.; Mauger, E.A.; Berling, D.P.; Jiang, K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993, 270, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Laupacis, A.; Keown, P.; Pus, N.; Krueger, H.; Ferguson, B.; Wong, C.; Muirhead, N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996, 50, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Howard, K.; Salkeld, G.; White, S.; McDonald, S.; Chadban, S.; Craig, J.C.; Cass, A. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology 2009, 14, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Van Walraven, C.; Manuel, D.G.; Knoll, G. Survival trends in ESRD patients compared with the general population in the United States. Am. J. Kidney Dis. 2014, 63, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hjelmesaeth, J.; Hartmann, A.; Leivestad, T.; Holdaas, H.; Sagedal, S.; Olstad, M.; Jenssen, T. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006, 69, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Pilmore, H.; Dent, H.; Chang, S.; McDonald, S.P.; Chadban, S.J. Reduction in cardiovascular death after kidney transplantation. Transplantation 2010, 89, 851–857. [Google Scholar] [CrossRef]
- Jalowiec, A.; Grady, K.L.; White-Williams, C. Mortality, rehospitalization, and post-transplant complications in gender-mismatched heart transplant recipients. Heart Lung 2017, 46, 265–272. [Google Scholar] [CrossRef]
- Weiss, E.S.; Allen, J.G.; Patel, N.D.; Russell, S.D.; Baumgartner, W.A.; Shah, A.S.; Conte, J.V. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation of 18;000 transplants in the modern era. Circ. Heart Fail. 2009, 2, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Csete, M. Gender Issues in Transplantation. Anesth. Analg. 2008, 107, 232–238. [Google Scholar] [CrossRef]
- Kouli, F.; Morrell, C.H.; Ratner, L.E.; Kraus, E.S. Impact of donor/recipient traits independent of rejection on long-term renal function. Am. J. Kidney Dis. 2001, 37, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Muller, V.; Szabo, A.; Viklicky, O.; Gaul, I.; Portl, S.; Philipp, T.; Heemann, U.W. Sex hormones and gender related differences: Their influence on chronic renal allograft rejection. Kidney Int. 1999, 55, 2011–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeier, M.; Dohler, B.; Opelz, G.; Ritz, E. The effect of donor gender on graft survival. J. Am. Soc. Nephrol. 2002, 13, 2570–2576. [Google Scholar] [CrossRef] [Green Version]
- Sanfey, H. Gender-specific issues in liver and kidney failure and transplantation: A review. J. Womens Health 2005, 14, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hecking, M.; Kainz, A.; Werzowa, J.; Haidinger, M.; Döller, D.; Tura, A.; Karaboyas, A.; Hörl, W.H.; Wolzt, M.; Sharif, A.; et al. Glucose Metabolism After Renal Transplantation. Diabetes Care 2013, 36, 2763–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an International Consensus Meeting on Posttransplantation Diabetes Mellitus: Recommendations and Future Directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, J.; Wilkinson, A.; Dantal, J.; Dotta, F.; Haller, H.; Hernández, D.; Kasiske, B.L.; Kiberd, B.; Krentz, A.; Legendre, C.; et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Transplantation 2003, 75, SS3–SS24. [Google Scholar] [CrossRef]
- Montori, V.M.; Basu, A.; Erwin, P.J.; Velosa, J.A.; Gabriel, S.E.; Kudva, Y.C. Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 2002, 25, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, E.; Díaz, J.M.; Moreso, F.; Lauzurrica, R.; Ibernon, M.; Torres, I.S.; Ruiz, R.B.; Rodríguez Rodríguez, A.E.; Mallén, P.D.; Bayés-Genís, B.; et al. Prediabetes is a risk factor for cardiovascular disease following renal transplantation. Kidney Int. 2019, 96, 1374–1380. [Google Scholar] [CrossRef]
- Guthoff, M.; Vosseler, D.; Langanke, J.; Nadalin, S.; Königsrainer, A.; Häring, H.-U.; Fritsche, A.; Heyne, N. Diabetes Mellitus and Prediabetes on Kidney Transplant Waiting List- Prevalence, Metabolic Phenotyping and Risk Stratification Approach. PLoS ONE 2015, 10, e0134971. [Google Scholar] [CrossRef] [Green Version]
- Chakkera, H.A.; Weil, E.J.; Swanson, C.M.; Dueck, A.C.; Heilman, R.L.; Reddy, K.S.; Hamawi, K.; Khamash, H.; Moss, A.A.; Mulligan, D.C.; et al. Pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care 2011, 34, 2141–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, G.; Dalla Man, C.; Campioni, M.; Chittilapilly, E.; Basu, R.; Toffolo, G.; Cobelli, C.; Rizza, R. Pathogenesis of Pre-Diabetes: Pathogenesis of pre-diabetes: Mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006, 55, 3536–3549. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.A.; Jenkinson, C.P.; Richardson, D.K.; Tripathy, D.; DeFronzo, R.A. Insulin Secretion and Action in Subjects with Impaired Fasting Glucose and Impaired Glucose Tolerance. Results Veterans Adm. Genet. Epidemiol. Study 2006, 55, 1430–1435. [Google Scholar] [CrossRef] [Green Version]
- Cehic, M.G.; Nundall, N.; Greenfield, J.R.; Macdonald, P.S. Management Strategies for Posttransplant Diabetes Mellitus after Heart Transplantation: A Review. J. Transplant. 2018, 2018, 1025893. [Google Scholar] [CrossRef] [Green Version]
- Pham, P.T.; Sidhu, H.S.; Pham, P.M.; Pham, P.C. Diabetes Mellitus After Solid Organ Transplantation. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK378977/ (accessed on 2 September 2020).
