Adiponectin Deregulation in Systemic Autoimmune Rheumatic Diseases
Abstract
:1. The Adiponectin Paradox
1.1. Adiponectin Expression, Structure, Isoforms and Signaling
1.2. What Affects Adiponectin Levels?
2. Adiponectin in Systemic Autoimmune Rheumatic Diseases
2.1. Rheumatoid Arthritis
2.1.1. Serum
2.1.2. Synovial Fluid
2.1.3. Cells/Tissues
2.2. Systemic Lupus Erythematosus
2.2.1. Serum
2.2.2. Urine
2.2.3. Cells
2.3. Ankylosing Spondylitis
Serum
2.4. Systemic Sclerosis
2.4.1. Serum
2.4.2. Skin
2.4.3. Other
2.5. Sjögren Syndrome
2.5.1. Serum
2.5.2. Salivary Gland and Saliva
2.6. Psoriatic Arthritis
Serum
2.7. Antiphospholipid Syndrome
Serum
3. Could SARDs Treatment Change Serum Adiponectin?
3.1. Anti-TNF
3.2. Glucocorticoids
3.3. Disease-Modifying Antirheumatic Drugs
3.4. Tocilizumab
3.5. JAK Inhibitors
3.6. Interleukin (IL)-1-Receptor Antagonists
3.7. Anti-Interleukin-17A
3.8. Cyclophosphamide
3.9. n-3 Fatty Acids
4. Divergent Changes in the Activation or Suppression of Adiponectin Gene Regulation by Transcription Factors Can Affect Its Serum Levels
4.1. PPAR-γ
4.2. Id3
4.3. ATF3
4.4. SIRT1, FoXO1 and C/EBPα
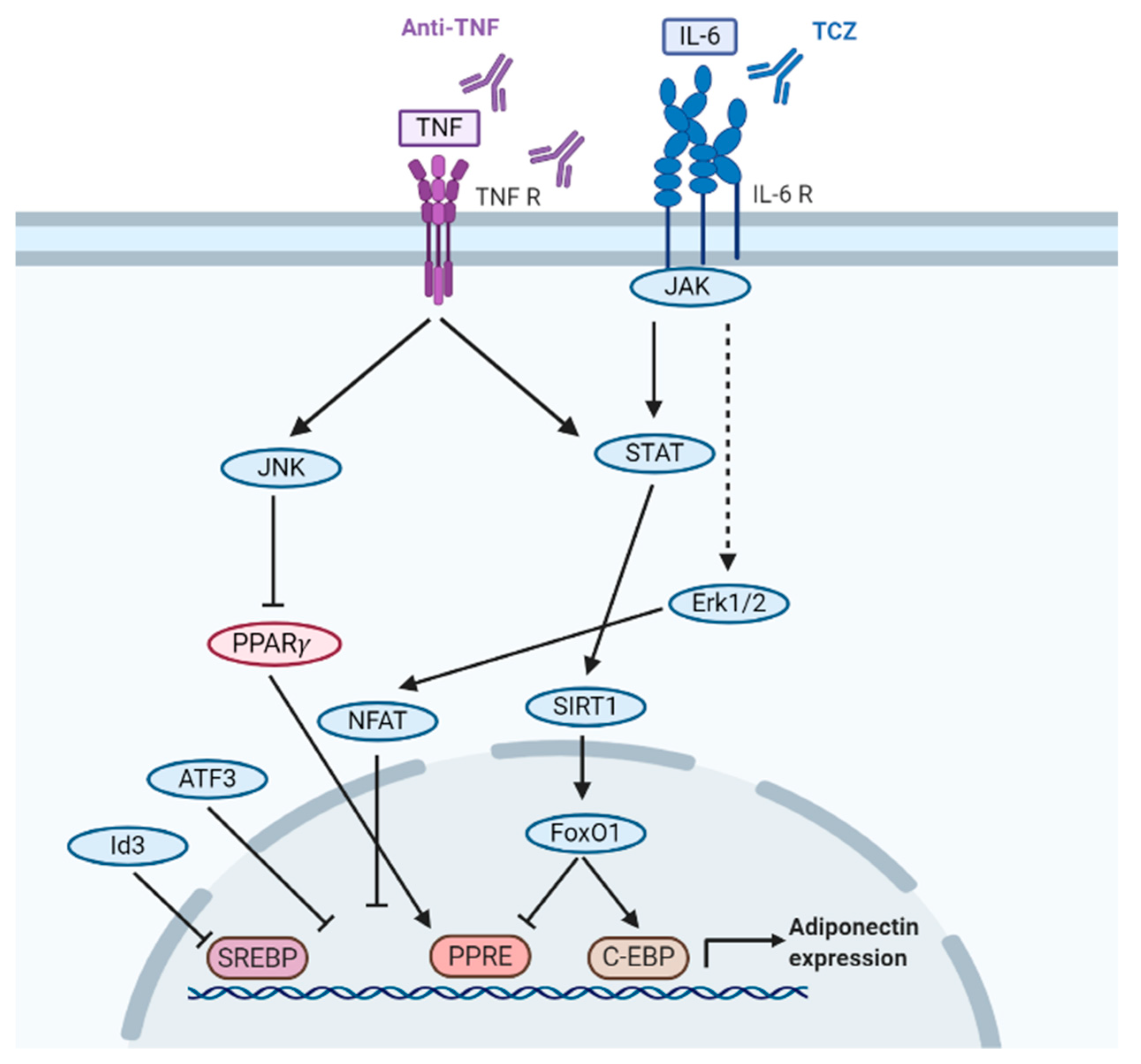
5. How Inflammatory Cytokines IL-6 and TNF-α Regulate Adiponectin Levels in SARDs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH | Aldehyde dehydrogenases |
| AMPK | AMP-activated protein kinase |
| AMT | Adipocyte mesenchymal transition |
| APS | Antiphospholipid syndrome |
| AS | Ankylosing spondylitis |
| ATF3 | Activating transcription factor 3 |
| ATV | Atorvastatin |
| CVD | Cardiovascular disease |
| CY | Cyclophosphamide |
| DAS28 | Disease Activity Score of 28 joints |
| dcSSc | Diffuse cutaneous SSc |
| DMARD | Disease-modifying antirheumatic drugs |
| dWAT | Dermal white adipose tissue |
| ESR | Erythrocyte sedimentation rate |
| FVC | Forced vital capacity |
| GC | Glucocorticoids |
| HC | Healthy controls |
| HCQ | Hydroxychloroquine |
| HMW | High molecular weight |
| IL | Interleukin |
| ILD | Interstitial lung disease |
| JAK | Janus kinase |
| lcSSc | Limited cutaneous SSc |
| LMW | Low molecular weight |
| LN | Lupus nephritis |
| MHAQ | Multidimensional Health Assessment Questionnaire |
| MMW | Medium molecular weight |
| mRSS | Modified Rodnan skin score |
| MTX | Methotrexate |
| PBMCs | Peripheral blood mononuclear cells |
| PPAR-γ | Peroxisome-proliferator-activated receptor gamma |
| PPAR-α | Peroxisome-proliferator-activated receptor alpha |
| PPRE | Peroxisome proliferator response element |
| PsA | Psoriatic arthritis |
| RA | Rheumatoid arthritis |
| RAPID3 | Routine Assessment of Patient Index Data 3 |
| RASF | RA synovial fibroblasts |
| SARDs | Systemic autoimmune rheumatic diseases |
| SGEC | Salivary glandular epithelial cells |
| SHS | Sharp-van der Heijde Score |
| SLE | Systemic lupus erythematosus |
| SLEDAI | Systemic Lupus Erythematosus Disease Activity Index |
| SMD | Standard mean difference |
| SS | Sjögren’s syndrome |
| SSA | Sulfasalazine |
| SSc | Systemic sclerosis |
| TCZ | Tocilizumab |
| Th17 | T helper 17 |
| TNF-α | Tumor necrosis factor α |
| TZD | Thiazolidinediones |
References
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzaghi, C.; Trischitta, V. Erratum. The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes 2018, 67, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.Y.; Dini, A.A.; Yang, X.K.; Li, L.J.; Wu, G.C.; Leng, R.X.; Pan, H.F.; Ye, D.Q. Association between serum/plasma adiponectin levels and immune-mediated diseases: A meta-analysis. Arch. Derm. Res. 2017, 309, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Dini, A.A.; Wang, P.; Ye, D.-Q. Serum adiponectin levels in patients with systemic lupus erythematosus: A Meta-analysis. J. Clin. Rheumatol. 2017, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-H.; Huang, X.-L.; Duan, Y.; Wang, Y.-J.; Chen, S.-Y.; Wang, J. Serum adipokines levels in patients with systemic sclerosis: A meta-analysis. Mod. Rheumatol. 2017, 27, 298–305. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.; Wu, M.; Hu, X.; Han, R.; Yuan, Y.; Wang, M.; Chen, M.; Jiang, S.; et al. Serum levels of leptin, adiponectin and resistin in patients with ankylosing spondylitis: A systematic review and meta-analysis. Int. Immunopharmacol. 2017, 52, 310–317. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int. J. Rheum. Dis. 2018, 21, 664–672. [Google Scholar] [CrossRef]
- Toussirot, E.; Binda, D.; Gueugnon, C.; Dumoulin, G. Adiponectin in autoimmune diseases. Curr. Med. Chem. 2012, 19, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Handzlik-Orlik, G.; Okopien, B. The role of adipokines in connective tissue diseases. Eur. J. Nutr. 2012, 51, 513–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotece, M.; Conde, J.; Lopez, V.; Gomez, R.; Lago, F.; Gomez Reino, J.J.; Gualillo, O. Adipokines and systemic rheumatic diseases: Linking inflammation, immunity and metabolism. In Insights and Perspectives in Rheumatology; Harrison, A., Ed.; InTech: London, UK, 2012; pp. 21–38. [Google Scholar]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Ye, J.; Bian, X.; Lim, J.; Medzhitov, R. Adiponectin and related C1q/TNF-related proteins bind selectively to anionic phospholipids and sphingolipids. Proc. Natl. Acad. Sci. USA 2020, 117, 17381–17388. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azamar-Llamas, D.; Hernandez-Molina, G.; Ramos-Avalos, B.; Furuzawa-Carballeda, J. Adipokine contribution to the pathogenesis of osteoarthritis. Mediat. Inflamm. 2017, 2017, 5468023. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, F. Transcriptional and post-translational regulation of adiponectin. Biochem. J. 2010, 425, 41–52. [Google Scholar] [CrossRef]
- Blaak, E. Gender differences in fat metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar]
- Katira, A.; Tan, P.H. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol. Med. 2016, 13, 101–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, R.B.; El Hachem, C. Adiponectin in eating disorders. Eat. Weight Disord. 2014, 19, 3–10. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Masui, Y.; Fang, F.; Korman, B.; Lord, G.; Lee, J.; Lakota, K.; Wei, J.; Scherer, P.E.; Otvos, L.; et al. Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Sci. Rep. 2017, 7, 4397. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, R.G.; Lu, T.T. The roles of dermal white adipose tissue loss in scleroderma skin fibrosis. Curr. Opin. Rheumatol. 2017, 29, 585–590. [Google Scholar] [CrossRef]
- Goldblatt, F.; O’Neill, S.G. Clinical aspects of autoimmune rheumatic diseases. Lancet 2013, 382, 797–808. [Google Scholar] [CrossRef]
- MᵃᶜDonald, I.; Liu, S.-C.; Huang, C.-C.; Kuo, S.-J.; Tsai, C.-H.; Tang, C.-H. Associations between adipokines in arthritic disease and implications for obesity. Int. J. Mol. Sci. 2019, 20, 1505. [Google Scholar] [CrossRef] [Green Version]
- Versini, M.; Jeandel, P.Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Khajoei, S.; Hassaninevisi, M.; Kianmehr, N.; Seif, F.; Khoshmirsafa, M.; Shekarabi, M.; Samei, A.; Haghighi, A. Serum levels of adiponectin and vitamin D correlate with activity of Rheumatoid Arthritis. Mol. Biol. Rep. 2019, 46, 2505–2512. [Google Scholar] [CrossRef]
