Estradiol-17β Regulates Expression of Luteal DNA Methyltransferases and Genes Involved in the Porcine Corpus Luteum Function In Vivo
Abstract
:1. Introduction
2. Results
2.1. The Effects on Intrauterine Estradiol-17β Administration In Vivo on Estradiol-17β and Progesterone Levels in CLs
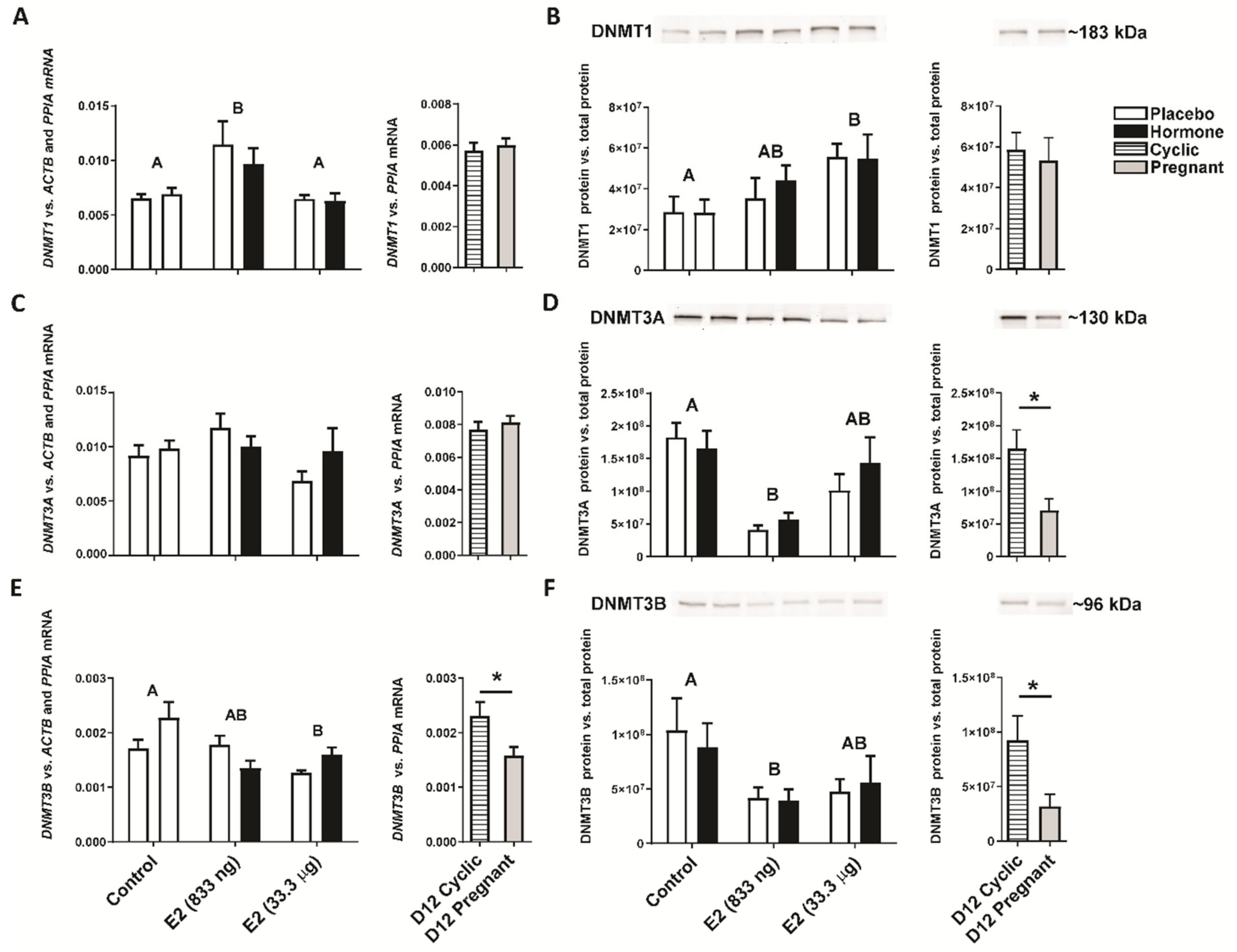
2.2. Intrauterine Infusion of E2 Affects DNMT Gene and Protein Expression In Vivo
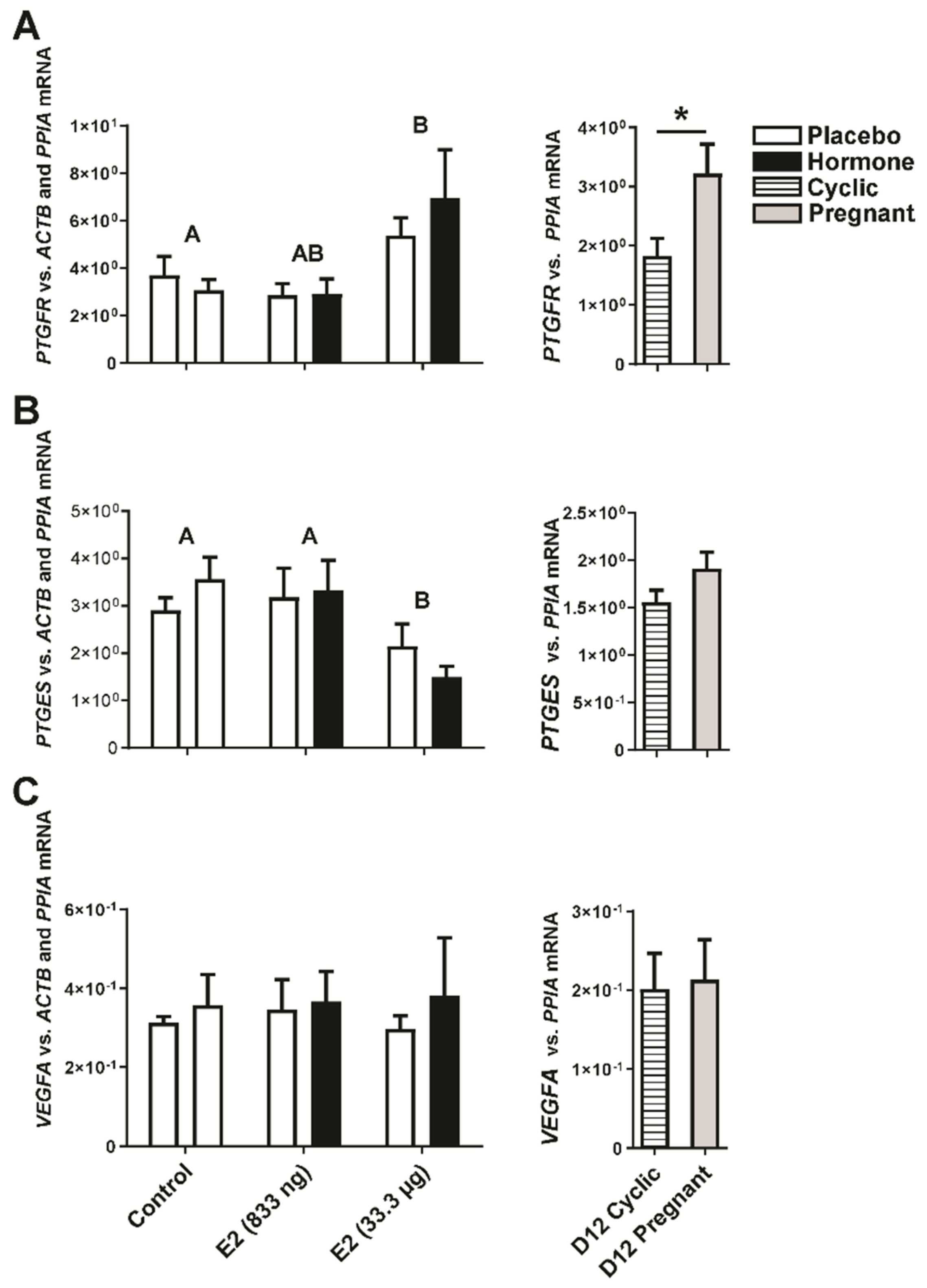
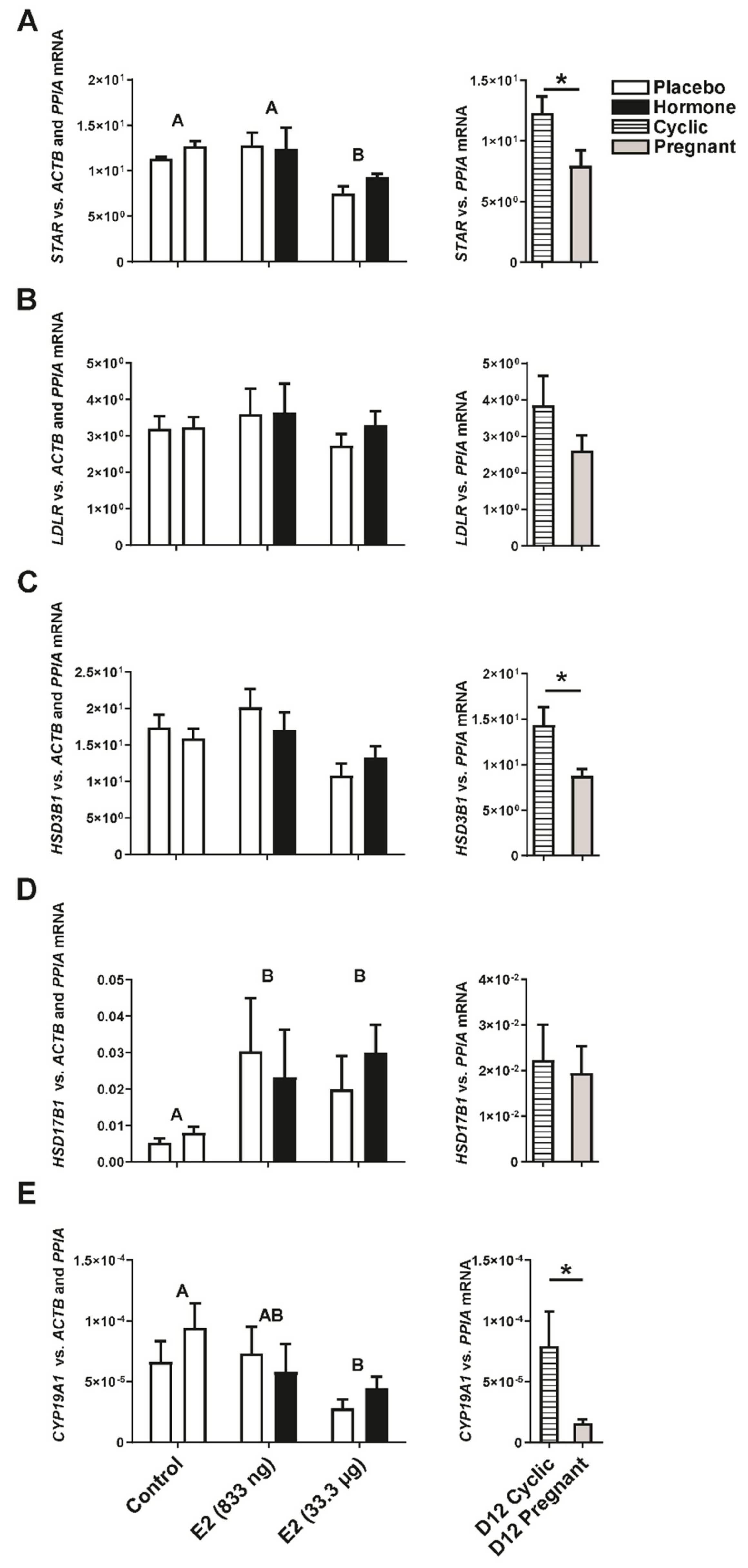
2.3. Estradiol-17β Alters Expression of Genes Involved in Luteal Function In Vivo
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.1.1. Animal Model In Vivo
4.1.2. Animal Model Ex Vivo
4.2. The Effect of E2 on Luteal Estradiol-17β and Progesterone Abundance
4.3. Western Blot Analyses
4.4. Quantitative PCR Analyses
4.4.1. Total RNA Isolation and cDNA Synthesis
4.4.2. Real-Time RT-PCR
| Gene | Primer Sequence/TaqMan® Assay ID | Reference |
|---|---|---|
| DNMT1 | Sense: 5′-TGGCGGGACCTACCAAACA-3′ Antisense: 5′-ACTTCCACGCAGGAGCAGA-3′ | [55] |
| DNMT2 | Sense: 5′-AAGAATGCCACCAAATCAGCC-3′ Antisense: 5′-AGAACTTGCCGTCTCCGAACCA-3′ | |
| DNMT3 | Sense: 5′-AGGTCTCCAGCCTCCTAAGTT-3′ Antisense: 5′-GTGTCTGAGCCATCTCCATCC-3′ | |
| ACTB | Sense: 5′-ACATCAAGGAGAAGCTCTGCTACG-3′ Antisense: 5′-GAGGGGCGATGATCTTGATCTTCA-3′ | [11] |
| GAPDH | Sense: 5′-CAGCAATGCCTCCTGTACCA-3′ Antisense: 5-GATGCCGAAGTTGTCATGGA-3′ | |
| PPIA | Sense: 5′-TAACCCCACCGTCTTCTT-3′ Antisense: 5′-TGCCATCCAACCACTCAG-3′ | |
| PTGFR | Ss03393819_s1 | [15] |
| PTGES | Ss03392129_m1 | |
| VEGFA | Ss03393993_m1 | |
| STAR | Ss03381250_u1 | |
| LDLR | Ss03373254_u1 | |
| HSD3B1 | Ss03391752_m1 | |
| HSD17B1 | Ss04245960_g1 | |
| CYP19A1 | Ss03384876_u1 | |
| PGRMC1 | Ss03392695_u1 | |
| PGRMC2 | Ss03388999_m1 | |
| NR5A1 | Ss03394295_m1 | |
| ACTB | Ss03376081_u1 | |
| GAPDH | Ss03373286_u1 | |
| PPIA | Ss03394782_g1 |
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Psychoyos, A. Uterine Receptivity for Nidation. Ann. N. Y. Acad. Sci. 1986, 476, 36–42. [Google Scholar] [CrossRef]
- Bazer, F.W.; Thatcher, W. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by the uterine endometrium. Prostaglandins 1977, 14, 397–401. [Google Scholar] [CrossRef]
- Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Kaczynski, P.; Ziecik, A.J. Embryo-maternal dialogue during pregnancy establishment and implantation in the pig. Mol. Reprod. Dev. 2017, 84, 842–855. [Google Scholar] [CrossRef] [Green Version]
- Geisert, R.D.; Zavy, M.T.; Moffatt, R.J.; Blair, R.M.; Yellin, T. Embryonic steroids and the establishment of pregnancy in pigs. J. Reprod. Fertil. Suppl. 1990, 40, 293–305. [Google Scholar]
- Spencer, T.E.; Bazer, F.W. Conceptus signals for establishment and maintenance of pregnancy. Reprod. Biol. Endocrinol. 2004, 2, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziecik, A.J.; Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Jalali, B.M.; Andronowska, A. Mechanisms for the Establishment of Pregnancy in the Pig. Reprod. Domest. Anim. 2011, 46, 31–41. [Google Scholar] [CrossRef]
- Diaz, F.J.; Wiltbank, M.C. Acquisition of Luteolytic Capacity: Changes in Prostaglandin F2α Regulation of Steroid Hormone Receptors and Estradiol Biosynthesis in Pig Corpora Lutea1. Biol. Reprod. 2004, 70, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Garverick, H.A.; Polge, C.; Flint, A.P.F. Oestradiol administration raises luteal LH receptor levels in intact and hysterectomized pigs. Reproduction 1982, 66, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conleyt, A.J.; Ford, S.P. Direct luteotrophic effect of oestradiol-17 on pig corpora lutea. Reproduction 1989, 87, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Waclawik, A.; Jabbour, H.N.; Blitek, A.; Ziecik, A.J. Estradiol-17β, Prostaglandin E2 (PGE2), and the PGE2 Receptor Are Involved in PGE2 Positive Feedback Loop in the Porcine Endometrium. Endocrinology 2009, 150, 3823–3832. [Google Scholar] [CrossRef] [Green Version]
- Kaczynski, P.; Waclawik, A. Effect of conceptus on expression of prostaglandin F2α receptor in the porcine endometrium. Theriogenology 2013, 79, 784–790. [Google Scholar] [CrossRef]
- Kaczynski, P.; Kowalewski, M.P.; Waclawik, A. Prostaglandin F2α promotes angiogenesis and embryo–maternal interactions during implantation. Reproduction 2016, 151, 539–552. [Google Scholar] [CrossRef] [Green Version]