- Starlz, T.E. Experience in Renal Transplantation; Saunders: Philadelphia, PA, USA, 1964. [Google Scholar]
- Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [CrossRef] [PubMed] [Green Version]
- Porrini, E.L.; Díaz, J.M.; Moreso, F.; Delgado Mallén, P.I.; Silva Torres, I.; Ibernon, M.; Bayés-Genís, B.; Benitez-Ruiz, R.; Lampreabe, I.; Lauzurrica, R.; et al. Clinical evolution of post-transplant diabetes mellitus. Nephrol. Dial. Transplant. 2015, 31, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Cosio, F.G.; Kudva, Y.; van der Velde, M.; Larson, T.S.; Textor, S.C.; Griffin, M.D.; Stegall, M.D. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005, 67, 2415–2421. [Google Scholar] [CrossRef] [Green Version]
- Copstein, L.A.; Zelmanovitz, T.; Gonçalves, L.F.; Manfro, R.C. Posttransplant diabetes mellitus in cyclosporine-treated renal allograft patients: A case-control study. Transplant. Proc. 2004, 36, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.C.; Lee, N.P.; Cohan, P.; Chuang, L.M. Beta cell function declines with age in glucose tolerant Caucasians. Clin. Endocrinol. 2000, 53, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Corpeleijn, E.; Deinum, J.; Stolk, R.P.; Gans, R.O.; Navis, G.; Bakker, S.J. Pancreatic β-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care 2013, 36, 1926–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lucena, D.D.; de Sá, J.R.; Medina-Pestana, J.O.; Rangel, É.B. Modifiable Variables Are Major Risk Factors for Posttransplant Diabetes Mellitus in a Time-Dependent Manner in Kidney Transplant: An Observational Cohort Study. J. Diabetes Res. 2020, 2020, 1938703. [Google Scholar] [CrossRef]
- Kuypers, D.R.J.; Claes, K.; Bammens, B.; Evenepoel, P.; Vanrenterghem, Y. Early clinical assessment of glucose metabolism in renal allograft recipients: Diagnosis and prediction of post-transplant diabetes mellitus (PTDM). Nephrol. Dial. Transplant. 2008, 23, 2033–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lucena, D.D.; Rangel, É.B. Glucocorticoids use in kidney transplant setting. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, G.; Ivani de Paula, M.; Rangel, É.B. mTOR inhibitors in pancreas transplant: Adverse effects and drug-drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 367–385. [Google Scholar] [CrossRef]
- Goldmannova, D.; Karasek, D.; Krystynik, O.; Zadrazil, J. New-onset diabetes mellitus after renal transplantation. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2016, 160, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrizi, F.; Martin, P.; Dixit, V.; Bunnapradist, S.; Kanwal, F.; Dulai, G. Posttransplant diabetes mellitus and HCV seropositive status after renal transplantation: Meta-analysis of clinical studies. Am. J. Transpl. 2005, 5, 2433–2440. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Wang, Y.; Han, X.; Chen, X. Hepatitis C virus induced a novel apoptosis-like death of pancreatic beta cells through a caspase 3-dependent pathway. PLoS ONE 2012, 7, e38522. [Google Scholar] [CrossRef] [Green Version]
- Einollahi, B.; Motalebi, M.; Salesi, M.; Ebrahimi, M.; Taghipour, M. The impact of cytomegalovirus infection on new-onset diabetes mellitus after kidney transplantation: A review on current findings. J. Nephropathol. 2014, 3, 139–148. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Biddle, K.; Augustine, T.; Shazli, A. Post-Transplantation Diabetes Mellitus. Diabetes Ther. 2020, 11, 779–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourishankar, S.; Jhangri, G.S.; Tonelli, M.; Wales, L.H.; Cockfield, S.M. Development of diabetes mellitus following kidney transplantation: A Canadian experience. Am. J. Transpl. 2004, 4, 1876–1882. [Google Scholar] [CrossRef]
- Cosio, F.G.; Pesavento, T.E.; Osei, K.; Henry, M.L.; Ferguson, R.M. Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001, 59, 732–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaswamy, V.; Bennett, R.G.; Clure, C.C.; Ottemann, B.; Davis, J.S.; Larsen, J.L.; Hamel, F.G. Tacrolimus and sirolimus have distinct effects on insulin signaling in male and female rats. Transl. Res. 2014, 163, 221–231. [Google Scholar] [CrossRef]
- Drachenberg, C.B.; Klassen, D.K.; Weir, M.R.; Wiland, A.; Fink, J.C.; Bartlett, S.T.; Cangro, C.B.; Blahut, S.; Papadimitriou, J.C. Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsies and clinical correlation. Transplantation 1999, 68, 396–402. [Google Scholar] [CrossRef]
- Soleimanpour, S.A.; Crutchlow, M.F.; Ferrari, A.M.; Raum, J.C.; Groff, D.N.; Rankin, M.M.; Liu, C.; De Leon, D.D.; Naji, A.; Kushner, J.A.; et al. Calcineurin signaling regulates human islet β-cell survival. J. Biol. Chem. 2010, 285, 40050–40059. [Google Scholar] [CrossRef] [Green Version]
- Asberg, A.; Midtvedt, K.; Voytovich, M.H.; Line, P.-D.; Narverud, J.; Reisaeter, A.V.; Mørkrid, L.; Jenssen, T.; Hartmann, A. Calcineurin inhibitor effects on glucose metabolism and endothelial function following renal transplantation. Clin. Transpl. 2009, 23, 511–518. [Google Scholar] [CrossRef]
- Rickels, M.R.; Naji, A.; Teff, K.L. Insulin sensitivity; glucose effectiveness; and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2138–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakkera, H.A.; Mandarino, L.J. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation 2013, 95, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Midtvedt, K.; Hjelmesæth, J.; Hartmann, A.; Lund, K.; Paulsen, D.; Egeland, T.; Jenssen, T. Insulin Resistance after Renal Transplantation: The Effect of Steroid Dose Reduction and Withdrawal. J. Am. Soc. Nephrol. 2004, 15, 3233–3239. [Google Scholar] [CrossRef] [Green Version]
- Leeaphorn, N.; Garg, N.; Khankin, E.V.; Cardarelli, F.; Pavlakis, M. Recurrence of IgA nephropathy after kidney transplantation in steroid continuation versus early steroid-withdrawal regimens: A retrospective analysis of the UNOS/OPTN database. Transpl. Int. 2018, 31, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Pascual, J.; Zamora, J.; Galeano, C.; Royuela, A.; Quereda, C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [Green Version]