- Hoffman, E.; Rahat, M.A.; Feld, J.; Elias, M.; Rosner, I.; Kaly, L.; Lavie, I.; Gazitt, T.; Zisman, D. Effects of tocilizumab, an anti-interleukin-6 receptor antibody, on serum lipid and adipokine levels in patients with rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 4633. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Xu, L.; Sun, X.; Wang, Y.; Xuan, W.; Zhang, Q.; Zhao, P.; Wu, Q.; Liu, R.; Che, N.; et al. Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro-Sanabria, J.A.; Bautista-Molano, W.; Bello-Gualtero, J.M.; Chila-Moreno, L.; Castillo, D.M.; Valle-Oñate, R.; Chalem, P.; Romero-Sánchez, C. Association of adipokines with rheumatic disease activity indexes and periodontal disease in patients with early rheumatoid arthritis and their first-degree relatives. Int. J. Rheum. Dis. 2019, 22, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- DeClercq, V.; Cui, Y.; Forbes, C.; Grandy, S.A.; Keats, M.; Parker, L.; Sweeney, E.; Yu, Z.M.; Dummer, T.J.B. Adiposity measures and plasma adipokines in females with rheumatoid and osteoarthritis. Mediat. Inflamm. 2017, 2017, 4302412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.-L.; Zhang, T.-P.; Wu, J.; Li, B.-Z.; Li, X.-M.; Pan, H.-F.; Ye, D.-Q. Association of adiponectin and adiponectin receptor gene polymorphisms with rheumatoid arthritis in a Chinese population. Postgrad. Med. J. 2020, 96, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Cansu, B.; Cansu, D.U.; Kaşifoğlu, T.; Gülbas, Z.; Korkmaz, C. Disease-modifying antirheumatic drugs increase serum adiponectin levels in patients with rheumatoid arthritis. J. Clin. Rheumatol. 2011, 17, 14–17. [Google Scholar] [CrossRef]
- Kontny, E.; Zielińska, A.; Skalska, U.; Księżopolska-Orłowska, K.; Głuszko, P.; Maśliński, W. Distinct secretory activity and clinical impact of subcutaneous abdominal adipose tissue in women with rheumatoid arthritis and osteoarthritis. Inflammation 2017, 40, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kontny, E.; Zielińska, A.; Księżopolska-Orłowska, K.; Głuszko, P. Secretory activity of subcutaneous abdominal adipose tissue in male patients with rheumatoid arthritis and osteoarthritis—Association with clinical and laboratory data. Reumatol. /Rheumatol. 2016, 5, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.; Sellam, J.; Fellahi, S.; Kotti, S.; Bastard, J.-P.; Meyer, O.; Lioté, F.; Simon, T.; Capeau, J.; Berenbaum, F. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: Results from the ESPOIR cohort. Arthritis Res. Ther. 2013, 15, R210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manrique-Arija, S.; Ureña, I.; Valdivielso, P.; Rioja, J.; Jiménez-Núñez, F.G.; Irigoyen, M.V.; Fernández-Nebro, A. Insulin resistance and levels of adipokines in patients with untreated early rheumatoid arthritis. Clin. Rheumatol. 2016, 35, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Erlandsson, M.C.; Doria Medina, R.; Töyrä Silfverswärd, S.; Bokarewa, M.I. Smoking Functions as a negative regulator of IGF1 and impairs adipokine network in patients with rheumatoid arthritis. Mediat. Inflamm. 2016, 2016, 3082820. [Google Scholar] [CrossRef] [Green Version]
- Minamino, H.; Katsushima, M.; Yoshida, T.; Hashimoto, M.; Fujita, Y.; Shirakashi, M.; Yamamoto, W.; Murakami, K.; Murata, K.; Nishitani, K.; et al. Increased circulating adiponectin is an independent disease activity marker in patients with rheumatoid arthritis: A cross-sectional study using the KURAMA database. PLoS ONE 2020, 15, e0229998. [Google Scholar] [CrossRef]
- Oranskiy, S.P.; Yeliseyeva, L.N.; Tsanaeva, A.V.; Zaytseva, N.V. Body composition and serum levels of adiponectin, vascular endothelial growth factor, and interleukin-6 in patients with rheumatoid arthritis. Croat. Med. J. 2012, 53, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, M.F.B.d.R.; de Andrade, M.V.M.; Machado, C.J.; Vieira, É.L.M.; Pinto, M.R.d.C.; Júnior, A.L.T.; Kakehasi, A.M. Leptin as an obesity marker in rheumatoid arthritis. Rheumatol. Int. 2018, 38, 1671–1677. [Google Scholar] [CrossRef]
- Zhang, Y.; Peltonen, M.; Andersson-Assarsson, J.; Svensson, P.A.; Herder, C.; Rudin, A.; Carlsson, L.; Maglio, C. Elevated adiponectin predicts the development of rheumatoid arthritis in subjects with obesity. Scand. J. Rheumatol. 2020, 49. [Google Scholar] [CrossRef]
- Ozgen, M.; Koca, S.S.; Dagli, N.; Balin, M.; Ustundag, B.; Isik, A. Serum adiponectin and vaspin levels in rheumatoid arthritis. Arch. Med. Res. 2010, 41, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Bustos Rivera-Bahena, C.; Xibillé-Friedmann, D.-X.; González-Christen, J.; Carrillo-Vázquez, S.M.; Montiel-Hernández, J.L. Peripheral blood leptin and resistin levels as clinical activity biomarkers in Mexican Rheumatoid Arthritis patients. Reumatol. Clínica 2016, 12, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Zhang, F.Z.; Xu, T.S.; Ding, R.; Li, P. Increasing production of matrix metalloproteinases, tumor necrosis factor-α, vascular endothelial growth factor and prostaglandin E2 in rheumatoid arthritis synovial fibroblasts by different adiponectin isoforms in a concentration-dependent manner. Cell. Mol. Biol. 2015, 61, 27–32. [Google Scholar] [PubMed]
- El-Hini, S.H.; Mohamed, F.I.; Hassan, A.A.; Ali, F.; Mahmoud, A.; Ibraheem, H.M. Visfatin and adiponectin as novel markers for evaluation of metabolic disturbance in recently diagnosed rheumatoid arthritis patients. Rheumatol. Int. 2013, 33, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Alkady, E.A.M.; Ahmed, H.M.; Tag, L.; Abdou, M.A. Adiponectin, resistin und visfatin in serum und gelenkflüssigkeit bei patienten mit rheumatoider arthritis: Zusammenhang mit der krankheitsaktivität. Z. Für Rheumatol. 2011, 70, 602–608. [Google Scholar] [CrossRef]
- Targońska-Stępniak, B.; Dryglewska, M.; Majdan, M. Adiponectin and leptin serum concentrations in patients with rheumatoid arthritis. Rheumatol. Int. 2010, 30, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Klein-Wieringa, I.R.; Andersen, S.N.; Herb-van Toorn, L.; Kwekkeboom, J.C.; van der Helm-van Mil, A.H.M.; Meulenbelt, I.; Huizinga, T.W.J.; Kloppenburg, M.; Toes, R.E.M.; Ioan-Facsinay, A. Are baseline high molecular weight adiponectin levels associated with radiographic progression in rheumatoid arthritis and osteoarthritis? J. Rheumatol. 2014, 41, 853–857. [Google Scholar] [CrossRef]
- Park, Y.-J.; Cho, C.-S.; Emery, P.; Kim, W.-U. LDL Cholesterolemia as a novel risk factor for radiographic progression of rheumatoid arthritis: A single-center prospective study. PLoS ONE 2013, 8, e68975. [Google Scholar] [CrossRef] [Green Version]
- Klein-Wieringa, I.R.; van der Linden, M.P.M.; Knevel, R.; Kwekkeboom, J.C.; van Beelen, E.; Huizinga, T.W.J.; van der Helm-van Mil, A.; Kloppenburg, M.; Toes, R.E.M.; Ioan-Facsinay, A. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2011, 63, 2567–2574. [Google Scholar] [CrossRef]
- Giles, J.T.; van der Heijde, D.M.; Bathon, J.M. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1562–1568. [Google Scholar] [CrossRef] [Green Version]
- Burgmaier, M.; Hoppe, S.; Krüger, T.; Mahnken, A.H.; Ketteler, M.; Reith, S.; Mühlenbruch, G.; Marx, N.; Brandenburg, V. Serum levels of C-peptide are associated with coronary artery calcification in patients with rheumatoid arthritis. Rheumatol. Int. 2015, 35, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Tsang, L.; Solomon, A.; Woodiwiss, A.J.; Millen, A.M.E.; Norton, G.R. Adiponectin and atherosclerosis in rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 358949. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Park, H.-J.; Kang, M.-I.; Lee, H.-S.; Lee, S.-W.; Lee, S.-K.; Park, Y.-B. Adipokines, inflammation, insulin resistance, and carotid atherosclerosis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R194. [Google Scholar] [CrossRef] [Green Version]
- Dessein, P.H.; Woodiwiss, A.J.; Norton, G.R.; Tsang, L.; Solomon, A. Independent associations of total and high molecular weight adiponectin with cardiometabolic risk and surrogate markers of enhanced early atherogenesis in black and white patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 2013, 15, R128. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.F.; Long, J.; Mostoufi-Moab, S.; Denburg, M.; Jorgenson, E.; Sharma, P.; Zemel, B.S.; Taratuta, E.; Ibrahim, S.; Leonard, M.B. Muscle deficits in rheumatoid arthritis contribute to inferior cortical bone structure and trabecular bone mineral density. J. Rheumatol. 2017, 44, 1777–1785. [Google Scholar] [CrossRef]