- Wacławik, A.; Blitek, A.; Ziecik, A.J. Oxytocin and tumor necrosis factor α stimulate expression of prostaglandin E2 synthase and secretion of prostaglandin E2 by luminal epithelial cells of the porcine endometrium during early pregnancy. Reproduction 2010, 140, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacławik, A. Novel insights into the mechanisms of pregnancy establishment: Regulation of prostaglandin synthesis and signaling in the pig. Reproduction 2011, 142, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przygrodzka, E.; Kaczmarek, M.M.; Kaczyński, P.; Ziecik, A.J. Steroid hormones, prostanoids, and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction 2016, 151, 135–147. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for De Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Roberts, R.J.; Bacolla, A.; Wells, R.D. Recombinant Human DNA (Cytosine-5) Methyltransferase. J. Biol. Chem. 1999, 274, 33002–33010. [Google Scholar] [CrossRef] [Green Version]
- Davalos, V.; Martinez-Cardus, A.; Esteller, M. The Epigenomic Revolution in Breast Cancer. Am. J. Pathol. 2017, 187, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Wen, Z.; Yang, Z.; Chen, J.; Wang, F. Estrogen regulates DNA methyltransferase 3B expression in Ishikawa endometrial adenocarcinoma cells. Mol. Biol. Rep. 2009, 36, 2201–2207. [Google Scholar] [CrossRef]
- Fürbass, R.; Said, H.M.; Schwerin, M.; Vanselow, J. Chromatin structure of the bovine Cyp19 promoter 1.1. JBIC J. Biol. Inorg. Chem. 2001, 268, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Spitschak, M.; Vanselow, J. Bovine large luteal cells show increasing de novo DNA methylation of the main ovarian CYP19A1 promoter P2. Gen. Comp. Endocrinol. 2012, 178, 37–45. [Google Scholar] [CrossRef]
- Kascheike, B.; Richardivell; Walther, N. Alterations in the Chromatin Structure of the Distal Promoter Region of the Bovine Oxytocin Gene Correlate with Ovarian Expression. DNA Cell Biol. 1997, 16, 1237–1248. [Google Scholar] [CrossRef]
- Meldi, K.M.; Gaconnet, G.A.; Mayo, K.E. DNA Methylation and Histone Modifications Are Associated with Repression of the Inhibin α Promoter in the Rat Corpus Luteum. Endocrinology 2012, 153, 4905–4917. [Google Scholar] [CrossRef] [Green Version]