- Rangel, E.B. Tacrolimus in pancreas transplant: A focus on toxicity, diabetogenic effect and drug–drug interactions. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1585–1605. [Google Scholar] [CrossRef]
- Boots, J.M.; van Duijnhoven, E.M.; Christiaans, M.H.; Wolffenbuttel, B.H.; van Hooff, J.P. Glucose metabolism in renal transplant recipients on tacrolimus: The effect of steroid withdrawal and tacrolimus trough level reduction. J. Am. Soc. Nephrol. 2002, 13, 221–227. [Google Scholar]
- Shivaswamy, V.; Boerner, B.; Larsen, J. Post-transplant diabetes mellitus: Causes; treatment; and impact on outcomes. Endocr. Rev. 2016, 37, 37–61. [Google Scholar] [CrossRef] [Green Version]
- Morrisett, J.D.; Abdel-Fattah, G.; Hoogeveen, R.; Mitchell, E.; Ballantyne, C.M.; Pownall, H.J.; Opekun, A.R.; Jaffe, J.S.; Oppermann, S.; Kahan, B.D. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J. Lipid Res. 2002, 43, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Teutonico, A.; Schena, P.F.; Di Paolo, S. Glucose Metabolism in Renal Transplant Recipients: Effect of Calcineurin Inhibitor Withdrawal and Conversion to Sirolimus. J. Am. Soc. Nephrol. 2005, 16, 3128–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kälble, F.; Seckinger, J.; Schaier, M.; Morath, C.; Schwenger, V.; Zeier, M.; Sommerer, C. Switch to an everolimus-facilitated cyclosporine A sparing immunosuppression improves glycemic control in selected kidney transplant recipients. Clin. Transplant. 2017, 31, e13024. [Google Scholar] [CrossRef]
- Sommerer, C.; Witzke, O.; Lehner, F.; Arns, W.; Reinke, P.; Eisenberger, U.; Vogt, B.; Heller, K.; Jacobi, J.; Guba, M.; et al. Onset and progression of diabetes in kidney transplant patients receiving everolimus or cyclosporine therapy: An analysis of two randomized, multicenter trials. BMC Nephrol. 2018, 19, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, E.; Cao, X.; Moibi, J.A.; Greene, S.R.; Young, R.; Trucco, M.; Gao, Z.; Matschinsky, F.M.; Deng, S.; Markman, J.F.; et al. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes 2003, 52, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Bussiere, C.T.; Lakey, J.R.T.; Shapiro, A.M.J.; Korbutt, G.S. The impact of the mTOR inhibitor sirolimus on the proliferation and function of pancreatic islets and ductal cells. Diabetologia 2006, 49, 2341–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyurus, E.; Kaposztas, Z.; Kahan, B.D. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: A long-term analysis of various treatment regimens. Transpl. Proc. 2011, 43, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Penfornis, A.; Kury-Paulin, S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006, 32, 539–546. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Hartmann, A.; Kofstad, J.; Stenstrøm, J.; Leivestad, T.; Egeland, T.; Fauchald, P. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation 1997, 64, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Torres-Romero, L.F.; Santiago-Delpín, E.A.; de Echegaray, S.; Solis, D.R.; Rodriguez-Trinidad, A.T.; Gonzalez-Caraballo, Z.A.; Morales-Otero, L.A. HLA Is Not Predictive of Posttransplant Diabetes Mellitus. Transplant. Proc. 2006, 38, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.; Matas, A.J. Diabetes Mellitus after Kidney Transplantation in the United States. Am. J. Transplant. 2003, 3, 178–185. [Google Scholar] [CrossRef]
- Baron, P.W.; Infante, S.; Peters, R.; Tilahun, J.; Weissman, J.; Delgado, L.; Kore, A.H.; Beeson, W.L.; de Vera, M.E. Post-Transplant Diabetes Mellitus After Kidney Transplant in Hispanics and Caucasians Treated with Tacrolimus-Based Immunosuppression. Ann. Transpl. 2017, 22, 309–314. [Google Scholar] [CrossRef]
- Sulanc, E.; Lane, J.T.; Puumala, S.E.; Groggel, G.C.; Wrenshall, L.E.; Stevens, R.B. New-onset diabetes after kidney transplantation: An application of 2003 International Guidelines. Transplantation 2005, 80, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, B.; Junik, R.; Kamińska, A.; Włodarczyk, Z.; Adamowicz, A. Factors associated with glucose metabolism disorders after kidney transplantation. Endokrynol. Pol. 2013, 64, 21–25. [Google Scholar] [PubMed]
- Marin, M.; Renoult, E.; Bondor, C.I.; Kessler, M. Factors influencing the onset of diabetes mellitus after kidney transplantation: A single French center experience. Transpl. Proc. 2005, 37, 1851–1856. [Google Scholar] [CrossRef]
- Mota-Zamorano, S.; Luna, E.; Garcia-Pino, G.; González, L.M.; Gervasini, G. Variability in the leptin receptor gene and other risk factors for post-transplant diabetes mellitus in renal transplant recipients. Ann. Med. 2019, 51, 164–173. [Google Scholar] [CrossRef]
- Zhang, X.; Men, T.; Liu, H.; Li, X.; Wang, J.; Lv, J. Genetic risk factors for post-transplantation diabetes mellitus in Chinese Han renal allograft recipients treated with tacrolimus. Transpl. Immunol. 2018, 49, 39–42. [Google Scholar] [CrossRef]
- Yang, J.; Hutchinson, I.I.; Shah, T.; Min, D.I. Genetic and clinical risk factors of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation 2011, 91, 1114–1119. [Google Scholar] [CrossRef]
- Romanowski, M.; Dziedziejko, V.; Maciejewska-Karlowska, A.; Sawczuk, M.; Safranow, K.; Domanski, L.; Pawlik, A. Adiponectin and leptin gene polymorphisms in patients with post-transplant diabetes mellitus. Pharmacogenomics 2015, 16, 1243–1251. [Google Scholar] [CrossRef]
- Berger, S.P.; Roos, A.; Mallat, M.J.; Schaapherder, A.F.; Doxiadis, I.I.; van Kooten, C.; Dekker, F.W.; Daha, M.R.; de Fijter, J.W. Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas-kidney transplantation. J. Am. Soc. Nephrol. 2007, 18, 2416–2422. [Google Scholar] [CrossRef] [Green Version]