- Baker, J.F.; Von Feldt, J.M.; Mostoufi-Moab, S.; Kim, W.; Taratuta, E.; Leonard, M.B. Insulin-like growth factor 1 and adiponectin and associations with muscle deficits, disease characteristics, and treatments in rheumatoid arthritis. J. Rheumatol. 2015, 42, 2038–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chedid, P.; Hurtado-Nedelec, M.; Marion-Gaber, B.; Bournier, O.; Hayem, G.; Gougerot-Pocidalo, M.-A.; Frystyk, J.; Flyvbjerg, A.; El Benna, J.; Marie, J.-C. Adiponectin and its globular fragment differentially modulate the oxidative burst of primary human phagocytes. Am. J. Pathol. 2012, 180, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Ehling, A.; Schaffler, A.; Herfarth, H.; Tarner, I.H.; Anders, S.; Distler, O.; Paul, G.; Distler, J.; Gay, S.; Scholmerich, J.; et al. The potential of adiponectin in driving arthritis. J. Immunol. 2006, 176, 4468–4478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontny, E.; Plebanczyk, M.; Lisowska, B.; Olszewska, M.; Maldyk, P.; Maslinski, W. Comparison of rheumatoid articular adipose and synovial tissue reactivity to proinflammatory stimuli: Contribution to adipocytokine network. Ann. Rheum. Dis. 2012, 71, 262–267. [Google Scholar] [CrossRef]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D. Manifestations of systemic lupus erythematosus. Maedica 2011, 6, 330–336. [Google Scholar]
- Anders, H.-J.; Saxena, R.; Zhao, M.-h.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.; Zhou, Z.; Zhao, L.; Fei, Y.; Chen, H.; Zhang, F.; Zhang, X. Management of severe refractory systemic lupus erythematosus: Real-world experience and literature review. Clin. Rev. Allergy Immunol. 2020, 60, 17–30. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Tsai, T.Y.; Huang, Y.C. Insulin resistance and serum levels of adipokines in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Lupus 2020, 29, 1078–1084. [Google Scholar] [CrossRef]
- Diaz-Rizo, V.; Bonilla-Lara, D.; Gonzalez-Lopez, L.; Sanchez-Mosco, D.; Fajardo-Robledo, N.S.; Perez-Guerrero, E.E.; Rodriguez-Jimenez, N.A.; Saldana-Cruz, A.M.; Vazquez-Villegas, M.L.; Gomez-Banuelos, E.; et al. Serum levels of adiponectin and leptin as biomarkers of proteinuria in lupus nephritis. PLoS ONE 2017, 12, e0184056. [Google Scholar] [CrossRef] [Green Version]
- Hutcheson, J.; Ye, Y.; Han, J.; Arriens, C.; Saxena, R.; Li, Q.Z.; Mohan, C.; Wu, T. Resistin as a potential marker of renal disease in lupus nephritis. Clin. Exp. Immunol. 2015, 179, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, F.M.; Telles, R.W.; Lanna, C.C.; Teixeira, A.L., Jr.; Miranda, A.S.; Rocha, N.P.; Ribeiro, A.L. Adipokines, tumor necrosis factor and its receptors in female patients with systemic lupus erythematosus. Lupus 2017, 26, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Montecucco, F.; Poggi, A.; Nobili, F.; Cacciapaglia, F.; Afeltra, A.; Moccetti, T.; Colombo, B.M. Serum adiponectin levels are associated with presence of carotid plaque in women with systemic lupus erythematosus. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1147–1151. [Google Scholar] [CrossRef]
- Gronwall, C.; Reynolds, H.; Kim, J.K.; Buyon, J.; Goldberg, J.D.; Clancy, R.M.; Silverman, G.J. Relation of carotid plaque with natural IgM antibodies in patients with systemic lupus erythematosus. Clin. Immunol. 2014, 153, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, H.R.; Buyon, J.; Kim, M.; Rivera, T.L.; Izmirly, P.; Tunick, P.; Clancy, R.M. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis 2010, 210, 569–574. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Skaggs, B.J.; Sahakian, L.; Grossman, J.; FitzGerald, J.; Ragavendra, N.; Charles-Schoeman, C.; Chernishof, M.; Gorn, A.; Witztum, J.L.; et al. High plasma leptin levels confer increased risk of atherosclerosis in women with systemic lupus erythematosus, and are associated with inflammatory oxidised lipids. Ann. Rheum. Dis. 2011, 70, 1619–1624. [Google Scholar] [CrossRef]
- McMahon, M.; Skaggs, B.J.; Grossman, J.M.; Sahakian, L.; Fitzgerald, J.; Wong, W.K.; Lourenco, E.V.; Ragavendra, N.; Charles-Schoeman, C.; Gorn, A.; et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrycek, E.; Banasiewicz-Szkróbka, I.; Żurakowski, A.; Dworak, J.; Błaszczak, E.; Franek, A.; Buszman, P. Selected adipokines and thickness of the intima-media complex in patients with systemic lupus erythematosus. Kardiol. Pol. 2018, 76, 917–919. [Google Scholar] [CrossRef] [Green Version]
- Vadacca, M.; Zardi, E.M.; Margiotta, D.; Rigon, A.; Cacciapaglia, F.; Arcarese, L.; Buzzulini, F.; Amoroso, A.; Afeltra, A. Leptin, adiponectin and vascular stiffness parameters in women with systemic lupus erythematosus. Intern. Emerg. Med. 2013, 8, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Loghman, M.; Haghighi, A.; Broumand, B.; Ataipour, Y.; Tohidi, M.; Marzbani, C.; Fakharran, M. Association between urinary adiponectin level and renal involvement in systemic lupus erythematous. Int. J. Rheum. Dis. 2016, 19, 678–684. [Google Scholar] [CrossRef]
- Landolt-Marticorena, C.; Prokopec, S.D.; Morrison, S.; Noamani, B.; Bonilla, D.; Reich, H.; Scholey, J.; Avila-Casado, C.; Fortin, P.R.; Boutros, P.C.; et al. A discrete cluster of urinary biomarkers discriminates between active systemic lupus erythematosus patients with and without glomerulonephritis. Arthritis Res. 2016, 18, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tao, Y.; Liu, Y.; Zhao, Y.; Song, C.; Zhou, B.; Wang, T.; Gao, L.; Zhang, L.; Hu, H. Rapid detection of urinary soluble intercellular adhesion molecule-1 for determination of lupus nephritis activity. Medicine 2018, 97, e11287. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.I.; Bennett, M.R.; Abulaban, K.; Klein-Gitelman, M.S.; O’Neil, K.M.; Tucker, L.; Ardoin, S.P.; Rouster-Stevens, K.A.; Onel, K.B.; Singer, N.G.; et al. Development of a novel renal activity index of lupus nephritis in children and young adults: Noninvasive measurement of lupus nephritis activity. Arthritis Care Res. 2016, 68, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Gulati, G.; Bennett, M.R.; Abulaban, K.; Song, H.; Zhang, X.; Ma, Q.; Brodsky, S.V.; Nadasdy, T.; Haffner, C.; Wiley, K.; et al. Prospective validation of a novel renal activity index of lupus nephritis. Lupus 2017, 26, 927–936. [Google Scholar] [CrossRef]
- Bennett, M.R.; Ma, Q.; Ying, J.; Devarajan, P.; Brunner, H. Effects of age and gender on reference levels of biomarkers comprising the pediatric Renal Activity Index for Lupus Nephritis (p-RAIL). Pediatr. Rheumatol. 2017, 15, 74. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.-P.; Zhao, Y.-L.; Li, X.-M.; Wu, C.-H.; Pan, H.-F.; Ye, D.-Q. Altered mRNA expression levels of vaspin and adiponectin in peripheral blood mononuclear cells of systemic lupus erythematosus patients. Clin. Exp. Rheumatol. 2019, 37, 458–464. [Google Scholar]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing spondylitis: Etiology, pathogenesis, and treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Lee, S.-G.; Jeon, Y.-K.; Park, E.-K.; Suh, Y.-S.; Kim, H.-O. Relationship between serum adipokine levels and radiographic progression in patients with ankylosing spondylitis: A preliminary 2-year longitudinal study. Medicine 2017, 96, e7854. [Google Scholar] [CrossRef]
- Syrbe, U.; Callhoff, J.; Conrad, K.; Poddubnyy, D.; Haibel, H.; Junker, S.; Frommer, K.W.; Müller-Ladner, U.; Neumann, E.; Sieper, J. Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression: Adipokines in ankylosing spondylitis. Arthritis Rheumatol. 2015, 67, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Hartl, A.; Sieper, J.; Syrbe, U.; Listing, J.; Hermann, K.-G.; Rudwaleit, M.; Poddubnyy, D. Serum levels of leptin and high molecular weight adiponectin are inversely associated with radiographic spinal progression in patients with ankylosing spondylitis: Results from the ENRADAS trial. Arthritis Res. Ther. 2017, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic sclerosis. Nat. Rev. Dis. Primers 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Tomčík, M.; Arima, K.; Hulejová, H.; Kuklová, M.; Filková, M.; Braun, M.; Beláček, J.; Novák, M.; Bečvář, R.; Vencovský, J.; et al. Adiponectin relation to skin changes and dyslipidemia in systemic sclerosis. Cytokine 2012, 58, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Meta-analysis of circulating adiponectin, leptin, and resistin levels in systemic sclerosis. Z. Rheumatol. 2017, 76, 789–797. [Google Scholar] [CrossRef]
