Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure
Abstract
:1. Introduction
2. Results
2.1. Water Resistance Stability
2.2. Chemical Stability
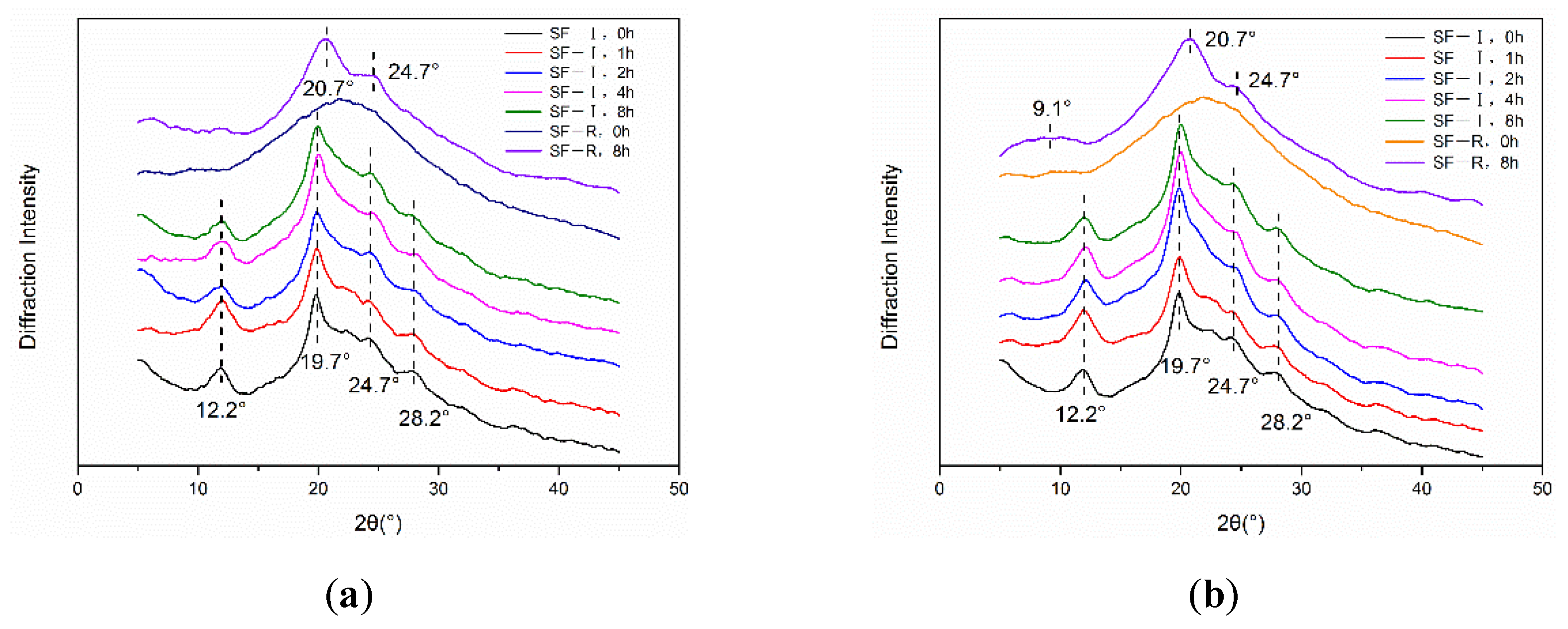
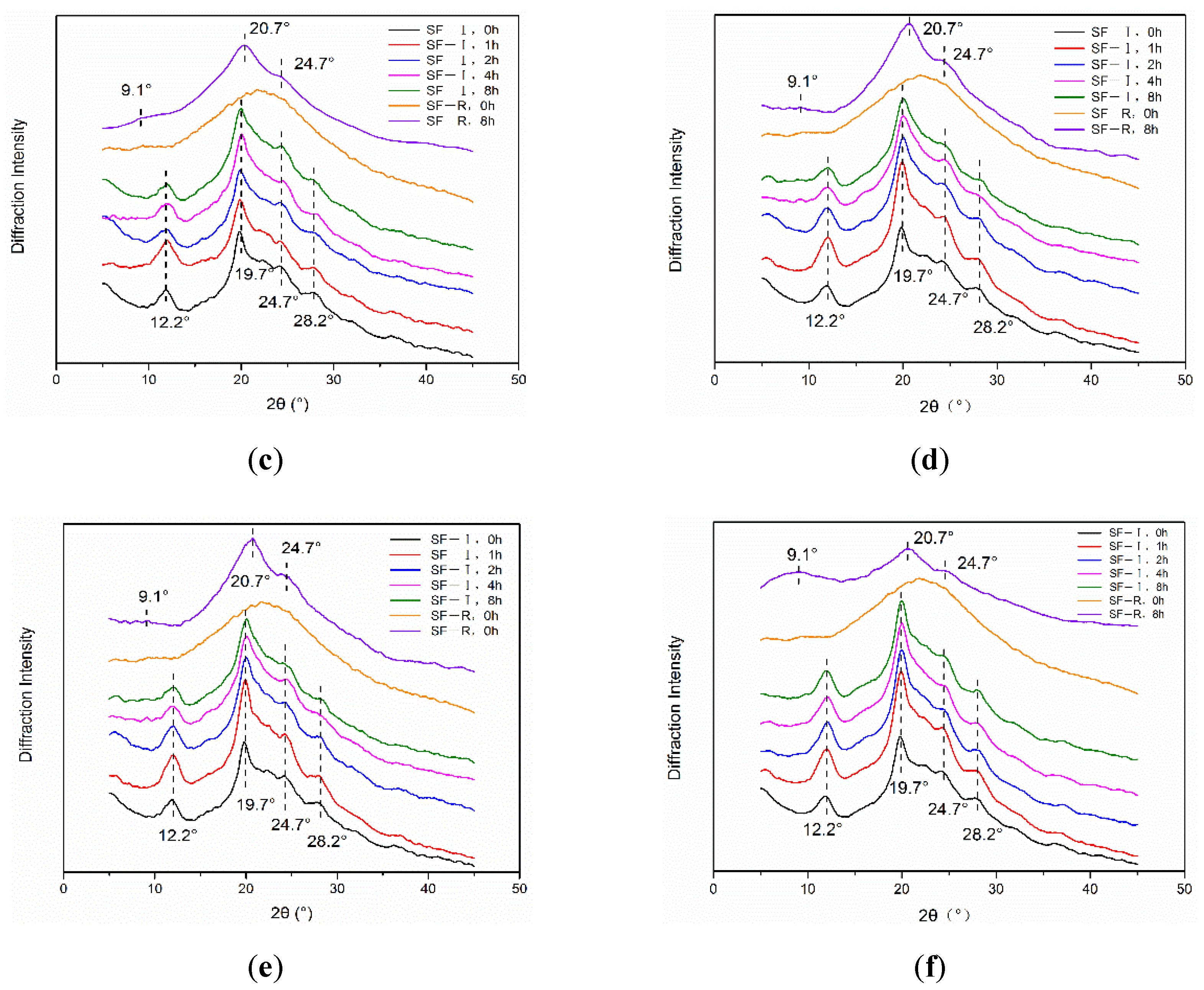
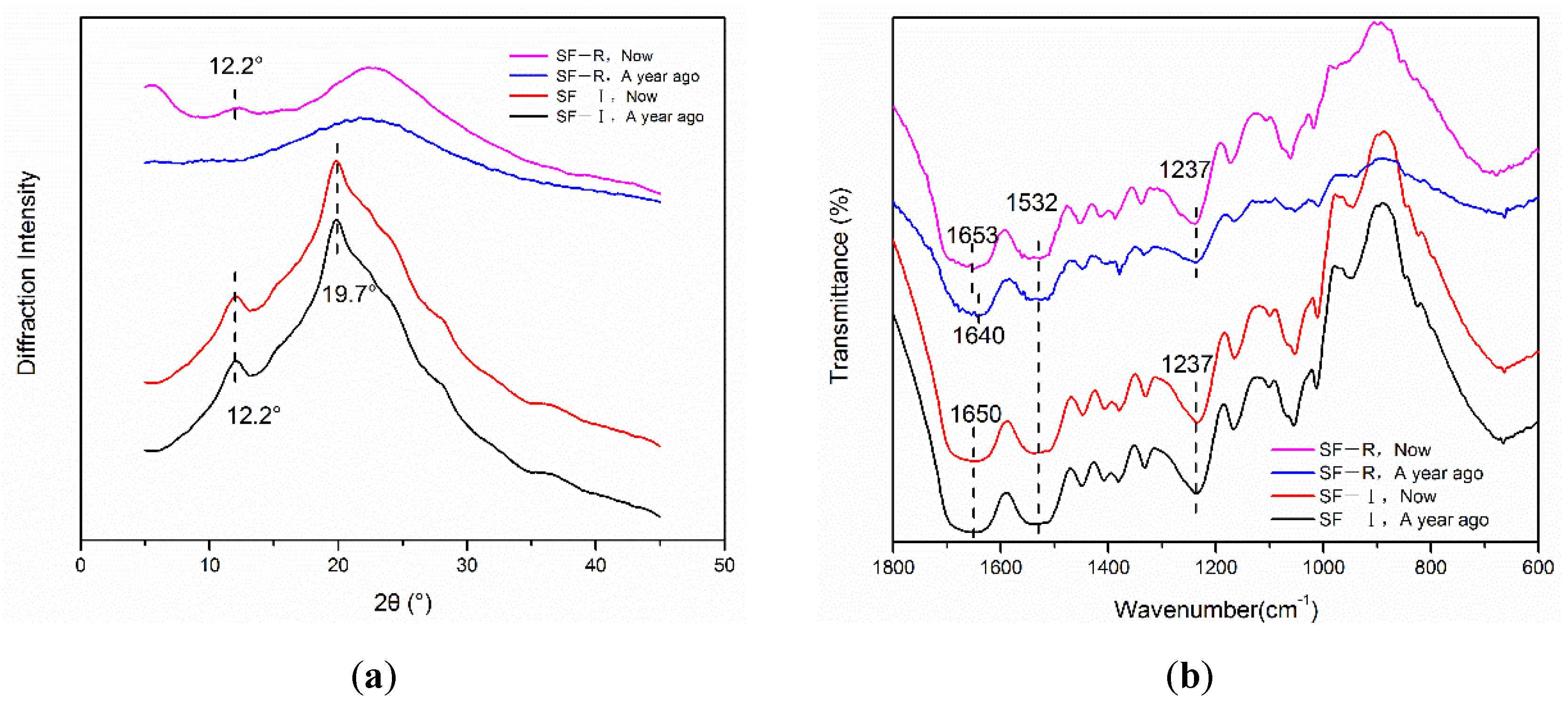
2.2.1. X-ray Diffraction Analysis
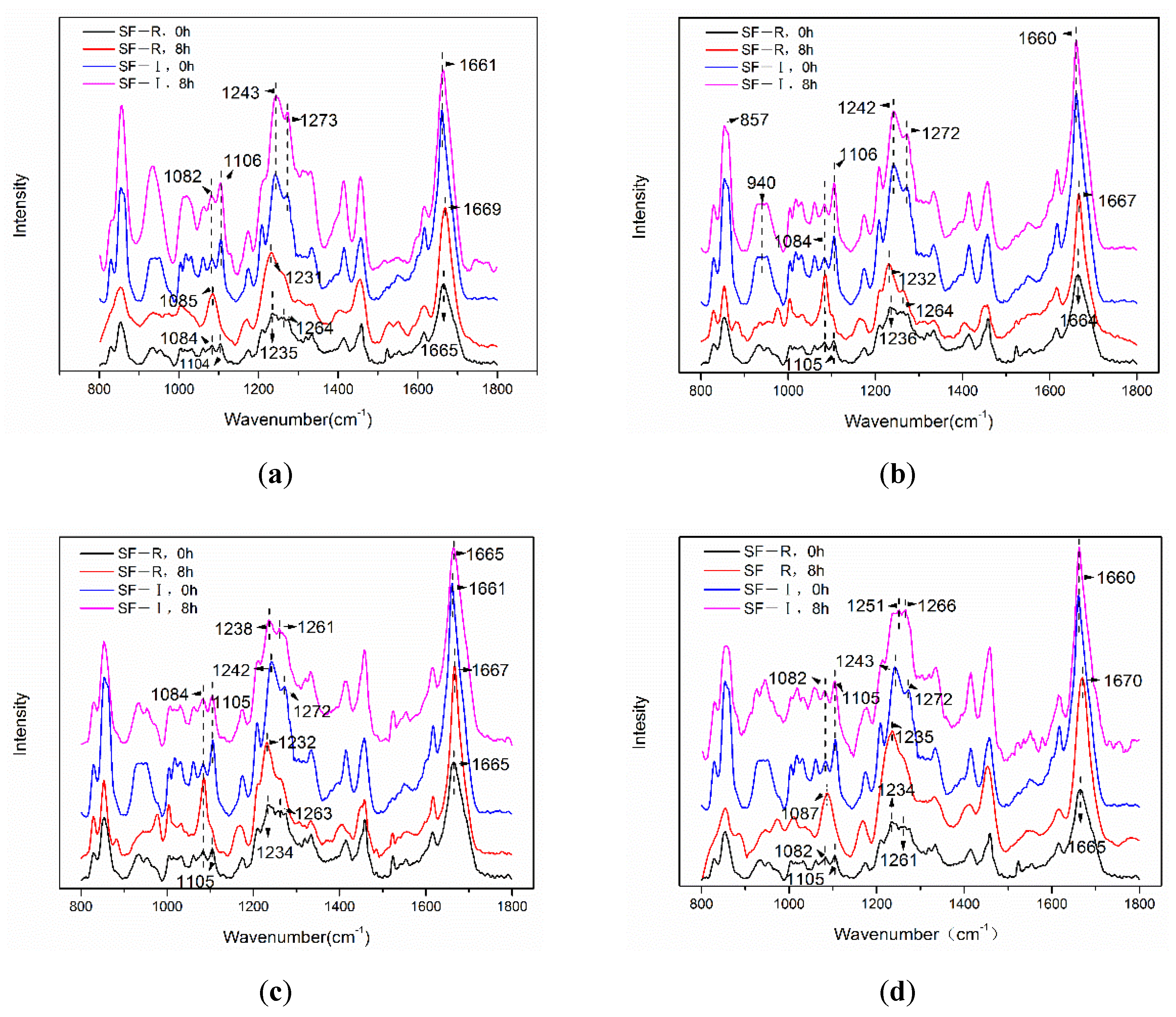
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.2.3. Raman Scattering Spectrum
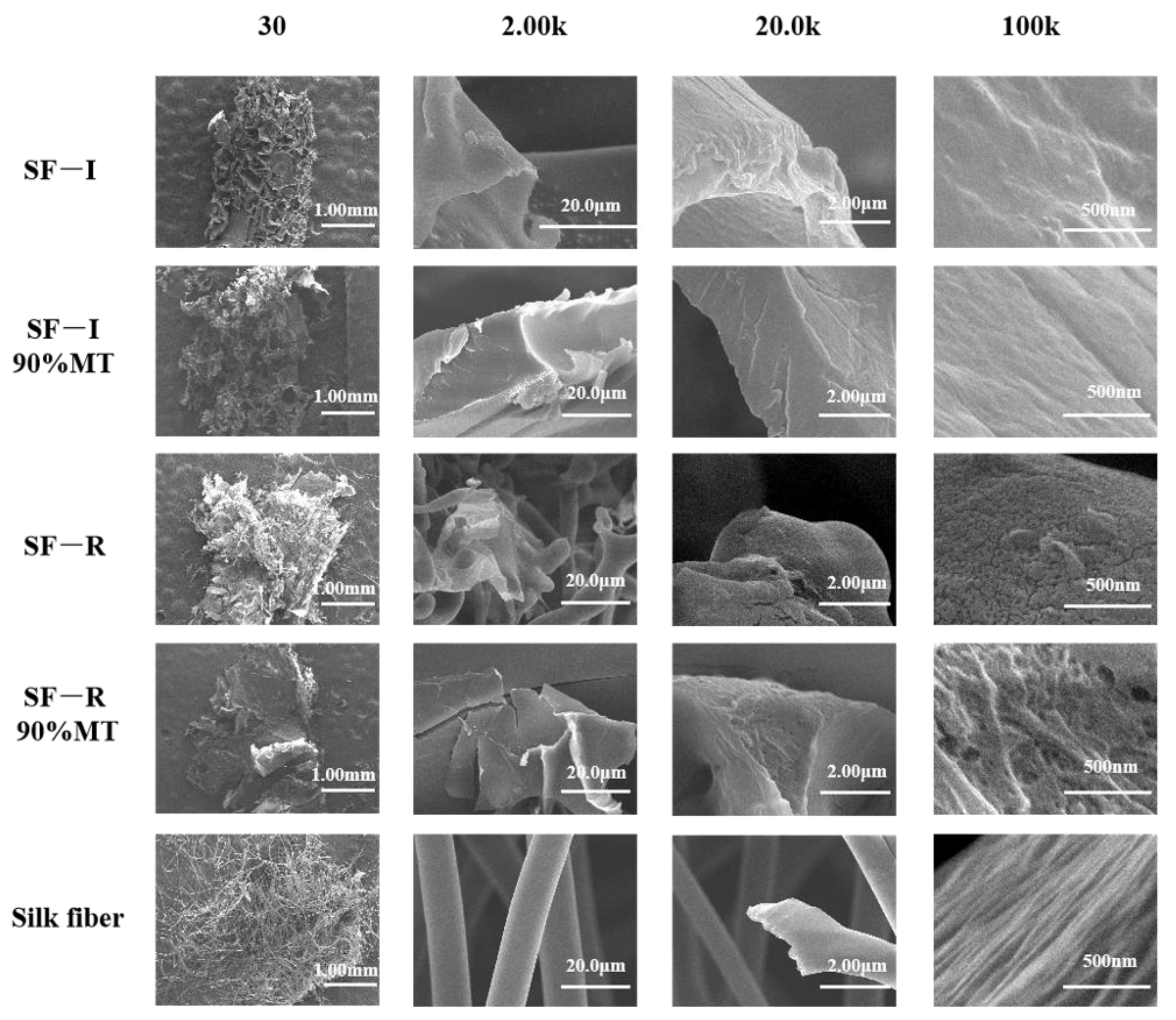
2.2.4. Scanning Electron Microscope (SEM) Images
2.3. Time Stability
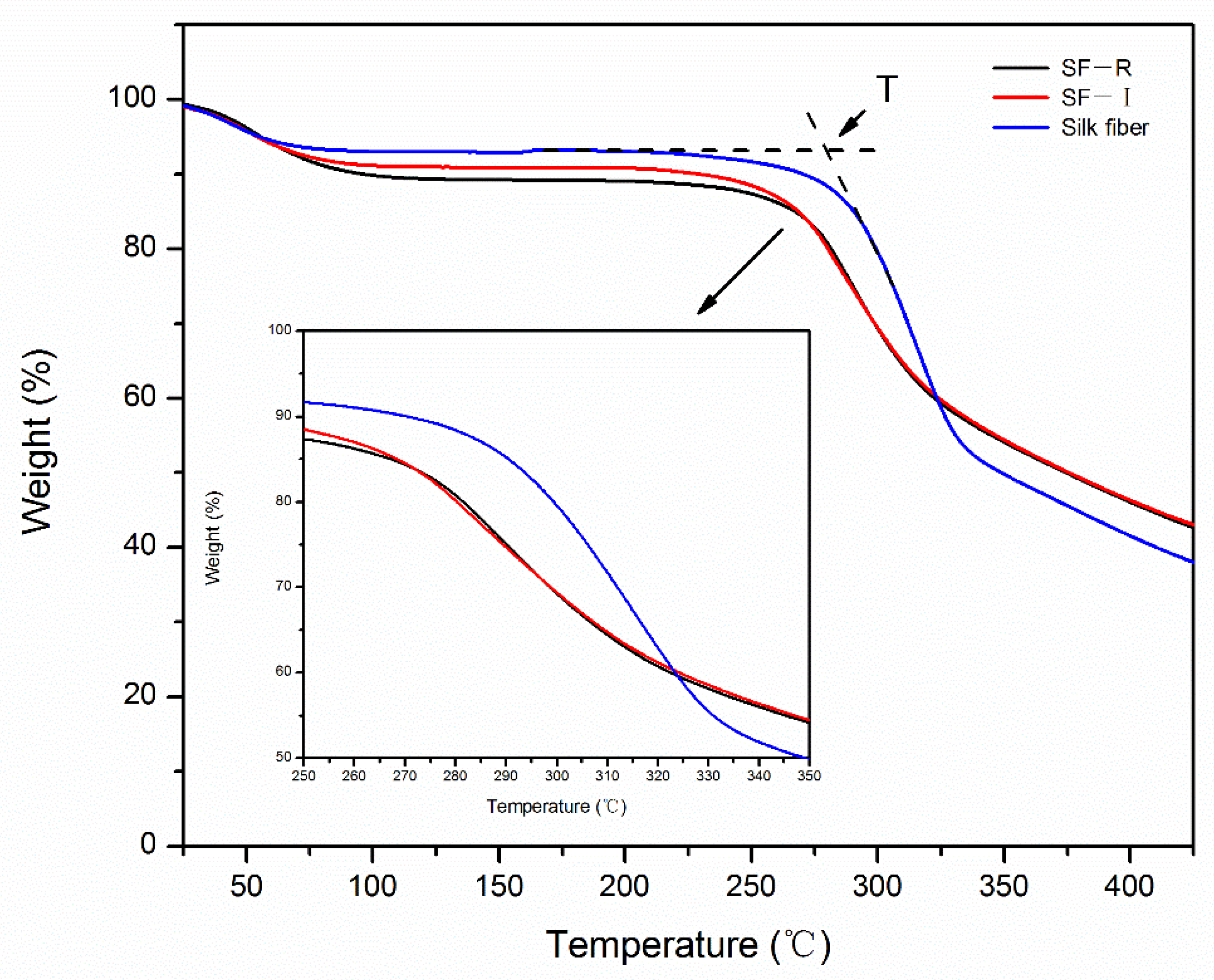
2.4. Thermal Stability
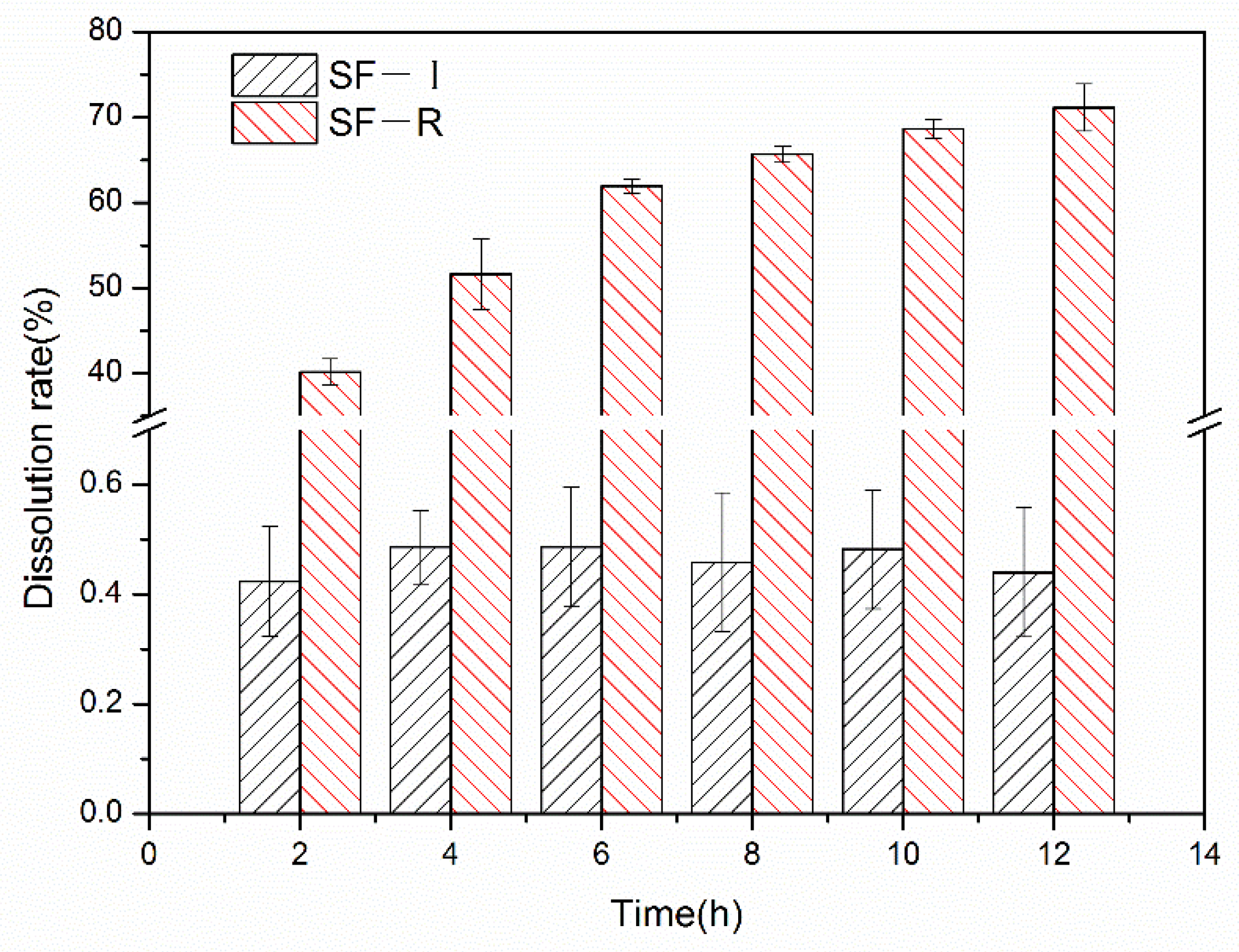
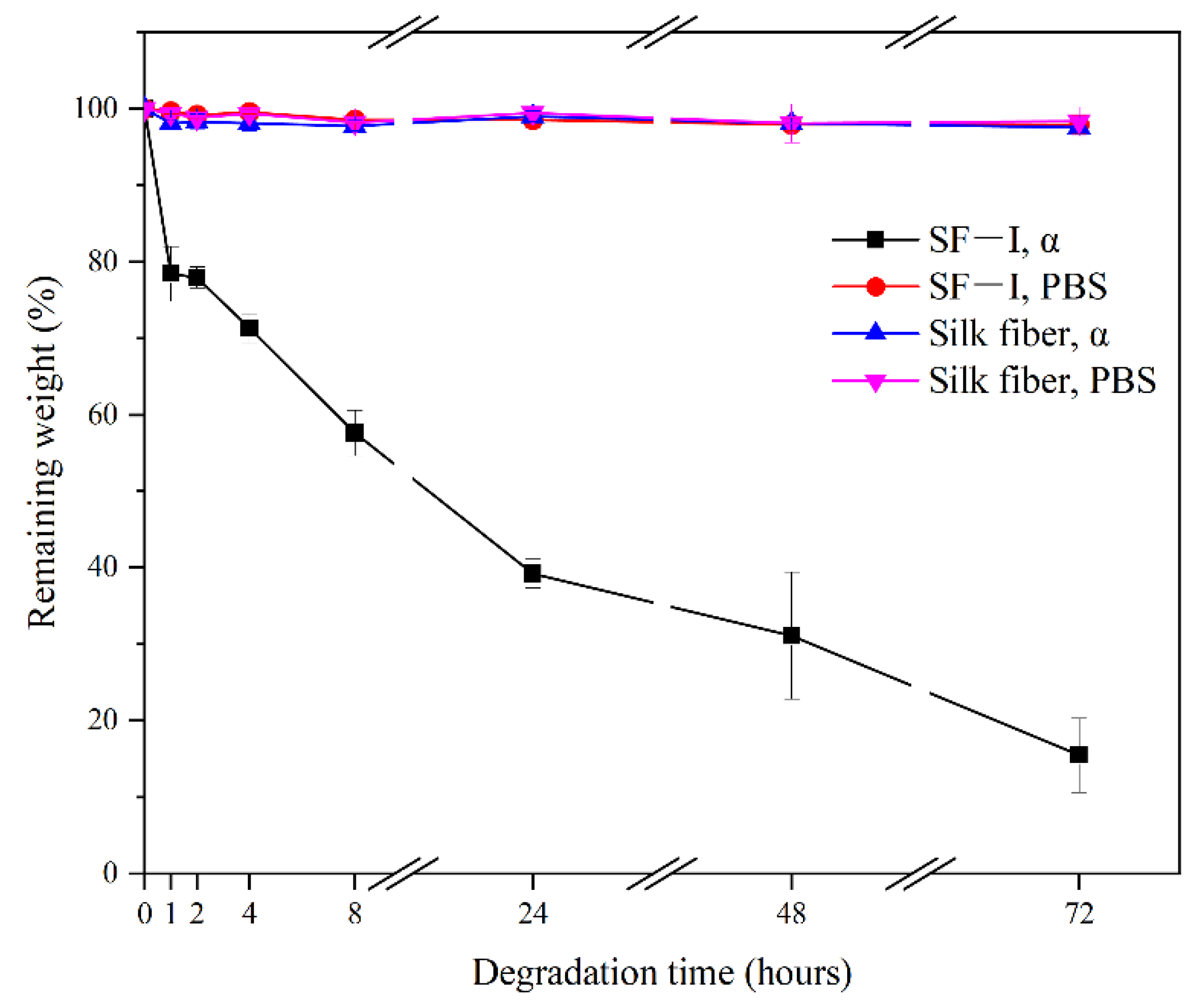
2.5. Enzyme Degradation Stability
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
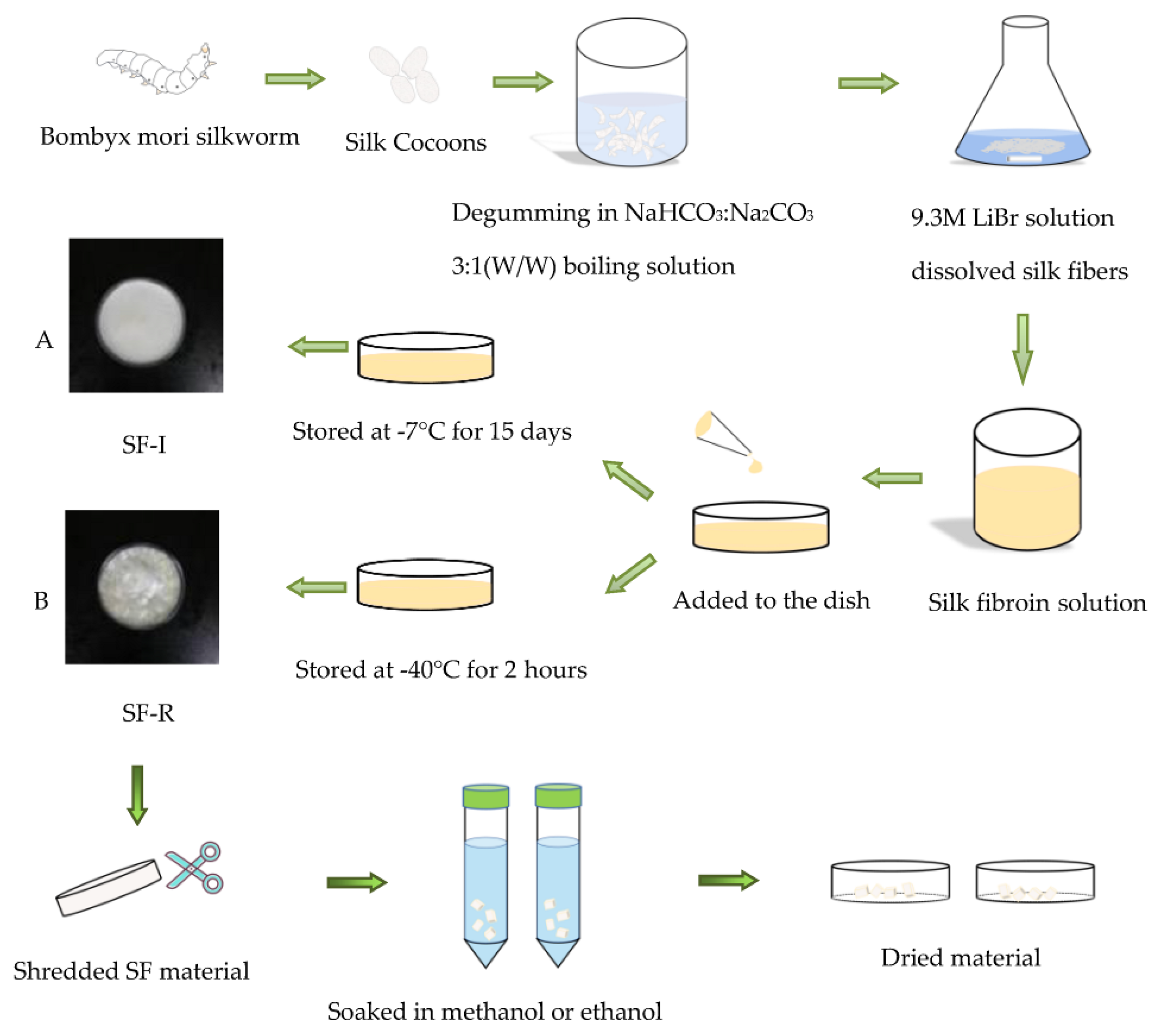
4.2. Preparation of Silk Fibroin Solution
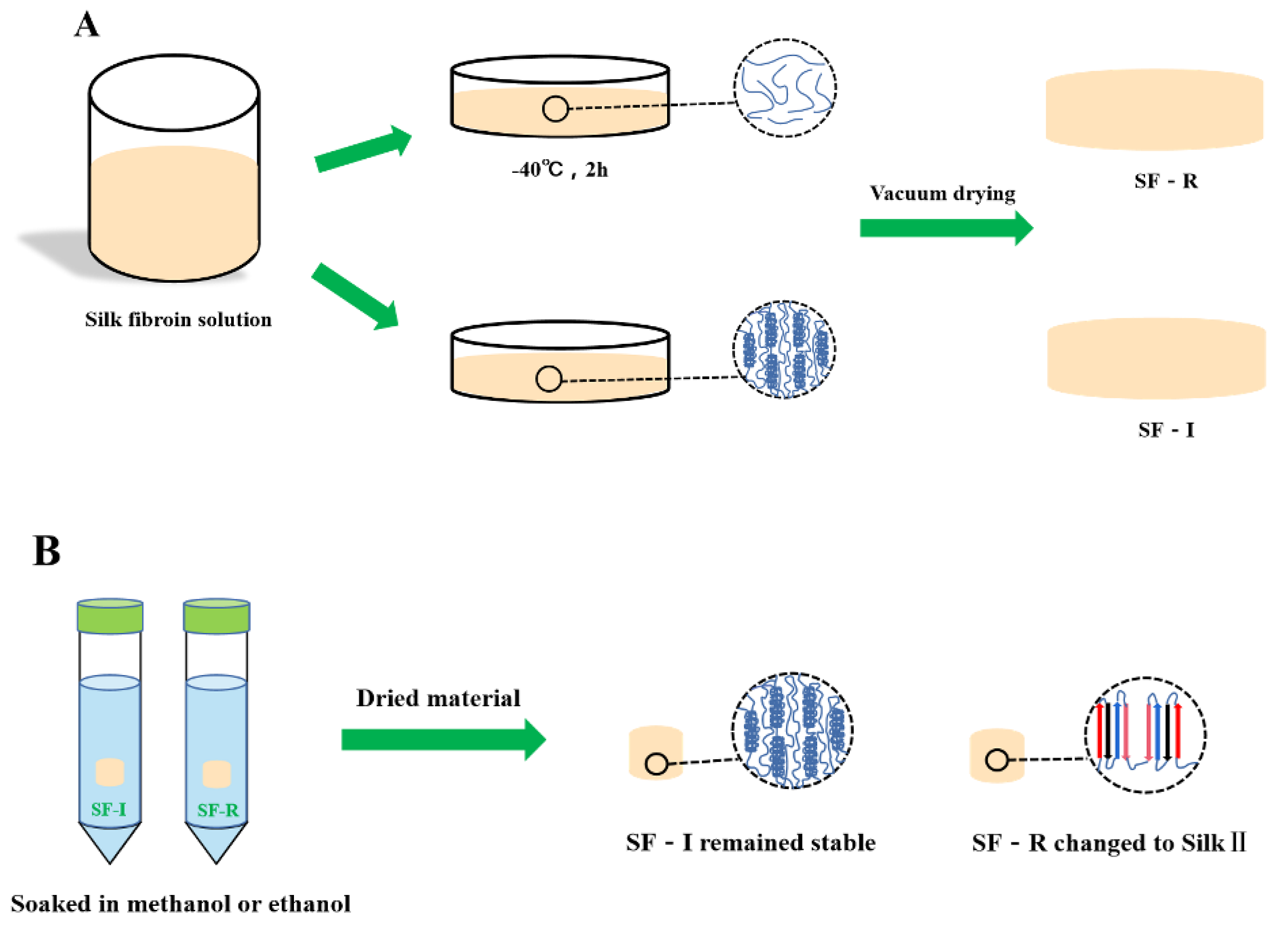