- Boniotto, M.; Braida, L.; Baldas, V.; Not, T.; Ventura, A.; Vatta, S.; Radillo, O.; Tedesco, F.; Percopo, S.; Montico, M.; et al. Evidence of a correlation between mannose binding lectin and celiac disease: A model for other autoimmune diseases. J. Mol. Med. 2005, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Guad, R.M.; Taylor-Robinson, A.W.; Wu, Y.S.; Gan, S.H.; Zaharan, N.L.; Basu, R.C.; Liew, C.; Wan Md Adnan, W. Clinical and genetic risk factors for new-onset diabetes mellitus after transplantation (NODAT) in major transplant centres in Malaysia. BMC Nephrol. 2020, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, M.; Słuczanowska-Głabowska, S.; Pawlik, A.; Mazurek-Mochol, M.; Dembowska, E. Genetic factors in pathogenesis of diabetes mellitus after kidney transplantation. Ther. Clin. Risk Manag. 2017, 13, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Bamoulid, J.; Courivaud, C.; Deschamps, M.; Mercier, P.; Ferrand, C.; Penfornis, A.; Tiberghien, P.; Chalopin, J.M.; Saas, P.; Ducloux, D. IL-6 promoter polymorphism -174 is associated with new-onset diabetes after transplantation. J. Am. Soc. Nephrol. 2006, 17, 2333–2340. [Google Scholar] [CrossRef]
- Kim, Y.G.; Ihm, C.G.; Lee, T.W.; Lee, S.H.; Jeong, K.H.; Moon, J.Y.; Chung, J.H.; Kim, S.K.; Kim, Y.H. Association of genetic polymorphisms of interleukins with new-onset diabetes after transplantation in renal transplantation. Transplantation 2012, 93, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.C.; Shu, K.H.; Tarng, D.C.; Wu, M.J.; Chen, C.H.; Yu, T.M.; Chuang, Y.W.; Huang, S.T.; Cheng, C.H. Gene polymorphisms are associated with posttransplantation diabetes mellitus among Taiwanese renal transplant recipients. Transpl. Proc. 2012, 44, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.A.; Maxwell, A.P.; McKnight, A.J. A HuGE review and meta-analyses of genetic associations in new onset diabetes after kidney transplantation. PLoS ONE 2016, 11, e0147323. [Google Scholar] [CrossRef] [Green Version]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. MAGIC investigators; GIANT Consortium Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef]
- Lyssenko, V.; Lupi, R.; Marchetti, P.; Del Guerra, S.; Orho-Melander, M.; Almgren, P.; Sjögren, M.; Ling, C.; Eriksson, K.F.; Lethagen, A.L.; et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Investig. 2007, 117, 2155–2163. [Google Scholar] [CrossRef] [Green Version]
- Kurzawski, M.; Dziewanowski, K.; Kędzierska, K.; Wajda, A.; Lapczuk, J.; Droździk, M. Association of transcription factor 7-like 2 (TCF7L2) gene polymorphism with posttransplant diabetes mellitus in kidney transplant patients medicated with tacrolimus. Pharmacol. Rep. 2011, 63, 826–833. [Google Scholar] [CrossRef]
- Ghisdal, L.; Baron, C.; Le Meur, Y.; Lionet, A.; Halimi, J.M.; Rerolle, J.P.; Glowacki, F.; Lebranchu, Y.; Drouet, M.; Noël, C.; et al. TCF7L2 polymorphism associates with new-onset diabetes after transplantation. J. Am. Soc. Nephrol. 2009, 20, 2459–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaughan, J.A.; McKnight, A.J.; Maxwell, A.P. Genetics of new-onset diabetes after transplantation. J. Am. Soc. Nephrol. 2014, 25, 1037–1049. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Sampaio, M.S.; Yang, J.W.; Min, D.; Hutchinson, I.V. Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation 2012, 93, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska-Zamojcin, E.; Romanowski, M.; Dziedziejko, V.; Maciejewska-Karlowska, A.; Sawczuk, M.; Safranow, K.; Domanski, L.; Pawlik, A. CCL2 gene polymorphism is associated with post-transplant diabetes mellitus. Int. Immunopharmacol. 2016, 32, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Moon, J.Y.; Chung, J.H.; Kim, Y.H.; Lee, T.W. Significant associations between CCL5 gene polymorphisms and post-transplantational diabetes mellitus in Korean renal allograft recipients. Am. J. Nephrol. 2010, 32, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Lee, T.W.; Kim, S.K.; Chung, J.H.; Kang, S.W.; Kim, T.H.; Park, S.J.; et al. Angiotensinogen polymorphisms and post-transplantation diabetes mellitus in Korean renal transplant subjects. Kidney Blood Press Res. 2013, 37, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, G.; Domanski, L.; Pawlik, A.; Binczak-Kuleta, A.; Safranow, K.; Ciechanowicz, A.; Dziedziejko, V.; Pietrzak-Nowacka, M.; Ciechanowski, K. Polymorphisms of super-oxide dismutase; glutathione peroxidase and catalase genes in patients with post-transplant diabetes mellitus. Arch. Med. Res. 2010, 41, 350–355. [Google Scholar] [CrossRef]
- Forgione, M.A.; Weiss, N.; Heydrick, S.; Cap, A.; Klings, E.S.; Bierl, C.; Eberhardt, R.T.; Farber, H.W.; Loscalzo, J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1255–H1261. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, T.; Furuta, H.; Kato, H.; Doi, A.; Tamai, M.; Shimomura, H.; Sakagashira, S.; Nishi, M.; Sasaki, H.; Sanke, T.; et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in Japanese type 2 diabetic patients. Diabetes 2004, 53, 2455–2460. [Google Scholar] [CrossRef] [Green Version]
- Elens, L.; Sombogaard, F.; Hesselink, D.A.; van Schaik, R.H.; van Gelder, T. Single-nucleotide polymorphisms in P450 oxidoreductase and peroxisome proliferator-activated receptor-α are associated with the development of new-onset diabetes after transplantation in kidney transplant recipients treated with tacrolimus. Pharm. Genom. 2013, 23, 649–657. [Google Scholar] [CrossRef]
- Hornum, M.; Lindahl, J.P.; von Zur-Mühlen, B.; Jenssen, T.; Feldt-Rasmussen, B. Diagnosis, management and treatment of glucometabolic disorders emerging after kidney transplantation. Transpl. Int. 2013, 26, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, H.A.; Weil, E.J.; Pham, P.T.; Pomeroy, J.; Knowler, W.C. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care 2013, 36, 1406–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, J.; Anderson-Haag, T.L.; Gustafson, S.; Snyder, J.J.; Kasiske, B.L.; Israni, A.K. Metformin use in kidney transplant recipients in the United States: An observational study. Am. J. Nephrol. 2014, 40, 546–553. [Google Scholar] [CrossRef]