- Stochmal, A.; Czuwara, J.; Zaremba, M.; Rudnicka, L. Altered serum level of metabolic and endothelial factors in patients with systemic sclerosis. Arch. Dermatol. Res. 2020, 312, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Jakubus, M.; Sawicka, K.; Potembska, E.; Kowal, M.; Krasowska, D. Clinical associations of serum leptin and leptin/adiponectin ratio in systemic sclerosis. Adv. Dermatol. Allergol. 2019, 36, 325–328. [Google Scholar] [CrossRef]
- Korman, B.D.; Marangoni, R.G.; Hinchcliff, M.; Shah, S.J.; Carns, M.; Hoffmann, A.; Ramsey-Goldman, R.; Varga, J. Brief report: Association of elevated adipsin levels with pulmonary arterial hypertension in systemic sclerosis: ELEVATED ADIPSIN LEVELS IN SSc-RELATED PAH. Arthritis Rheumatol. 2017, 69, 2062–2068. [Google Scholar] [CrossRef]
- Zuo, W.; Wu, Z.-H.; Wu, N.; Duan, Y.-H.; Wu, J.-T.; Wang, H.; Qiu, G.-X. Adiponectin receptor 1 mediates the difference in adiponectin- induced prostaglandin E2 production in rheumatoid arthritis and osteoarthritis synovial fibroblasts. Chin. Med. J. 2011, 124, 3919–3924. [Google Scholar]
- Bellocchi, C.; Ying, J.; Goldmuntz, E.A.; Keyes-Elstein, L.; Varga, J.; Hinchcliff, M.E.; Lyons, M.A.; McSweeney, P.; Furst, D.E.; Nash, R.; et al. Large-scale characterization of systemic sclerosis serum protein profile: Comparison to peripheral blood cell transcriptome and correlations with skin/lung fibrosis. Arthritis Rheumatol. 2020, 73, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Olewicz-Gawlik, A.; Danczak-Pazdrowska, A.; Kuznar-Kaminska, B.; Batura-Gabryel, H.; Katulska, K.; Wojciech, S.; Trzybulska, D.; Hrycaj, P. Circulating adipokines and organ involvement in patients with systemic sclerosis. Acta Reum. Port. 2015, 40, 156–162. [Google Scholar]
- Lakota, K.; Wei, J.; Carns, M.; Hinchcliff, M.; Lee, J.; Whitfield, M.L.; Sodin-Semrl, S.; Varga, J. Levels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: Potential utility as a biomarker? Arthritis Res. Ther. 2012, 14, R102. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, H.; Jinnin, M.; Muchemwa, F.C.; Makino, T.; Kajihara, I.; Makino, K.; Honda, N.; Sakai, K.; Fukushima, S.; Ihn, H. Adiponectin expression is decreased in the involved skin and sera of diffuse cutaneous scleroderma patients: Letter to the Editor. Exp. Dermatol. 2011, 20, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Masui, Y.; Asano, Y.; Shibata, S.; Noda, S.; Aozasa, N.; Akamata, K.; Yamada, D.; Tamaki, Z.; Tada, Y.; Sugaya, M.; et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis: Significance of serum adiponectin levels in SSc. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Winsz-Szczotka, K.; Kuźnik-Trocha, K.; Komosińska-Vassev, K.; Kucharz, E.; Kotulska, A.; Olczyk, K. Relationship between adiponectin, leptin, IGF-1 and total lipid peroxides plasma concentrations in patients with systemic sclerosis: Possible role in disease development. Int. J. Rheum. Dis. 2016, 19, 706–714. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.; Varga, J. Adipocytic progenitor cells give rise to pathogenic myofibroblasts: Adipocyte-to-mesenchymal transition and its emerging role in fibrosis in multiple organs. Curr. Rheumatol. Rep. 2020, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Lepper, N.; Vasile, M.; Riccieri, V.; Peters, M.; Meier, F.; Hülser, M.-L.; Distler, O.; Gay, S.; Mahavadi, P.; et al. Adipokine expression in systemic sclerosis lung and gastrointestinal organ involvement. Cytokine 2019, 117, 41–49. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Tenta, R.; Skopouli, F.N. Autoimmune epithelitis (Sjögren’s syndrome); the impact of metabolic status of glandular epithelial cells on auto-immunogenicity. J. Autoimmun. 2019, 104, 102335. [Google Scholar] [CrossRef]
- Augusto, K.L.; Bonfa, E.; Pereira, R.M.R.; Bueno, C.; Leon, E.P.; Viana, V.S.T.; Pasoto, S.G. Metabolic syndrome in Sjögren’s syndrome patients: A relevant concern for clinical monitoring. Clin. Rheumatol. 2016, 35, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Zinkevičienė, A.; Dumalakienė, I.; Mieliauskaitė, D.; Vilienė, R.; Narkevičiūtė, I.; Girkontaitė, I. sICAM-1 as potential additional parameter in the discrimination of the Sjögren syndrome and non-autoimmune sicca syndrome: A pilot study. Clin. Rheumatol. 2019, 38, 2803–2809. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Tenta, R.; Skopouli, F.N. Activation of AMP-activated protein kinase (AMPK) by adiponectin rescues salivary gland epithelial cells from spontaneous and IFNγ-induced apoptosis. Arthritis Rheum. 2010, 62, 414–419. [Google Scholar]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2019, 23, 1407–1414. [Google Scholar] [CrossRef]
- Kumthekar, A.; Ogdie, A. Obesity and psoriatic arthritis: A narrative review. Rheumatol. Ther. 2020, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dikbas, O.; Tosun, M.; Bes, C.; Tonuk, S.B.; Aksehirli, O.Y.; Soy, M. Serum levels of visfatin, resistin and adiponectin in patients with psoriatic arthritis and associations with disease severity. Int. J. Rheum. Dis. 2016, 19, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Jiang, L.; Cheng, Q.; Chen, H.; Yu, Y.; Lin, Y.; Yang, X.; Kong, N.; Zhu, X.; Xu, X.; et al. Adipokines in psoriatic arthritis patients: The correlations with osteoclast precursors and bone erosions. PLoS ONE 2012, 7, e46740. [Google Scholar] [CrossRef] [Green Version]
- Eder, L.; Jayakar, J.; Pollock, R.; Pellett, F.; Thavaneswaran, A.; Chandran, V.; Rosen, C.F.; Gladman, D.D. Serum adipokines in patients with psoriatic arthritis and psoriasis alone and their correlation with disease activity. Ann. Rheum. Dis. 2013, 72, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.; Nissan, S.; Eder, L.; Rahat, M.A.; Elias, M.; Rimar, D.; Laor, A.; Bitterman, H.; Zisman, D. Increased prevalence of metabolic syndrome and adipocytokine levels in a psoriatic arthritis cohort. J. Clin. Rheumatol. 2018, 24, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Abji, F.; Perruccio, A.V.; Gandhi, R.; Li, S.; Cook, R.J.; Gladman, D.D. Serum-based soluble markers differentiate psoriatic arthritis from osteoarthritis. Ann. Rheum. Dis. 2019, 78, 796–801. [Google Scholar] [CrossRef]
- Negrini, S.; Pappalardo, F.; Murdaca, G.; Indiveri, F.; Puppo, F. The antiphospholipid syndrome: From pathophysiology to treatment. Clin. Exp. Med. 2017, 17, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.E.M.; Vendramini, M.B.; Bueno, C.; Bonfá, E.; de Carvalho, J.F. Adipocytokines in primary antiphospholipid syndrome: Potential markers of low-grade inflammation, insulin resistance and metabolic syndrome. Clin. Exp. Rheumatol. 2012, 30, 871–878. [Google Scholar] [PubMed]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Colia, R.; Rotondo, C.; Sanpaolo, E.; Cantatore, F.P. Changes in serum adipokines profile and insulin resistance in patients with rheumatoid arthritis treated with anti-TNF-α. Curr. Med. Res. Opin. 2019, 35, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Engvall, I.-L.; Tengstrand, B.; Brismar, K.; Hafström, I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: A randomised study over 21 months. Arthritis Res. Ther. 2010, 12, R197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Choi, H.-M.; Ji, H.-I.; Song, R.; Yang, H.-I.; Lee, S.-K.; Yoo, M.C.; Park, Y.-B. Serum adipokine levels in rheumatoid arthritis patients and their contributions to the resistance to treatment. Mol. Med. Rep. 2014, 9, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Ferraz-Amaro, I.; Arce-Franco, M.; Muñiz, J.; López-Fernández, J.; Hernández-Hernández, V.; Franco, A.; Quevedo, J.; Martínez-Martín, J.; Díaz-González, F. Systemic blockade of TNF-α does not improve insulin resistance in humans. Horm. Metab. Res. 2011, 43, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, R.; Herenius, M.M.J.; Wijbrandts, C.A.; de Jager, W.; van Tuyl, L.H.; Nurmohamed, M.T.; Prakken, B.J.; Gerlag, D.M.; Tak, P.P. Treatment-specific changes in circulating adipocytokines: A comparison between tumour necrosis factor blockade and glucocorticoid treatment for rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 1510–1516. [Google Scholar] [CrossRef]