- Kaczynski, P.; Bauersachs, S.; Baryla, M.; Goryszewska, E.; Muszak, J.; Grzegorzewski, W.J.; Waclawik, A. Estradiol-17β-Induced Changes in the Porcine Endometrial Transcriptome In Vivo. Int. J. Mol. Sci. 2020, 21, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisert, R.D.; Zavy, M.T.; Wettemann, R.P.; Biggers, B.G. Length of pseudopregnancy and pattern of uterine protein release as influenced by time and duration of oestrogen administration in the pig. Reproduction 1987, 79, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Pusateri, A.E.; Smith, J.M.; Smith, J.W.; Thomford, P.J.; Diekman, M.A. Maternal Recognition of Pregnancy in Swine. I. Minimal Requirement for Exogenous Estradiol-17β to Induce Either Short or Long Pseudopregnancy in Cycling Gilts1. Biol. Reprod. 1996, 55, 582–589. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Witek, K.; Kaczmarek, M.; Andronowska, A.; Ziecik, A. Expression of factors associated with apoptosis in the porcine corpus luteum throughout the luteal phase of the estrous cycle and early pregnancy: Their possible involvement in acquisition of luteolytic sensitivity. Theriogenology 2015, 83, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P.; Magness, R.R.; Farley, D.B.; Van Orden, D.E. Local and Systemic Effects of Intrauterine Estradiol-17β on Luteal function of Nonpregnant Sows. J. Anim. Sci. 1982, 55, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Bökenkamp, D.; Jungblut, P.W.; Thole, H.H. The C-terminal half of the porcine estradiol receptor contains no post-translational modification: Determination of the primary structure. Mol. Cell. Endocrinol. 1994, 104, 163–172. [Google Scholar] [CrossRef]
- Lavoie, H.A.; DeSimone, D.C.; Gillio-Meina, C.; Hui, Y.Y. Cloning and Characterization of Porcine Ovarian Estrogen Receptor β Isoforms1. Biol. Reprod. 2002, 66, 616–623. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, B.F.; Nguyen, T.; Marchese, A.; Cheng, R.; Lynch, K.R.; Heng, H.H.; Kolakowski, L.F.; George, S.R. Discovery of Three Novel G-Protein-Coupled Receptor Genes. Genome 1998, 47, 310–313. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The many faces of estrogen signaling. Biochem. Medica 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Samaranayake, M.; Pradhan, S. Epigenetic mechanisms in mammals. Cell. Mol. Life Sci. 2008, 66, 596–612. [Google Scholar] [CrossRef] [Green Version]