- Kurian, B.; Joshi, R.; Helmuth, A. Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr. Pract. 2008, 14, 979–984. [Google Scholar] [CrossRef]
- Dedinská, I.; Graňák, K.; Vnučák, M.; Skálová, P.; Kováčiková, L.; Laca, Ľ.; Miklušica, J.; Prídavková, D.; Galajda, P.; Mokáň, M. Role of sex in post-transplant diabetes mellitus development: Are men and women equal? J. Diabetes Complicat. 2019, 33, 315–322. [Google Scholar] [CrossRef]
- Madhav, D.; Ram, R.; Dakshinamurty, K.V. Posttransplant Diabetes Mellitus: Analysis of Risk Factors, Effects on Biochemical Parameters and Graft Function 5 Years after Renal Transplantation. Transplant. Proc. 2010, 42, 4069–4071. [Google Scholar] [CrossRef]
- González-Posada, J.M.; Hernández, D.; Bayés Genís, B.; García Perez, J.; Rivero Sanchez, M. Impact of diabetes mellitus on kidney transplant recipients in Spain. Nephrol. Dial. Transpl. 2004, 19 (Suppl. 3), iii57–iii61. [Google Scholar] [CrossRef] [Green Version]
- Prasad, G.V.; Kim, S.J.; Huang, M.; Nash, M.M.; Zalzman, J.S.; Fenton, S.S.; Cattran, D.C.; Cole, E.H.; Cardella, C.J. Reduced incidence of new onset diabetes mellitus after renal transplantation with 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins). Am. J. Transpl. 2004, 4, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Lê, K.-A.; Mahurkar, S.; Alderete, T.L.; Hasson, R.E.; Adam, T.C.; Kim, J.S.; Beale, E.; Xie, C.; Greenberg, A.S.; Allayee, H.; et al. Subcutaneous Adipose Tissue Macrophage Infiltration Is Associated with Hepatic and Visceral Fat Deposition, Hyperinsulinemia, and Stimulation of NF-κB Stress Pathway. Diabetes 2011, 60, 2802–2809. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Wu, M.; Xing, Y. Pretransplant metabolic syndrome and its components predict post-transplantation diabetes mellitus in Chinese patients receiving a first renal transplant. Ther. Clin. Risk Manag. 2019, 15, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, E.; Fernández-Fresnedo, G.; Valero, R.; Ruiz, J.C.; Piñera, C.; Palomar, R.; González-Cotorruelo, J.; Gómez-Alamillo, C.; Arias, M. New-onset diabetes after kidney transplantation: Risk factors. J. Am. Soc. Nephrol. 2006, 17 (Suppl. 3), S291–S295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, M.; Gatault, P.; Al-Najjar, A.; Doute, C.; Barbet, C.; Chatelet, V.; Laouad, I.; Marlière, J.F.; Nivet, H.; Büchler, M.; et al. Early pulse pressure and low-grade proteinuria as independent long-term risk factors for new-onset diabetes mellitus after kidney transplantation. Am. J. Transpl. 2008, 8, 1719–1728. [Google Scholar] [CrossRef]
- Hermans, M.P.; Ahn, S.A.; Rousseau, M.F. The atherogenic dyslipidemia ratio [log(TG)/HDL-C] is associated with residual vascular risk, beta-cell function loss and microangiopathy in type 2 diabetes females. Lipids Health Dis. 2012, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Bayés, B.; Granada, M.L.; Pastor, M.C.; Lauzurica, R.; Salinas, I.; Sanmartí, A.; Espinal, A.; Serra, A.; Navarro, M.; Bonal, J.; et al. Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am. J. Transpl. 2007, 7, 416–422. [Google Scholar] [CrossRef]
- de Lourdes Lima, M.; Cruz, T.; Rodrigues, L.E.; Bomfim, O.; Melo, J.; Correia, R.; Porto, M.; Cedro, A.; Vicente, E. Serum and intracellular magnesium deficiency in patients with metabolic syndrome-Evidences for its relation to insulin resistance. Diabetes Res. Clin. Pract. 2009, 83, 257–262. [Google Scholar] [CrossRef]
- Schweer, T.; Gwinner, W.; Scheffner, I.; Schwarz, A.; Haller, H.; Blume, C. High impact of rejection therapy on the incidence of post-transplant diabetes mellitus after kidney transplantation. Clin. Transpl. 2014, 28, 512–519. [Google Scholar] [CrossRef]
- Alnasrallah, B.; Goh, T.L.; Chan, L.W.; Manley, P.; Pilmore, H. Transplantation and diabetes (Transdiab): A pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation. BMC Nephrol. 2019, 20, 147. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Hartmann, A.; Midtvedt, K.; Aakhus, S.; Stenstrøm, J.; Mørkrid, L.; Egeland, T.; Tordarson, H.; Fauchald, P. Metabolic cardiovascular syndrome after renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, T.A.; Wahba, M.; Mallik, R.; Peracha, J.; Patel, D.; De, P.; Fogarty, D.; Frankel, A.; Karalliedde, J.; Mark, P.B.; et al. Association of British Clinical Diabetologists and Renal Association guidelines on the detection and management of diabetes post solid organ transplantation. Diabet. Med. 2021, e14523. [Google Scholar] [CrossRef]
- Wauters, R.P.; Cosio, F.G.; Suarez Fernandez, M.L.; Kudva, Y.; Shah, P.; Torres, V.E. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation 2012, 94, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldal, T.F.; Ueland, T.; Jenssen, T.; Hartmann, A.; Reisaeter, A.V.; Aukrust, P.; Michelsen, A.; Åsberg, A. Inflammatory and related biomarkers are associated with post-transplant diabetes mellitus in kidney recipients: A retrospective study. Transpl. Int. 2018, 31, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Kim, S.K.; Park, J.Y.; Kim, Y.G.; Moon, J.Y.; Lee, S.H.; Ihm, C.G.; Lee, T.W.; Kim, S.K.; Chung, J.H.; et al. Significant association between toll-like receptor gene polymorphisms and posttransplantation diabetes mellitus. Nephron 2016, 133, 279. [Google Scholar] [CrossRef]
- Cieniawski, D.; Miarka, P.; Ignacak, E.; Bętkowska-Prokop, A.; Waluś-Miarka, M.; Idzior-Waluś, B.; Kuźniewski, M.; Sułowicz, W. ognostic value of proinflammatory markers in patients after kidney transplantation in relation to the presence of diabetes. Transpl. Proc. 2016, 48, 1604. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Hagen, M.; Hartmann, A.; Midtvedt, K.; Egeland, T.; Jenssen, T. The impact of impaired insulin release and insulin resistance on glucose intolerance after renal transplantation. Clin. Transpl. 2002, 16, 389. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Asberg, A.; Muller, F.; Hartmann, A.; Jenssen, T. New-onset posttransplantation diabetes mellitus: Insulin resistance or insulinopenia? Impact of immunosuppressive drugs, cytomegalovirus and hepatitis C virus infection. Curr. Diabetes Rev. 2005, 1, 1–10. [Google Scholar] [CrossRef]