- Peters, M.J.L.; Watt, P.; Cherry, L.; Welsh, P.; Henninger, E.; Dijkmans, B.A.C.; McInnes, I.B.; Nurmohamed, M.T.; Sattar, N. Lack of effect of TNF blockade therapy on circulating adiponectin levels in patients with autoimmune disease: Results from two independent prospective studies. Ann. Rheum. Dis. 2010, 69, 1687–1690. [Google Scholar] [CrossRef]
- Virone, A.; Bastard, J.-P.; Fellahi, S.; Capeau, J.; Rouanet, S.; Sibilia, J.; Ravaud, P.; Berenbaum, F.; Gottenberg, J.-E.; Sellam, J. Comparative effect of tumour necrosis factor inhibitors versus other biological agents on cardiovascular risk-associated biomarkers in patients with rheumatoid arthritis. RMD Open 2019, 5, e000897. [Google Scholar] [CrossRef] [Green Version]
- Toussirot, É.; Mourot, L.; Dehecq, B.; Wendling, D.; Grandclément, É.; Dumoulin, G. TNFα blockade for inflammatory rheumatic diseases is associated with a significant gain in android fat mass and has varying effects on adipokines: A 2-year prospective study. Eur. J. Nutr. 2014, 53, 951–961. [Google Scholar] [CrossRef]
- Sikorska, D.; Rutkowski, R.; Łuczak, J.; Samborski, W.; Witowski, J. Serum adiponectin as a predictor of laboratory response to anti-TNF-α therapy in rheumatoid arthritis. Cent. Eur. J. Immunol. 2018, 43, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Ursini, F.; Cipriani, P.; Greco, M.; Alvaro, S.; Vasiliki, L.; Di Benedetto, P.; Carubbi, F.; Berardicurti, O.; Gulletta, E.; et al. IL-1 inhibition improves insulin resistance and adipokines in rheumatoid arthritis patients with comorbid type 2 diabetes: An observational study. Medicine 2019, 98, e14587. [Google Scholar] [CrossRef]
- Wagner, C.L.; Visvanathan, S.; Elashoff, M.; McInnes, I.B.; Mease, P.J.; Krueger, G.G.; Murphy, F.T.; Papp, K.; Gomez-Reino, J.J.; Mack, M.; et al. Markers of inflammation and bone remodelling associated with improvement in clinical response measures in psoriatic arthritis patients treated with golimumab. Ann. Rheum. Dis. 2013, 72, 83–88. [Google Scholar] [CrossRef]
- Derdemezis, C.S.; Filippatos, T.D.; Voulgari, P.V.; Tselepis, A.D.; Drosos, A.A.; Kiortsis, D.N. Leptin and adiponectin levels in patients with ankylosing spondylitis. The effect of infliximab treatment. Clin. Exp. Rheumatol. 2010, 28, 880–883. [Google Scholar]
- Miranda-Filloy, J.A.; López-Mejias, R.; Genre, F.; Carnero-López, B.; Ochoa, R.; Diaz de Terán, T.; González-Juanatey, C.; Blanco, R.; Llorca, J.; González-Gay, M.A. Adiponectin and resistin serum levels in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clin. Exp. Rheumatol. 2013, 31, 365–371. [Google Scholar] [PubMed]
- Shapiro, L.; Scherer, P.E. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 1998, 8, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Kishore, U.; Gaboriaud, C.; Waters, P.; Shrive, A.K.; Greenhough, T.J.; Reid, K.B.M.; Sim, R.B.; Arlaud, G.J. C1q and tumor necrosis factor superfamily: Modularity and versatility. Trends Immunol. 2004, 25, 551–561. [Google Scholar] [CrossRef]
- Frommer, K.W.; Schäffler, A.; Büchler, C.; Steinmeyer, J.; Rickert, M.; Rehart, S.; Brentano, F.; Gay, S.; Müller-Ladner, U.; Neumann, E. Adiponectin isoforms: A potential therapeutic target in rheumatoid arthritis? Ann. Rheum. Dis. 2012, 71, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Hua, C.; Buttgereit, F.; Combe, B. Glucocorticoids in rheumatoid arthritis: Current status and future studies. RMD Open 2020, 6, e000536. [Google Scholar] [CrossRef] [Green Version]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; DuBois, D.C.; Jusko, W.J.; Almon, R.R. Glucocorticoid effects on adiponectin expression. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2012; Volume 90, pp. 163–186. [Google Scholar]
- Yaşar Bilge, N.Ş.; Kaşifoğlu, N.; Kaşifoğlu, T.; Şahin, F.; Gönüllü, E.; Korkmaz, C. The role of methotrexate and low-dose prednisolone on adiponectine levels and insulin resistance in patients with rheumatoid arthritis naïve to disease-modifying antirheumatic drugs. Int. J. Rheum. Dis. 2016, 19, 665–671. [Google Scholar] [CrossRef] [PubMed]
- El-Barbary, A.M.; Hussein, M.S.; Rageh, E.M.; Hamouda, H.E.; Wagih, A.A.; Ismail, R.G. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J. Rheumatol. 2011, 38, 229–235. [Google Scholar] [CrossRef]
- The Signaling Pathways Project. Available online: https://www.signalingpathways.org/index.jsf (accessed on 23 November 2020).
- Xibillé-Friedmann, D.-X.; Ortiz-Panozo, E.; Bustos Rivera-Bahena, C.; Sandoval-Ríos, M.; Hernández-Góngora, S.-E.; Dominguez-Hernandez, L.; Montiel-Hernández, J.-L. Leptin and adiponectin as predictors of disease activity in rheumatoid arthritis. Clin. Exp. Rheumatol. 2015, 33, 471–477. [Google Scholar]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheumatoid arthritis: Past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, Y.-A.; Ji, H.-I.; Song, R.; Kim, J.Y.; Lee, S.-H.; Hong, S.-J.; Yoo, M.C.; Yang, H.-I. Increased expression of endocan in arthritic synovial tissues: Effects of adiponectin on the expression of endocan in fibroblast-like synoviocytes. Mol. Med. Rep. 2015, 11, 2695–2702. [Google Scholar] [CrossRef] [Green Version]
- Fioravanti, A.; Tenti, S.; Bacarelli, M.R.; Damiani, A.; Li Gobbi, F.; Bandinelli, F.; Cheleschi, S.; Galeazzi, M.; Benucci, M. Tocilizumab modulates serum levels of adiponectin and chemerin in patients with rheumatoid arthritis: Potential cardiovascular protective role of IL-6 inhibition. Clin. Exp. Rheumatol. 2019, 37, 293–300. [Google Scholar]
- Choi, I.A.; Sagawa, A.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Tocilizumab Increases body weight and serum adipokine levels in patients with rheumatoid arthritis independently of their treatment response: A retrospective cohort study. J. Korean Med. Sci. 2020, 35, e155. [Google Scholar] [CrossRef] [PubMed]
- Kubler, P. Experimental and clinical pharmacology: Janus kinase inhibitors: Mechanisms of action. Aust. Prescr. 2014, 37, 154–157. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Perillo, F.; Metella Refini, R.; Bergantini, L.; Bellisai, F.; Selvi, E.; Cameli, P.; Manganelli, S.; Conticini, E.; Cantarini, L.; et al. Efficacy of baricitinib in treating rheumatoid arthritis: Modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int. Immunopharmacol. 2020, 86, 106748. [Google Scholar] [CrossRef]
- Jandus, C.; Bioley, G.; Rivals, J.-P.; Dudler, J.; Speiser, D.; Romero, P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008, 58, 2307–2317. [Google Scholar] [CrossRef]
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat. Commun. 2015, 6, 7687. [Google Scholar] [CrossRef] [Green Version]
- Piccio, L.; Cantoni, C.; Henderson, J.G.; Hawiger, D.; Ramsbottom, M.; Mikesell, R.; Ryu, J.; Hsieh, C.-S.; Cremasco, V.; Haynes, W.; et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis: Immunomodulation. Eur. J. Immunol. 2013, 43, 2089–2100. [Google Scholar] [CrossRef] [Green Version]
- Fassio, A.; Gatti, D.; Gisondi, P.; Girolomoni, G.; Viapiana, O.; Giollo, A.; Zamboni, M.; Rossini, M.; Idolazzi, L. Effects of secukinumab on serum adipocytokines: Preliminary data. Reumatismo 2017, 69, 105. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.G.; Tilby, M.J. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992, 6, 163–173. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef]
- GTEx Portal. Available online: https://gtexportal.org/home/gene/ALDH1A1 (accessed on 15 November 2020).
- Available online: https://www.gtexportal.org/home/gene/PPARG (accessed on 15 November 2020).