- Waclawik, A.; Rivero-Muller, A.; Blitek, A.; Kaczmarek, M.M.; Brokken, L.J.S.; Watanabe, K.; Rahman, N.A.; Ziecik, A.J. Molecular Cloning and Spatiotemporal Expression of Prostaglandin F Synthase and Microsomal Prostaglandin E Synthase-1 in Porcine Endometrium. Endocrinology 2006, 147, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Waclawik, A.; Ziecik, A.J. Differential expression of prostaglandin (PG) synthesis enzymes in conceptus during peri-implantation period and endometrial expression of carbonyl reductase/PG 9-ketoreductase in the pig. J. Endocrinol. 2007, 194, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Nitta, A.; Shirasuna, K.; Nibuno, S.; Bollwein, H.; Shimizu, T.; Miyamoto, A. Downregulation of Lymphatic Vessel Formation Factors in PGF2^|^alpha;-induced Luteolysis in the Cow. J. Reprod. Dev. 2013, 59, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Zalman, Y.; Klipper, E.; Farberov, S.; Mondal, M.; Wee, G.; Folger, J.K.; Smith, G.W.; Meidan, R. Regulation of Angiogenesis-Related Prostaglandin F2alpha-Induced Genes in the Bovine Corpus Luteum1. Biol. Reprod. 2012, 86, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczynski, P.; Goryszewska, E.; Baryla, M.; Waclawik, A. Prostaglandin F2α stimulates angiogenesis at the embryo-maternal interface during early pregnancy in the pig. Theriogenology 2020, 142, 169–176. [Google Scholar] [CrossRef]
- Gruber, C.J.; Gruber, D.M.; Gruber, I.M.; Wieser, F.; Huber, J.C. Anatomy of the estrogen response element. Trends Endocrinol. Metab. 2004, 15, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, S.; Yan, F.; He, K.; Zhao, A. Genome-wide DNA methylation profiles of porcine ovaries in estrus and proestrus. Physiol. Genom. 2018, 50, 714–723. [Google Scholar] [CrossRef]
- Wacławik, A.; Kaczmarek, M.M.; Kowalczyk, A.; Bogacki, M.; Ziecik, A.J. Expression of prostaglandin synthesis pathway enzymes in the porcine corpus luteum during the oestrous cycle and early pregnancy. Theriogenology 2008, 70, 145–152. [Google Scholar] [CrossRef]