- Yepes-Calderón, M.; Sotomayor, C.G.; Gomes-Neto, A.W.; Gans, R.O.B.; Berger, S.P.; Rimbach, G.; Esatbeyoglu, T.; Rodrigo, R.; Geleijnse, J.M.; Navis, G.J.; et al. Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 453. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, D.; Ruggenenti, P.; Baldelli, S.; Motterlini, N.; Gotti, E.; Sandrini, S.; Salvadori, M.; Segoloni, G.; Rigotti, P.; Donati, D.; et al. ABCB1 genotypes predict cyclosporine-related adverse events and kidney allograft outcome. J. Am. Soc. Nephrol. 2009, 20, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Dormans, J.P.; Gunawardena, A.T.; Hakonarson, H.; Hecht, J.T. The type 2 diabetes associated rs7903146 T allele within TCF7L2 is significantly under-represented in hereditary multiple exostoses: Insights into pathogenesis. Bone 2015, 72, 123–127. [Google Scholar]
- Dehwah, M.S.; Wang, M.; Huang, Q.Y. CDKAL1 and type 2 diabetes: A global meta-analysis. Genet. Mol. Res. 2010, 9, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Schultz, B.; Gallicio, G.; Cesaroni, M.; Lupey, L.; Engel, N. Enhancers compete with a long non-coding RNA for regulation of the Kcnq1 domain. Nucl. Acids Res. 2015, 43, 745–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Valverde, S.L.; Taft, R.J.; Mattick, J.S. MicroRNAs in β-cell biology, insulin resistance, diabetes and its complications. Diabetes 2011, 60, 1825–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Liu, Y.; Li, L.; Su, B.; Yang, L.; Fan, W.; Yin, Q.; Chen, L.; Cui, T.; Zhang, J.; et al. Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: Implications for glomerular endothelial injury. BMC Nephrol. 2014, 15, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The role of circulating microRNA-126 (miR-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Zhong, M.; Zhao, W.; Wang, C.; Zhang, J.; Liu, X.; Li, Y.; Paudel, S.D.; Wang, Q.; Lou, T. Urinary miR-29 correlates with albuminuria and carotid intima-media thickness in type 2 diabetes patients. PLoS ONE 2013, 8, e82607. [Google Scholar]
- Celen, E.; Ertosun, M.G.; Kocak, H.; Dinckan, A.; Yoldas, B. Expression Profile of MicroRNA Biogenesis Components in Renal Transplant Patients. Transplant. Proc. 2017, 49, 472–476. [Google Scholar] [CrossRef]
- Ulbing, M.; Kirsch, A.H.; Leber, B.; Lemesch, S.; Münzker, J.; Schweighofer, N.; Hofer, D.; Trummer, O.; Rosenkranz, A.R.; Müller, H.; et al. MicroRNAs 223-3p and 93-5p in patients with chronic kidney disease before and after renal transplantation. Bone 2017, 95, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Boerner, B.P.; Shivaswamy, V.; Desouza, C.V.; Larsen, J.L. Diabetes and cardiovascular disease following kidney transplantation. Curr. Diabetes Rev. 2011, 7, 221–234. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neale, J.; Smith, A.C. Cardiovascular risk factors following renal transplant. World J. Transpl. 2015, 5, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Beto, J.A.; Coronado, B.E.; Eknoyan, G.; Foley, R.N.; Kasiske, B.L.; Klag, M.J.; Mailloux, L.U.; Manske, C.L.; Meyer, K.B.; et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am. J. Kidney Dis. 1998, 32, 853–906. [Google Scholar] [CrossRef]
- Rao, P.S.; Merion, R.M.; Ashby, V.B.; Port, F.K.; Wolfe, R.A.; Kayler, L.K. Renal transplantation in elderly patients older than 70 years of age: Results from the Scientific Registry of Transplant Recipients. Transplantation 2007, 83, 1069–1074. [Google Scholar] [CrossRef] [Green Version]
- Liefeldt, L.; Budde, K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl. Int. 2010, 23, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am. J. Transpl. 2004, 4, 1662–1668. [Google Scholar] [CrossRef]
- Lentine, K.L.; Brennan, D.C.; Schnitzler, M.A. Incidence and predictors of myocardial infarction after kidney transplantation. J. Am. Soc. Nephrol. 2005, 16, 496–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aftab, W.; Varadarajan, P.; Rasool, S.; Pai, R.G. Predictors and prognostic implications of major adverse cardiovascular events after renal transplant: 10 years outcomes in 321 patients. Int. J. Angiol. 2014, 23, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, C.J.; Fourlanos, S.; Hjelmesæth, J.; Colman, P.G.; Cohney, S.J. New-Onset Diabetes After Kidney Transplantation—Changes and Challenges. Am. J. Transplant. 2012, 12, 820–828. [Google Scholar] [CrossRef]
- Kahn, R.; Buse, J.; Ferrannini, E.; Stern, M. The metabolic syndrome: Time for a critical appraisal. Diabetologia 2005, 48, 1684–1699. [Google Scholar] [CrossRef] [PubMed]
- Alshamsi, S. The risk factors and their relative strengths for new onset diabetes after transplantation (NODAT). Nephrol. Dial. Transplant. 2015, 30, iii653. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Cai, X.; Mai, W.; Li, M.; Hu, Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ 2016, 355, i5953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderhaug, T.G.; Hjelmesæth, J.; Hartmann, A.; Røislien, J.; Bergrem, H.A.; Leivestad, T.; Line, P.D.; Jenssen, T. The association of early post-transplant glucose levels with long-term mortality. Diabetologia 2011, 54, 1341–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, E.; Santos, L.; Piñera, C.; Millán, J.C.; Quintela, M.E.; Toyos, C.; Allende, N.; Gómez-Alamillo, C.; Arias, M. Prediction at first year of incident new-onset diabetes after kidney transplantation by risk prediction models. Diabetes Care 2012, 35, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Cashion, A.K.; Sánchez, Z.V.; Cowan, P.A.; Hathaway, D.K.; Lo Costello, A.; Gaber, A.O. Changes in weight during the first year after kidney transplantation. Prog. Transpl. 2007, 17, 40–47. [Google Scholar] [CrossRef]
- Type 2 Diabetes in Adults: Management. NICE Guideline [NG28]. Available online: https://www.nice.org.uk/guidance/ng28 (accessed on 1 October 2020).