- Huscher, D.; Siegert, E.; Allanore, Y.; Czirják, L.; DelGaldo, F.; Denton, C.P.; Distler, O.; Frerix, M.; Matucci-Cerinic, M.; EUSTAR co-workers on behalf of the DeSScipher Project Research Group within the EUSTAR Network; et al. Systemic sclerosis associated interstitial lung disease—Individualized immunosuppressive therapy and course of lung function: Results of the EUSTAR group. Arthritis Res. Ther. 2018, 20, 17. [Google Scholar]
- Tashkin, D.P.; Elashoff, R.; Clements, P.J.; Goldin, J.; Roth, M.D.; Furst, D.E.; Arriola, E.; Silver, R.; Strange, C.; Bolster, M.; et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 2006, 354, 2655–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masui, Y.; Asano, Y.; Takahashi, T.; Shibata, S.; Akamata, K.; Aozasa, N.; Noda, S.; Taniguchi, T.; Ichimura, Y.; Toyama, T.; et al. Clinical significance of monitoring serum adiponectin levels during intravenous pulse cyclophosphamide therapy in interstitial lung disease associated with systemic sclerosis. Mod. Rheumatol. 2013, 23, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.; Unal, R.; Tripathi, P.; Pokrovskaya, I.; Owens, R.J.; Kern, P.A.; Ranganathan, G. Adiponectin translation is increased by the PPARγ agonists pioglitazone and ω-3 fatty acids. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E480–E489. [Google Scholar] [CrossRef] [Green Version]
- Bernatsky, S.; Boivin, J.F.; Joseph, L.; Manzi, S.; Ginzler, E.; Gladman, D.D.; Urowitz, M.; Fortin, P.R.; Petri, M.; Barr, S.; et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2550–2557. [Google Scholar] [CrossRef]
- Curado Borges, M.; De Miranda Moura Dos Santos, F.; Weiss Telles, R.; Melo De Andrade, M.V.; Toulson Davisson Correia, M.I.; Lanna, C.C.D. Omega-3 fatty acids, inflammatory status and biochemical markers of patients with systemic lupus erythematosus: A pilot study. Rev. Bras. Reumatol. 2017, 57, 526–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozovoy, M.; Simão, A.; Morimoto, H.; Scavuzzi, B.; Iriyoda, T.; Reiche, E.; Cecchini, R.; Dichi, I. Fish oil N-3 fatty acids increase adiponectin and decrease leptin levels in patients with systemic lupus erythematosus. Mar. Drugs 2015, 13, 1071–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedenko, L.; Lamina, C.; Kiesslich, T.; Kapur, K.; Bergmann, S.; Waterworth, D.; Heid, I.M.; Wichmann, H.E.; Kedenko, I.; Kronenberg, F.; et al. Genetic polymorphisms of the main transcription factors for adiponectin gene promoter in regulation of adiponectin levels: Association analysis in three european cohorts. PLoS ONE 2012, 7, e52497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARgamma and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun 2020, 113, 102510. [Google Scholar] [CrossRef]
- Li, X.F.; Sun, Y.Y.; Bao, J.; Chen, X.; Li, Y.H.; Yang, Y.; Zhang, L.; Huang, C.; Wu, B.M.; Meng, X.M.; et al. Functional role of PPAR-gamma on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Sci. Rep. 2017, 7, 12671. [Google Scholar] [CrossRef] [Green Version]
- Oxer, D.S.; Godoy, L.C.; Borba, E.; Lima-Salgado, T.; Passos, L.A.; Laurindo, I.; Kubo, S.; Barbeiro, D.F.; Fernandes, D.; Laurindo, F.R.; et al. PPARgamma expression is increased in systemic lupus erythematosus patients and represses CD40/CD40L signaling pathway. Lupus 2011, 20, 575–587. [Google Scholar] [CrossRef]
- Zhao, W.; Berthier, C.C.; Lewis, E.E.; McCune, W.J.; Kretzler, M.; Kaplan, M.J. The peroxisome-proliferator activated receptor-gamma agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin. Immunol. 2013, 149, 119–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aprahamian, T.R.; Bonegio, R.G.; Weitzner, Z.; Gharakhanian, R.; Rifkin, I.R. Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus. Immunology 2014, 142, 363–373. [Google Scholar] [CrossRef] [PubMed]
- FDA. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/medication-guides (accessed on 13 November 2020).
- Mallano, T.; Palumbo-Zerr, K.; Zerr, P.; Ramming, A.; Zeller, B.; Beyer, C.; Dees, C.; Huang, J.; Hai, T.; Distler, O.; et al. Activating transcription factor 3 regulates canonical TGFbeta signalling in systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korman, B.; Marangoni, R.G.; Lord, G.; Olefsky, J.; Tourtellotte, W.; Varga, J. Adipocyte-specific repression of PPAR-gamma by NCoR contributes to scleroderma skin fibrosis. Arthritis Res. 2018, 20, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmouni, K.; Sigmund, C.D. Id3, E47, and SREBP-1c: Fat factors controlling adiponectin expression. Circ. Res. 2008, 103, 565–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindahl, G.E.; Stock, C.J.; Shi-Wen, X.; Leoni, P.; Sestini, P.; Howat, S.L.; Bou-Gharios, G.; Nicholson, A.G.; Denton, C.P.; Grutters, J.C.; et al. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir Res. 2013, 14, 80. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Li, H.; Han, M.; Xu, T.; Wu, X.; Zhuang, Y. Modeling Sjögren’s syndrome with Id3 conditional knockout mice. 2011, 135, 34–42. Immunol. Lett. 2011, 135, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, D.; Yamaguchi, A.; Tsuchiya, N.; Yamamoto, K.; Tokunaga, K. Expression of ID family genes in the synovia from patients with rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2001, 284, 436–442. [Google Scholar] [CrossRef]