4.3. Preparation of Silk Fibroin Materials
4.3.1. Preparation of Silk I Crystalline Structure Materials
4.3.2. Preparation of Random coil Structure Materials
4.4. Water Resistance Stability Determination
4.5. Determination of Stability of Monohydric Alcohol Treatment
4.5.1. Methanol Treatment
4.5.2. Ethanol Treatment
4.6. Long Time Stability
4.7. Thermal Stability Determination
4.8. Enzymatic Degradation
4.9. Structural Characterization
4.9.1. X-ray Diffraction Analysis
4.9.2. Fourier Transform Infrared Spectroscopy
4.9.3. Raman Scattering Spectroscopy
4.10. Morphology Characterization
5. Conclusions
Abbreviation
| SF | Silk Fibroin |
| SF-R | Silk Fibroin Random coil porous material |
| SF-I | Silk Fibroin Silk I crystalline structure porous material |
| GAGAGS | Amino acid sequence Gly-Ala-Gly-Ala-Gly-Ser |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscope |
| TGA | Thermogravimetric analysis |
| MT | Methanol treatment. |
| Tg | Glass transition temperature |
| Tm | Melting temperature |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H.J.; Kaplan, D.L.; Rutledge, G.C. Mechanical Properties of Electrospun Silk Fibers. Macromolecules 2004, 37, 6856–6864. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins Struct. Funct. Bioinform. 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Cong-Zhao, Z.; Fabrice, C.; Nadine, M.; Yvan, Z.; Catherine, E.; Tie, Y.; Michel, J.; Joel, J.; Michel, D.; Roland, P. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhu, H.; Zhang, C.; Zhang, F.; Zhang, B.; Kaplan, D.L. Silk Self-Assembly Mechanisms and Control-From Thermodynamics to Kinetics. Biomacromolecules 2012, 13, 826–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.X.; Hirabayashi, K. Improvements of the physical properties of fibroin membranes with sodium alginate. J. Appl. Polym. Sci. 1992, 45, 1937–1943. [Google Scholar] [CrossRef]