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010, 2010, Cd002967. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Chakkera, H.A.; Weil, E.J.; Castro, J.; Heilman, R.L.; Reddy, K.S.; Mazur, M.J.; Hamawi, K.; Mulligan, D.C.; Moss, A.A.; Mekeel, K.L.; et al. Hyperglycemia during the immediate period after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2009, 4, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.C.; Mathew, T.H.; Russ, G.R.; Rao, M.M.; Moran, J. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: A pilot study. Transplantation 2001, 72, 1321–1324. [Google Scholar] [CrossRef]
- Hecking, M.; Haidinger, M.; Döller, D.; Werzowa, J.; Tura, A.; Zhang, J.; Tekoglu, H.; Pleiner, J.; Wrba, T.; Rasoul-Rockenschaub, S.; et al. Early Basal Insulin Therapy Decreases New-Onset Diabetes after Renal Transplantation. J. Am. Soc. Nephrol. 2012, 23, 739–749. [Google Scholar] [CrossRef] [Green Version]
- Gallwitz, B.; Bretzel, R.G. How do we continue treatment in patients with type 2 diabetes when therapeutic goals are not reached with oral antidiabetes agents and lifestyle? Incretin versus insulin treatment. Diabetes Care 2013, 36 (Suppl. 2), S180–S189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Jun, H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Padmasekar, M.; Lingwal, N.; Samikannu, B.; Chen, C.; Sauer, H.; Linn, T. Exendin-4 protects hypoxic islets from oxidative stress and improves islet transplantation outcome. Endocrinology 2013, 154, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Farber, S.J.; Berger, E.Y.; Earle, D.P. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J. Clin. Investig. 1951, 30, 125–129. [Google Scholar] [CrossRef]
- Norton, L.; Shannon, C.E.; Fourcaudot, M.; Hu, C.; Wang, N.; Ren, W.; Song, J.; Abdul-Ghani, M.; DeFronzo, R.A.; Ren, J.; et al. Sodium-glucose co-transporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes Obes. Metab. 2017, 19, 1322–1326. [Google Scholar] [CrossRef]
- Wright, E.M.; Turk, E. The sodium/glucose cotransport family SLC5. Pflug. Arch. 2004, 447, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M. Renal Na(+)-glucose cotransporters. Am. J. Physiol. Ren. Physiol. 2001, 280, F10–F18. [Google Scholar] [CrossRef]
- Turk, E.; Martín, M.G.; Wright, E.M. Structure of the human Na+/glucose cotransporter gene SGLT1. J. Biol. Chem. 1994, 269, 15204–15209. [Google Scholar] [CrossRef]
- Mogensen, C.E. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand. J. Clin. Lab. Investig. 1971, 28, 101–109. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Davidson, J.A.; Del Prato, S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes. Metab. 2012, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; Defronzo, R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef] [Green Version]
- Freitas, H.S.; Anhê, G.F.; Melo, K.F.; Okamoto, M.M.; Oliveira-Souza, M.; Bordin, S.; Machado, U.F. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: Involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 2008, 149, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, L.A.; Ward, M.S.; Fotheringham, A.K.; Zhuang, A.; Borg, D.J.; Flemming, N.B.; Harvie, B.M.; Kinneally, T.L.; Yeh, S.M.; McCarthy, D.A.; et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci. Rep. 2016, 6, 26428. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A.; Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes 2013, 62, 3324–3328. [Google Scholar] [CrossRef] [Green Version]
- Danne, T.; Edelman, S.; Frias, J.P.; Ampudia-Blasco, F.J.; Banks, P.; Jiang, W.; Davies, M.J.; Sawhney, S. Efficacy and safety of adding sotagliflozin, a dual sodium-glucose co-transporter (SGLT)1 and SGLT2 inhibitor, to optimized insulin therapy in adults with type 1 diabetes and baseline body mass index ≥ 27 kg/m(2). Diabetes Obes. Metab. 2021, 23, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Sotagliflozin: A Review in Type 1 Diabetes. Drugs 2019, 79, 1977–1987. [Google Scholar] [CrossRef]
- Zou, H.; Liu, L.; Guo, J.; Wang, H.; Liu, S.; Xing, Y.; Deng, C.; Xiao, Y.; Zhou, Z. Sodium-glucose cotransporter inhibitors as add-on therapy in addition to insulin for type 1 diabetes mellitus: A meta-analysis of randomized controlled trials. J. Diabetes Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.F.; Clegg, D.J.; Taylor, S.I.; Weir, M.R. Diabetic ketoacidosis, sodium glucose transporter-2 inhibitors and the kidney. J. Diabetes Complicat. 2016, 30, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Novikov, A.; Vallon, V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes/Metab. Res. Rev. 2017, 33, e2886. [Google Scholar] [CrossRef]
- Dashora, U.; Patel, D.C.; Gregory, R.; Winocour, P.; Dhatariya, K.; Rowles, S.; Macklin, A.; Rayman, G.; Nagi, D. Association of British Clinical Diabetologists (ABCD) and Diabetes UK joint position statement and recommendations on the use of sodium-glucose cotransporter inhibitors with insulin for treatment of type 1 diabetes (Updated October 2020). Diabet. Med. 2021, 38, e14458. [Google Scholar] [CrossRef] [PubMed]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transpl. 2009, 9 (Suppl. 3), S1–S155. [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Rodrigo, E.; Piñera, C.; Quintella, E.; Ruiz, J.C.; Fernández-Fresnedo, G.; Palomar, R.; Gómez-Alamillo, C.; de Francisco, A.; Arias, M. New-onset diabetes after transplantation: Drug-related risk factors. Transpl. Proc. 2012, 44, 2585–2587. [Google Scholar] [CrossRef]
- Palepu, S.; Prasad, G.V. New-onset diabetes mellitus after kidney transplantation: Current status and future directions. World J. Diabetes 2015, 6, 445–455. [Google Scholar] [CrossRef]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Neel, B.A.; Sargis, R.M. The paradox of progress: Environmental disruption of metabolism and the diabetes epidemic. Diabetes 2011, 60, 1838–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, O.; Kim, H.L.; Weon, J.-I.; Seo, Y.R. Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. J. Cancer Prev. 2015, 20, 12–24. [Google Scholar] [CrossRef]
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diab. Rep. 2018, 18, 98. [Google Scholar] [CrossRef]

| Type of Study | Study Group | Most Important Results | S—Strong Evidence, A—Association | References |
|---|---|---|---|---|
| Risk Factors | ||||
| Prospective cohort study | A total of 487 RTR (age 50 ± 12 years, 55% men); 16% developed PTDM |
Confirmation of the role of β-cell dysfunction in the pathophysiology of NODAT in RTR. | S | [32] |
| Single-center cohort study | 450 recipients of living and deceased donor kidney transplants on immunosuppressive therapy; 13.5% developed PTDM |
| A | [33] |
| Observational study | 359 de novo renal allograft recipients; 17.8% developed PTDM (follow-up 42.8 ± 16.9 months) |
| A | [34] |
| Systematic review of the published medical literature of the relationship between anti-HCV seropositive status and DM after RT | 2502 unique RT recipients were identified. The incidence of PTDM after RT ranged between 7.9% and 50% |
| S | [38] |
| In vitro study | Virus infection system/insulinoma cell line, MIN6 |