- Liu, C.; Yu, S.; Jin, R.; Long, Y.; Lu, S.; Song, Y.; Sun, X.; Sun, X.H.; Zhang, Y. Correlation of the levels of DNA-binding inhibitor Id3 and regulatory T cells with SLE disease severity. J. Autoimmun. 2020, 113, 102498. [Google Scholar] [CrossRef]
- Hai, T.; Wolford, C.C.; Chang, Y.S. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: Is modulation of inflammation a unifying component? Gene Expr. 2010, 15, 1–11. [Google Scholar] [CrossRef]
- Engler, A.; Tange, C.; Frank-Bertoncelj, M.; Gay, R.E.; Gay, S.; Ospelt, C. Regulation and function of SIRT1 in rheumatoid arthritis synovial fibroblasts. J. Mol. Med. 2016, 94, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivares, D.; Perez-Hernandez, J.; Forner, M.J.; Perez-Soriano, C.; Tormos, M.C.; Saez, G.T.; Chaves, F.J.; Redon, J.; Cortes, R. Urinary levels of sirtuin-1 associated with disease activity in lupus nephritis. Clin. Sci. 2018, 132, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Jiang, S.; Liu, Q.; Ma, Y.; Zhu, X.; Liang, M.; Shi, X.; Ding, W.; Zhou, X.; Zou, H.; et al. Sirtuin1 protects against systemic sclerosis-related pulmonary fibrosis by decreasing proinflammatory and profibrotic processes. Am. J. Respir Cell Mol. Biol. 2018, 58, 28–39. [Google Scholar] [CrossRef]
- Fasshauer, M.; Kralisch, S.; Klier, M.; Lossner, U.; Bluher, M.; Klein, J.; Paschke, R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2003, 301, 1045–1050. [Google Scholar] [CrossRef]
- He, Y.; Lu, L.; Wei, X.; Jin, D.; Qian, T.; Yu, A.; Sun, J.; Cui, J.; Yang, Z. The multimerization and secretion of adiponectin are regulated by TNF-alpha. Endocrine 2016, 51, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, W.H.; Park, S.I. GO6976 prevents TNF-alpha-induced suppression of adiponectin expression in 3T3-L1 adipocytes: Putative involvement of protein kinase C. FEBS Lett. 2008, 582, 3473–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, M.; Baron, B. The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Appenzeller, S. The role of Tumor Necrosis Factor-alpha (TNF-α) in the pathogenesis of systemic lupus erythematosus. Cytokine 2011, 56, 537–543. [Google Scholar] [CrossRef]
- Shehzad, A.; Iqbal, W.; Shehzad, O.; Lee, Y.S. Adiponectin: Regulation of its production and its role in human diseases. Hormones 2012, 11, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Imamura, T.; Sonoda, N.; Sears, D.D.; Patsouris, D.; Kim, J.J.; Olefsky, J.M. FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J. Biol. Chem. 2009, 284, 12188–12197. [Google Scholar] [CrossRef] [Green Version]
- Standiford, T.J.; Keshamouni, V.G.; Reddy, R.C. Peroxisome proliferator-activated receptor-{gamma} as a regulator of lung inflammation and repair. Proc. Am. Thorac. Soc. 2005, 2, 226–231. [Google Scholar] [CrossRef]
- Zhang, X.; Young, H.A. PPAR and immune system—What do we know? Int. Immunopharmacol. 2002, 2, 1029–1044. [Google Scholar] [CrossRef]
- Chinetti, G.; Griglio, S.; Antonucci, M.; Torra, I.P.; Delerive, P.; Majd, Z.; Fruchart, J.-C.; Chapman, J.; Najib, J.; Staels, B. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. J. Biol. Chem. 1998, 273, 25573–25580. [Google Scholar] [CrossRef] [Green Version]
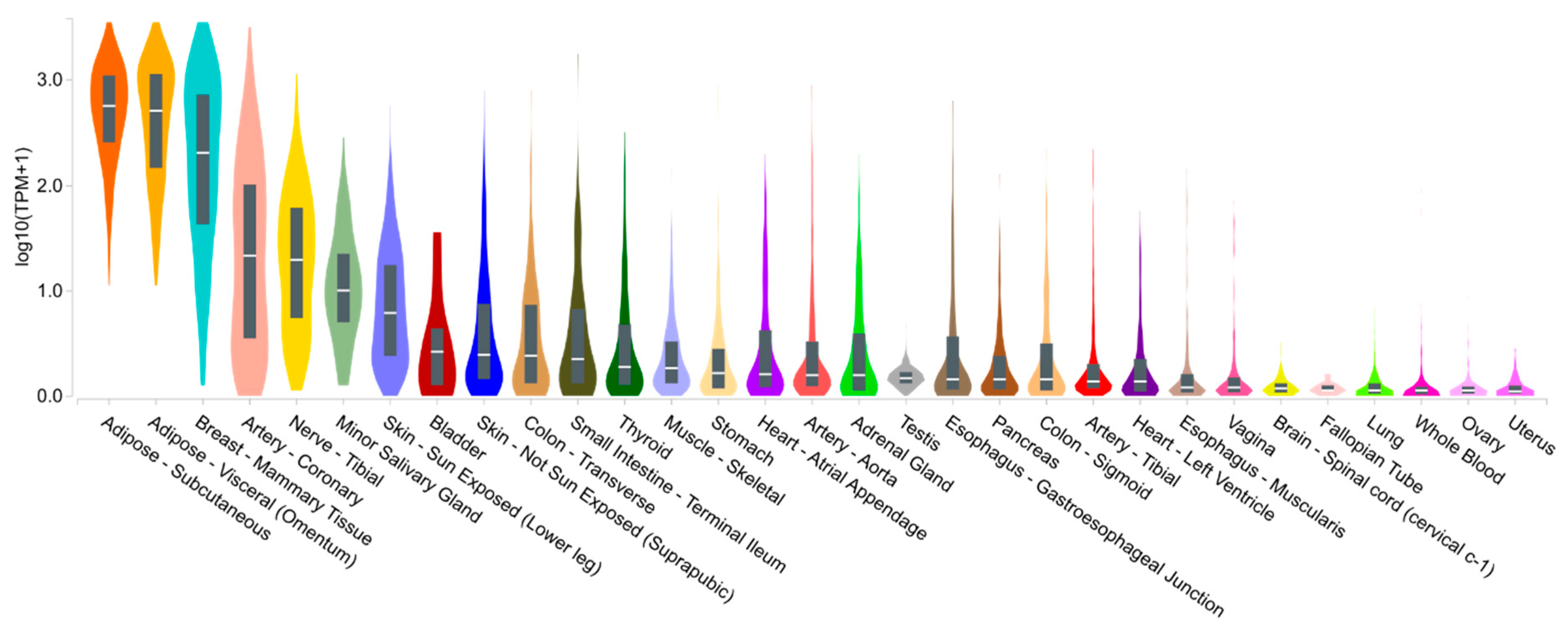
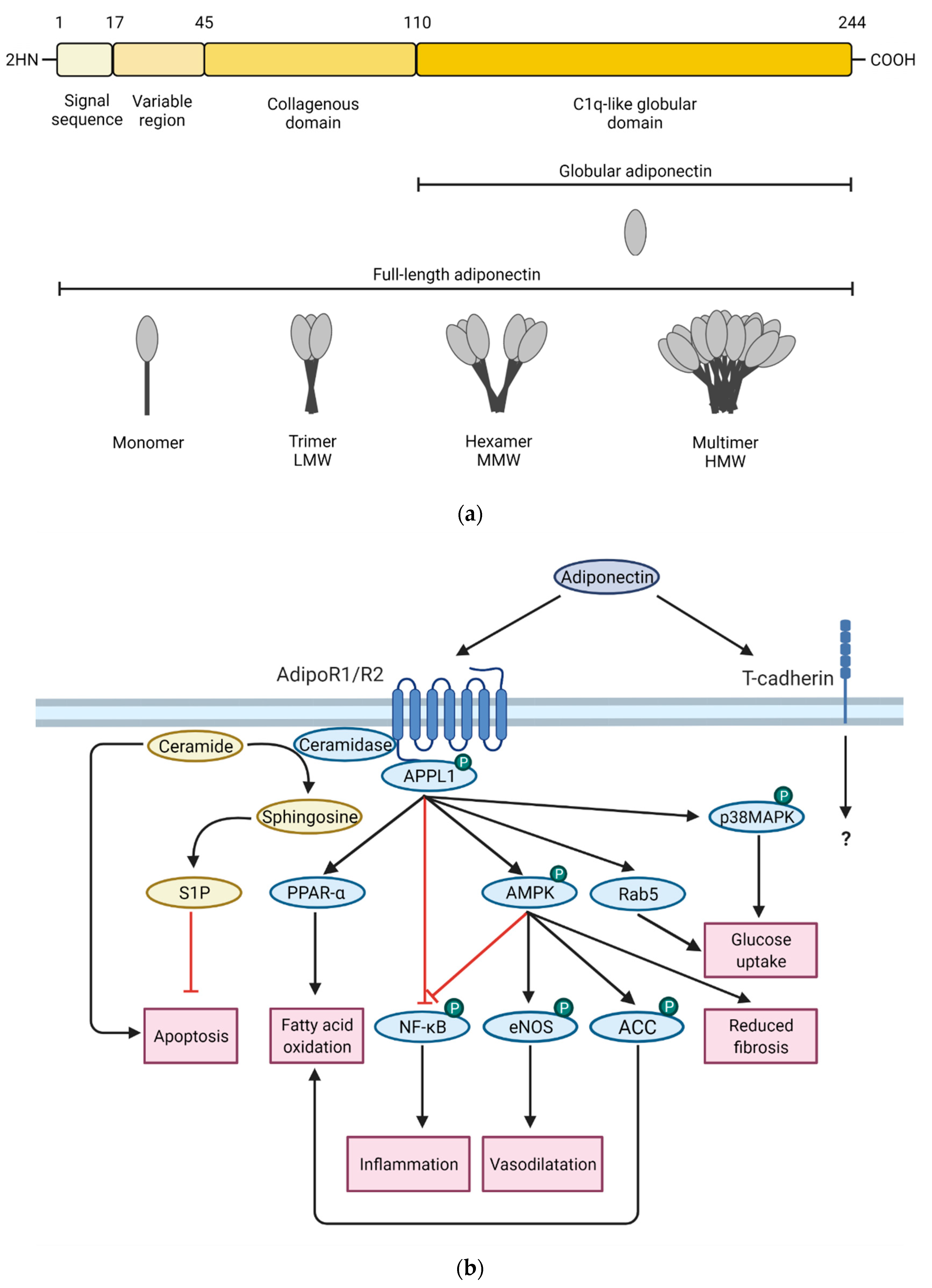
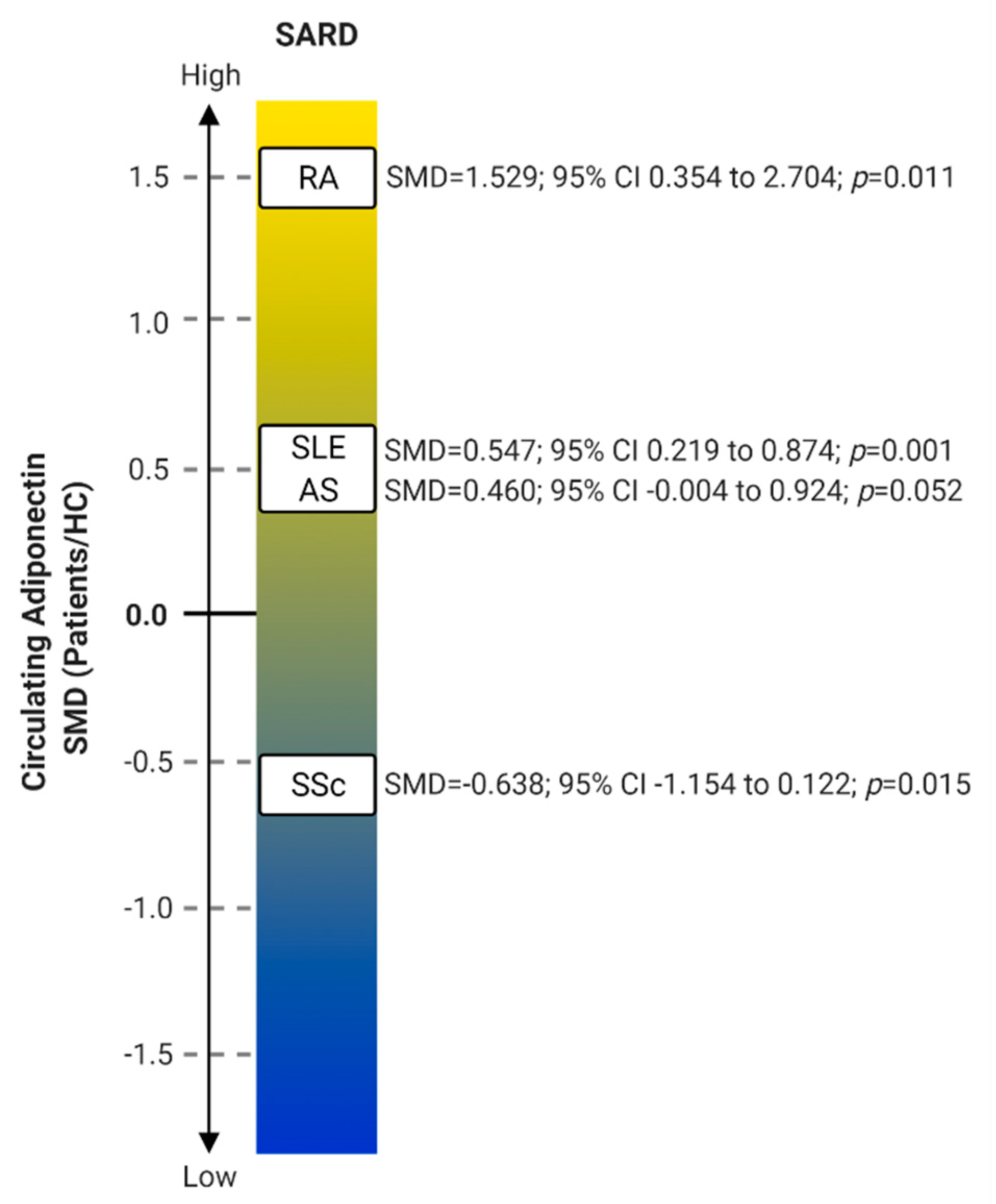
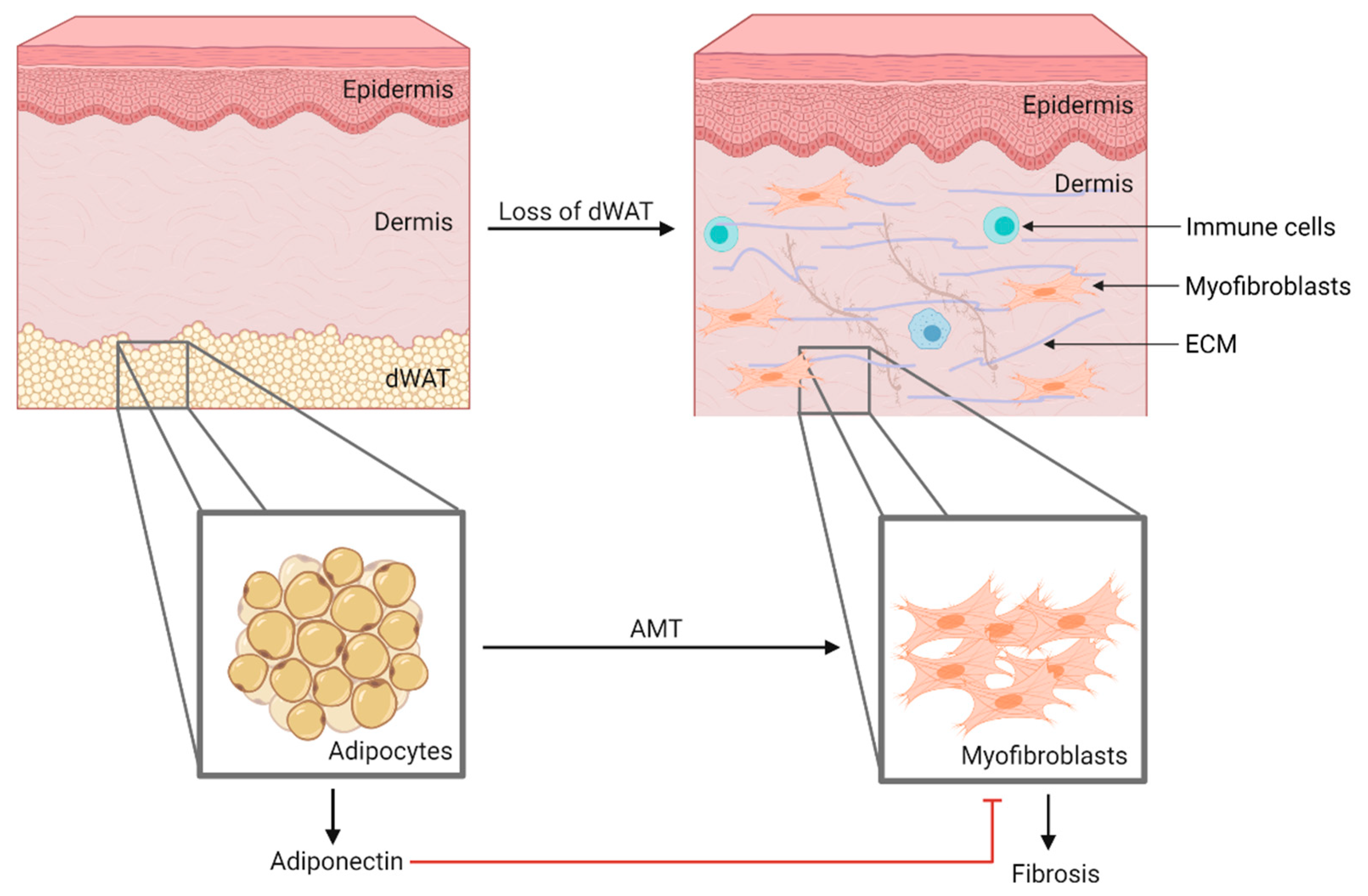


| Clinical Feature | RA Patients(N) | Serum Adiponectin Levels Associations/Correlations | Reference |
|---|---|---|---|
| Disease activity | 351/90/52 | Positive association with DAS28-ESR. | [26,38,42] |
| 51 | Early RA with high adiponectin levels was less likely to have MHAQ score > 3 and RAPID3 score > 12. | [29] | |
| 121 | Stratifying according to DAS28 (low, moderate and high activity), there were no differences seen for adiponectin. | [43] | |
| 180 | Negative correlation of total, HMW, MMW, and LMW adiponectin with the DAS28. | [44] | |
| 40 | Negative correlation with DAS28. | [45] | |
| 70 | Positive correlation with DAS28-ESR in active disease. | [46] | |
| 80 | Negative correlation with the number of swollen joints. | [47] | |
| Radiographic severity/progression | 324 | Positive association of total, but not HMW adiponectin with radiographic progression. | [48] |
| 242 | Positive correlation with radiographic severity. | [49] | |
| 632 | Independent association with baseline total SHS, ΔSHS ≥ 1 and predicted ΔSHS ≥ 5. | [36] | |
| 253 | Positive association with radiographic progression over 4 years. | [50] | |
| 152 | Positive association with radiographic progression. | [51] | |
| CV-related | 54 | No correlation with coronary artery calcification. | [52] |