- Stefańczyk-Krzymowska, S.; Wąsowska, B.; Chłopek, J.; Gilun, P.; Grzegorzewski, W.; Radomski, M. Retrograde and local destination transfer of uterine prostaglandin E2 in early pregnant sow and its physiological consequences. Prostaglandins Other Lipid Mediat. 2006, 81, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Akinlosotu, B.; Diehl, J.; Gimenez, T. Sparing effects of intrauterine treatment with prostaglandin E2 on luteal function in cycling gilts. Prostaglandins 1986, 32, 291–299. [Google Scholar] [CrossRef]
- Christenson, L.; Anderson, L.; Ford, S.; Farley, D. Luteal maintenance during early pregnancy in the pig: Role for prostaglandin E2. Prostaglandins 1994, 47, 61–75. [Google Scholar] [CrossRef]
- Kallen, C.B.; Arakane, F.; Christenson, L.K.; Watari, H.; Devoto, L.; Strauss, J.F. Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Mol. Cell. Endocrinol. 1998, 145, 39–45. [Google Scholar] [CrossRef]
- An, X.; Ma, H.; Han, P.; Zhu, C.; Cao, B.; Bai, Y. Genome-wide differences in DNA methylation changes in caprine ovaries between oestrous and dioestrous phases. J. Anim. Sci. Biotechnol. 2018, 9, 85. [Google Scholar] [CrossRef]
- Nguyen, B.-L.; Chetrite, G.; Pasqualini, J.R. Transformation of estrone and estradiol in hormone-dependent and hormone-independent human breast cancer cells. Breast Cancer Res. Treat. 1995, 34, 139–146. [Google Scholar] [CrossRef]
- Pasqualini, J.R. The selective estrogen enzyme modulators in breast cancer: A review. Biochim. Biophys. Acta (BBA) Bioenerg. 2004, 1654, 123–143. [Google Scholar] [CrossRef]
- Hughes, A.L.; Powell, D.W.; Bard, M.; Eckstein, J.; Barbuch, R.; Link, A.J.; Espenshade, P.J. Dap1/PGRMC1 Binds and Regulates Cytochrome P450 Enzymes. Cell Metab. 2007, 5, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Engmann, L.; Losel, R.; Wehling, M.; Peluso, J.J. Progesterone Regulation of Human Granulosa/Luteal Cell Viability by an RU486-Independent Mechanism. J. Clin. Endocrinol. Metab. 2006, 91, 4962–4968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goryszewska, E.; Kaczynski, P.; Baryla, M.; Waclawik, A. Pleiotropic role of prokineticin 1 in the porcine endometrium during pregnancy establishment and embryo implantation. Biol. Reprod. 2021, 104, 181–196. [Google Scholar] [CrossRef]