| S | [39] |
| Observational study | 386 adult kidney transplant recipients from Canadian kidney transplant population; cumulative incidence rate of PTDM—9.8% |
| S | [41] |
| Case–control study | 2078 non-DM renal allograft recipients; 21% developed PTDM |
| S | [43] |
| Retrospective study | Group 1, SIR plus full-exposure CSA/S (n = 118); group 2, full-exposure CSA/S/no SIR ± antiproliferative drug (n = 141); group 3, SIR plus reduced CSA exposure/S (n = 212); group 4, no SIR/full-exposure CSA/S ± antiproliferative drug (n = 43) |
| S | [62] |
| Prospective study | 173 consecutive kidney transplant recipients |
| S | [64] |
| Retrospective study | 11,659 Medicare beneficiaries from the United Renal Data System who received their first kidney transplant |
| S | [66] |
| Retrospective study | 177 adult patients, without previously known diabetes who underwent transplantation |
| S | [70] |
| Case–control study | 315 renal transplant recipients |
| A | [71] |
| Case–control study | 129 nondiabetic, primary, Chinese Han renal allograft recipients treated with TAC; 13.2% developed PTDM |
| A | [72] |
| Case–control study | Hispanic kidney allograft recipients without evidence of preexisting diabetes who developed NODAT |
| A | [73] |
| Case–control study | 323 patients who received kidney transplants and treated with tacrolimus or cyclosporine |
| A | [74] |
| Comparative study | 168 nondiabetic patients (58% males, 69% of Chinese ethnicity) who received renal transplantation |
| A | [77] |
| Comparative study | 306 renal transplants recipients without a history of diabetes |
| A | [80] |
| Comparative study | 278 renal transplant participants, including 251 subjects free of diabetes and 27 with PTDM |
| A | [81] |
| Comprehensive meta-analysis of data from 36 publications | Kidney transplant recipients |
| A | [82] |
| Comparative study | Hispanic renal transplant patients |
| A | [88] |
| Comparative study | 315 patients who received kidney transplants treated with calcineurin inhibitors, with PTDM (n = 43) and without PTDM (n = 272) |
| A | [89] |
| Comparative study | 311 patients who had received kidney transplants without a prior history of diabetes; 18% developed PTDM |
| A | [90] |
| Comparative study | 302 subjects without previously diagnosed diabetes who had received kidney transplants; PTDM developed in 16.2% |
| A | [91] |
| Comparative study | 159 patients receiving kidney transplants, 21 developed PTDM |
| A | [92] |
| Comparative study | 101 renal transplant recipients receiving tacrolimus-based immunosuppressive therapy |
| A | [95] |
| Retrospective study | 218 records of postrenal transplant patients who had a minimum follow-up for 5 years. Patients with diabetes mellitus (DM; n = 21), PTDM (n = 58) |
| S | [101] |
| Comparative study | 3365 adult kidney allograft recipients, group I (DM; n = 156), Group II (PTDM; n = 251) and Group III (nondiabetic; n = 2958) |
| S | [102] |
| Comparative study | 314 nondiabetic adults who received a renal allograft; PTDM developed in 16% |
| A | [103] |
| Single-center retrospective study | 633 nondiabetic patients receiving a first kidney transplant; 26.2% of recipients developed PTDM |
| A | [105] |
| Retrospective study | 828 Caucasian renal transplant recipients |
| A | [106] |
| Comparative study | 199 nondiabetic patients (128 men; age: 53 ± 11 years; body mass index (BMI) 24.98 ± 3.76 kg/m2); 45 developed PTDM |
| S | [109] |
| Systematic study | 526 kidney transplant recipients; 16.7% of patients developed PTDM |
| [111] | |
| Pathophysiology | ||||
| In vivo/animal study | Male and female Sprague-Dawley rats receiving TAC, SIR, TAC and SIR, or control for 2 weeks. All rats were administered an oral glucose challenge at the end of treatment |
| A | [44] |
| In vitro | 26 pancreas allograft biopsies, performed 1–8 months post-transplantation, from 20 simultaneous kidney-pancreas transplant recipients, randomized to receive either TAC or CSA |
| S | [45] |
| In vitro/In vivo### | Human islets/rat islets/ INS-1 rat insulinoma cells/male C57BL/6 mice |
| S | [46] |
| Predefined substudy of a previously published randomized trial | Renal transplant recipients on CNI treatment (n = 23) vs. CNI-avoidance (n = 21) |
| A | [47] |
| Prospective study | 26 kidney transplant patients who discontinued CSA to take sirolimus, 15 recipients of suboptimal kidneys treated with tacrolimus + sirolimus for the first 3 mo after grafting and then with sirolimus alone |
| S | [57] |
| Retrospective study | 146 renal transplant recipients |
| S | [58] |
| Analysis of two randomized, multicenter trials | Kidney transplant recipients switched (at month 4.5) to everolimus, receiving standard cyclosporine (CsA)-based regimen (ZEUS, n = 300), or switched (at month 3) to everolimus, remaining on standard CNI therapy or convert to everolimus with reduced-exposure CsA (HERAKLES, n = 497) |
| S | [59] |
| In vitro study | MIN-6 insulinoma cells |
| A | [60] |
| Population and Study Design | Result | References |
|---|---|---|
| Single-center, unblinded, pilot randomized controlled trial (19 patients) assessing the feasibility, tolerability and efficacy of metformin after renal transplantation in patients with impaired glucose tolerance (IGT) |
| [1,162] |
| 191 kidney transplants who had at least 1-year follow-up post-transplant |
| [148] |
| Randomly assigned 3234 nondiabetic persons with elevated fasting and post-load plasma glucose concentrations to placebo, metformin, or a lifestyle-modification program |
| [149] |
| Randomly assigned 522 middle-aged, overweight subjects (172 men and 350 women) with impaired glucose tolerance to either the intervention group (individualized counselling aimed at reducing weight, total intake of fat, and intake of saturated fat and increasing intake of fiber and physical activity) or the control group |
| [150] |
| Randomized controlled trial of conventional policy, primarily with diet alone (n = 411) versus intensive blood-glucose control policy with metformin in 753 patients recruited to UKPDS in 15 centers |
| [154] |
| 154 consecutive patients with a body mass index ≥27 kg/m2 |
| [155] |
| 138 patients on active kidney transplant waiting list at the Tübingen University Hospital Collaborative Transplant Center |
| [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysz, J.; Franczyk, B.; Radek, M.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Diabetes and Cardiovascular Risk in Renal Transplant Patients. Int. J. Mol. Sci. 2021, 22, 3422. https://doi.org/10.3390/ijms22073422
Rysz J, Franczyk B, Radek M, Ciałkowska-Rysz A, Gluba-Brzózka A. Diabetes and Cardiovascular Risk in Renal Transplant Patients. International Journal of Molecular Sciences. 2021; 22(7):3422. https://doi.org/10.3390/ijms22073422
Chicago/Turabian StyleRysz, Jacek, Beata Franczyk, Maciej Radek, Aleksandra Ciałkowska-Rysz, and Anna Gluba-Brzózka. 2021. "Diabetes and Cardiovascular Risk in Renal Transplant Patients" International Journal of Molecular Sciences 22, no. 7: 3422. https://doi.org/10.3390/ijms22073422