| 210 | In RA patients with abdominal obesity or no clinically evident joint damage associated with decreased carotid atherosclerosis. | [53] | |
| 192 | Leptin: adiponectin ratio associated with common carotid artery resistive index. | [54] | |
| 210 | Positive associations of total and HMW adiponectin with increased blood pressure parameters, and in white patients additionally with endothelial activation. | [55] | |
| Bone-related | 112 | Negative association with trabecular volumetric bone mineral density and cortical thickness. | [56] |
| 38 | Positive correlation with osteopontin in serum. | [28] | |
| Muscle-related | 50 | Negative association with appendicular lean mass index and muscle cross-sectional area. | [57] |
| SARD | Patients (N) | Anti-TNF | Treatment Regimen | Study Duration | Influence on Adiponectin Levels | Ref. |
|---|---|---|---|---|---|---|
| RA | 16 | adalimumab | 40 mg every 2 weeks | 1 year | No change | [118] |
| etanercept | 25 mg twice a week | |||||
| Infliximab | 3 mg/kg every 8 weeks | |||||
| 48 | adalimumab | 40 mg every 2 weeks | 16 weeks | No change | [119] | |
| 171 | adalimumab | 40 mg every 2 weeks | 16 weeks | No change | [120] | |
| 96 | adalimumab | at approved doses | 24 weeks | No change | [121] | |
| certolizumab | at approved doses | |||||
| etanercept | at approved doses | |||||
| infliximab | at approved doses | |||||
| 8 | adalimumab | 40 mg every 2 weeks | 2 years | No change | [122] | |
| etanercept | 50 mg every week | |||||
| infliximab | 3 mg/kg | |||||
| 21 | adalimumab | at approved doses | 12 weeks | No change | [123] | |
| certolizumab | at approved doses | |||||
| etanercept | at approved doses | |||||
| golimumab | at approved doses | |||||
| infliximab | at approved doses | |||||
| 15 | anti-TNF | at approved doses | 6 months | No change | [124] | |
| 33 | adalimumab | 40 mg every 2 weeks | 12 and | Increase | [115] | |
| etanercept | 50 mg every week | 24 weeks | ||||
| infliximab | 5 mg/kg every 8 weeks | |||||
| 16 | infliximab | 3 mg/kg in weeks 0, 2 and 6 and every 8 weeks after | 24 months | No change | [116] | |
| 3–12 months | Increase | |||||
| 16 | adalimumab | - | 6 months | Increase | [117] | |
| eternacept | - | |||||
| infliximab | - | |||||
| PsA | 126 | onercept | 50 mg or 100 mg three times a week | 12 weeks | No change | [120] |
| 405 | golimumab | 50 mg or 100 mg every 4 weeks | 14 weeks | No change | [125] | |
| AS | 30 | infliximab | 5 mg/kg in weeks 0, 2, 6 and every 8 weeks after | 6 months | No change | [126] |
| 29 | infliximab | Infusion (120 min) | before and right after | No change | [127] | |
| 12 | adalimumab | 40 mg every 2 weeks | 2 years | No change | [122] | |
| etanercept | 50 mg every week | |||||
| infliximab | 5 mg/kg |
| Patients (N) | Treatment Regimen | Additional Therapy | Additional Therapy Regimen | Study Duration | Influence on APN Levels | Ref. |
|---|---|---|---|---|---|---|
| 65 | 7.5–15.0 mg/day | MTX | 10–15 mg/week | 3 months | Increase | [134] |
| 15 | 10 mg/day | MTX | 0.2 mg/kg/week | 6 months | Increase | [135] |
| 15 | 10 mg/day | MTX + ATV | 0.2 mg/kg/week 40 mg/day | 6 months | Increase | [135] |
| 9 | 60 mg/day (week 1); 40 mg/day (week 2) | - | - | 2 weeks | Increase | [119] |
| 19 | Tapered high dose: 60 mg/day (week 1); 40 mg/day (week 2); 30 mg/day (week 3); 20 mg/day (week 4); 15 mg/day (week 5); 10 mg/day (week 6); 7.5 mg/day (thereafter) | HCQ, SSA MTX | 400 mg/day 2 g/day 10 mg/week | 22 weeks | No change | [119] |
| 127 | 10 mg/day or less | DMARD | Stable therapy | 6 months | No change | [137] |
| 91 | 1 year | |||||
| 52 | 2 years |
| N of Patients | Treatment Regimen | Additional Therapy | Study Duration | Influence on APN Levels | Ref. |
|---|---|---|---|---|---|
| 41 | 8 mg/kg | ± MTX, GC, NSAID | 6 months | Increase | [141] |
| 40 | 8 mg/kg every 4 weeks | ± MTX, SSA, HCQ, Leflunomide, GC, statins, anti-diabetics | 4 months | Decrease | [27] |
| 20 | 8 mg/kg every 4 weeks | ± NSAID, coxibs, GC | 6 months | Increase | [140] |
| 24 | 8 mg/kg every 4 weeks | MTX (± NSAID, coxibs, GC) | 6 months | Increase | [140] |
| 47 | - | - | 6 months | Increase | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezovec, N.; Perdan-Pirkmajer, K.; Čučnik, S.; Sodin-Šemrl, S.; Varga, J.; Lakota, K. Adiponectin Deregulation in Systemic Autoimmune Rheumatic Diseases. Int. J. Mol. Sci. 2021, 22, 4095. https://doi.org/10.3390/ijms22084095
Brezovec N, Perdan-Pirkmajer K, Čučnik S, Sodin-Šemrl S, Varga J, Lakota K. Adiponectin Deregulation in Systemic Autoimmune Rheumatic Diseases. International Journal of Molecular Sciences. 2021; 22(8):4095. https://doi.org/10.3390/ijms22084095
Chicago/Turabian StyleBrezovec, Neža, Katja Perdan-Pirkmajer, Saša Čučnik, Snežna Sodin-Šemrl, John Varga, and Katja Lakota. 2021. "Adiponectin Deregulation in Systemic Autoimmune Rheumatic Diseases" International Journal of Molecular Sciences 22, no. 8: 4095. https://doi.org/10.3390/ijms22084095