- Kratky, O.; Schauenstein, E.; Sekora, A. An Unstable Lattice in Silk Fibroin. Nature 1950, 165, 319–320. [Google Scholar] [CrossRef]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.S.; Park, S.H.; Cebe, P.; Kaplan, D.L. Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef] [Green Version]
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Silk I and Silk II studied by fast scanning calorimetry. Acta Biomater. 2017, 55, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Valluzzi, R.; Gido, S.P.; Muller, W.; Kaplan, D.L. Orientation of silk III at the air-water interface. Int. J. Biol. Macromol. 1999, 24, 237–242. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The influence of the hydrophilic–lipophilic environment on the structure of silk fibroin protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, S.; Strey, H.H.; Gido, S.P. Phase Behavior and Hydration of Silk Fibroin. Biomacromolecules 2004, 5, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Feng, L.; Li, M.; Di, C.; Bai, L. Study on Silk I Porous 3-D Scaffolds. Key Eng. Mater. 2007, 342-343, 233–236. [Google Scholar] [CrossRef]
- Jin, H.; Park, J.; Karageorgiou, V.; Kim, U.; Valluzzi, R.; Kaplan, D. Water—Stable Silk Films with Reduced β—Sheet Content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Jiang, F.; Cao, J.; Lu, S. Combined Silk Fibroin Microneedles for Insulin Delivery. ACS Biomater. Sci. Eng. 2020, 6, 3422–3429. [Google Scholar] [CrossRef]
- Riekel, C.; Burghammer, M.; Rosenthal, M. Nanoscale X-Ray Diffraction of Silk Fibers. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Li, M.; Lu, S.; Wu, Z.; Yan, H.; And, J.M.; Wang, L. Study on porous silk fibroin materials. I. Fine structure of freeze dried silk fibroin. J. Appl. Polym. Sci. 2001, 79, 2185–2191. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Taddei, P.; Freddi, G.; Asakura, T.; Tsukada, M. Raman spectroscopic characterization of Bombyx mori silk fibroin: Raman spectrum of Silk I. J. Raman Spectrosc. 2001, 32, 103–107. [Google Scholar] [CrossRef]
- Tu, A.T. Raman Spectroscopie in Biology. In Principles and Applications; A Wiley-Interscience Publication, John Wiley and Sons: Hoboken, NJ, USA, 1982. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, X.; Knight, D.P.; Zong, X.H.; Deng, F.; Yao, W.H. Effects of pH and Calcium Ions on the Conformational Transitions in Silk Fibroin Using 2D Raman Correlation Spectroscopy and 13C Solid-State NMR. Biochemistry 2004, 43, 11302–11311. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Effect of water on the thermal properties of silk fibroin. Thermochim. Acta 2007, 461, 137–144. [Google Scholar] [CrossRef]
- Ak, F.; Oztoprak, Z.; Karakutuk, I.; Okay, O. Macroporous Silk Fibroin Cryogels. Biomacromolecules 2013, 14, 719–727. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.; Lu, Q.; Zhang, X.; Kluge, J.A.; Uppal, N.; Omenetto, F.; Kaplan, D.L. Insoluble and Flexible Silk Films Containing Glycerol. Biomacromolecules 2010, 11, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation Mechanism and Control of Silk Fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freddi, G.; Monti, P.; Nagura, M.; Gotoh, Y.; Tsukada, M. Structure and molecular conformation of tussah silk fibroin films: Effect of heat treatment. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 841–847. [Google Scholar] [CrossRef]
- Puerta, M.; Arango, M.C.; Jaramillo-Quiceno, N.; Lvarez-López, C.; Restrepo-Osorio, A. Influence of ethanol post-treatments on the properties of silk protein materials. SN Appl. Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Numata, K.; Cebe, P.; Kaplan, D.L. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials 2010, 31, 2926–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Qi, Z.; Tao, X.; Newkirk, C.; Hu, X.; Lu, S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. Int. J. Mol. Sci. 2021, 22, 4136. https://doi.org/10.3390/ijms22084136
Zhao M, Qi Z, Tao X, Newkirk C, Hu X, Lu S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. International Journal of Molecular Sciences. 2021; 22(8):4136. https://doi.org/10.3390/ijms22084136
Chicago/Turabian StyleZhao, Meihui, Zhenzhen Qi, Xiaosheng Tao, Chad Newkirk, Xiao Hu, and Shenzhou Lu. 2021. "Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure" International Journal of Molecular Sciences 22, no. 8: 4136. https://doi.org/10.3390/ijms22084136