- Diaz, F.; Crenshaw, T.; Wiltbank, M. Prostaglandin F2α Induces Distinct Physiological Responses in Porcine Corpora Lutea after Acquisition of Luteolytic Capacity1. Biol. Reprod. 2000, 63, 1504–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shen, L.; Xia, Y.; Yang, Q.; Li, X.; Tang, G.; Jiang, Y.; Wang, J.; Li, M.; Zhu, L. DNA methylation landscape of fat deposits and fatty acid composition in obese and lean pigs. Sci. Rep. 2016, 6, 35063. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog No., or Name of Source | Species Raised in Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| DNMT1 | EKDDREDKENAFKR | DNMT1 Antibody | NB100-56519 Novus Biologicals | Mouse, monoclonal | 1:1000 |
| DNMT3A | N/A | DNMT3A (D23G1) Rabbit mAb | #3598 Cell Signaling Technology | Rabbit, monoclonal | 1:300 |
| DNMT3B | RGRRSSSRLSKREVSSC | DNMT3B Antibody | orb372330 Biorbyt | Rabbit, polyclonal | 1:100 |
| Anti-mouse, secondary antibodies | N/A | Immun-Star Goat Anti-Mouse (GAM)-HRP Conjugate | Bio-Rad; 1705047 | Goat, polyclonal | 1:20 000 |
| Anti-rabbit, secondary antibodies | N/A | Immun-Star Goat Anti-Rabbit (GAM)-HRP Conjugate | Bio-Rad; 1706515 | Goat, poyclonal | 1:20 000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczynski, P.; Baryla, M.; Goryszewska, E.; Waclawik, A. Estradiol-17β Regulates Expression of Luteal DNA Methyltransferases and Genes Involved in the Porcine Corpus Luteum Function In Vivo. Int. J. Mol. Sci. 2021, 22, 3655. https://doi.org/10.3390/ijms22073655
Kaczynski P, Baryla M, Goryszewska E, Waclawik A. Estradiol-17β Regulates Expression of Luteal DNA Methyltransferases and Genes Involved in the Porcine Corpus Luteum Function In Vivo. International Journal of Molecular Sciences. 2021; 22(7):3655. https://doi.org/10.3390/ijms22073655
Chicago/Turabian StyleKaczynski, Piotr, Monika Baryla, Ewelina Goryszewska, and Agnieszka Waclawik. 2021. "Estradiol-17β Regulates Expression of Luteal DNA Methyltransferases and Genes Involved in the Porcine Corpus Luteum Function In Vivo" International Journal of Molecular Sciences 22, no. 7: 3655. https://doi.org/10.3390/ijms22073655







