MicroRNA-30b Is Both Necessary and Sufficient for Interleukin-21 Receptor-Mediated Angiogenesis in Experimental Peripheral Arterial Disease
Abstract
:1. Introduction
2. Results
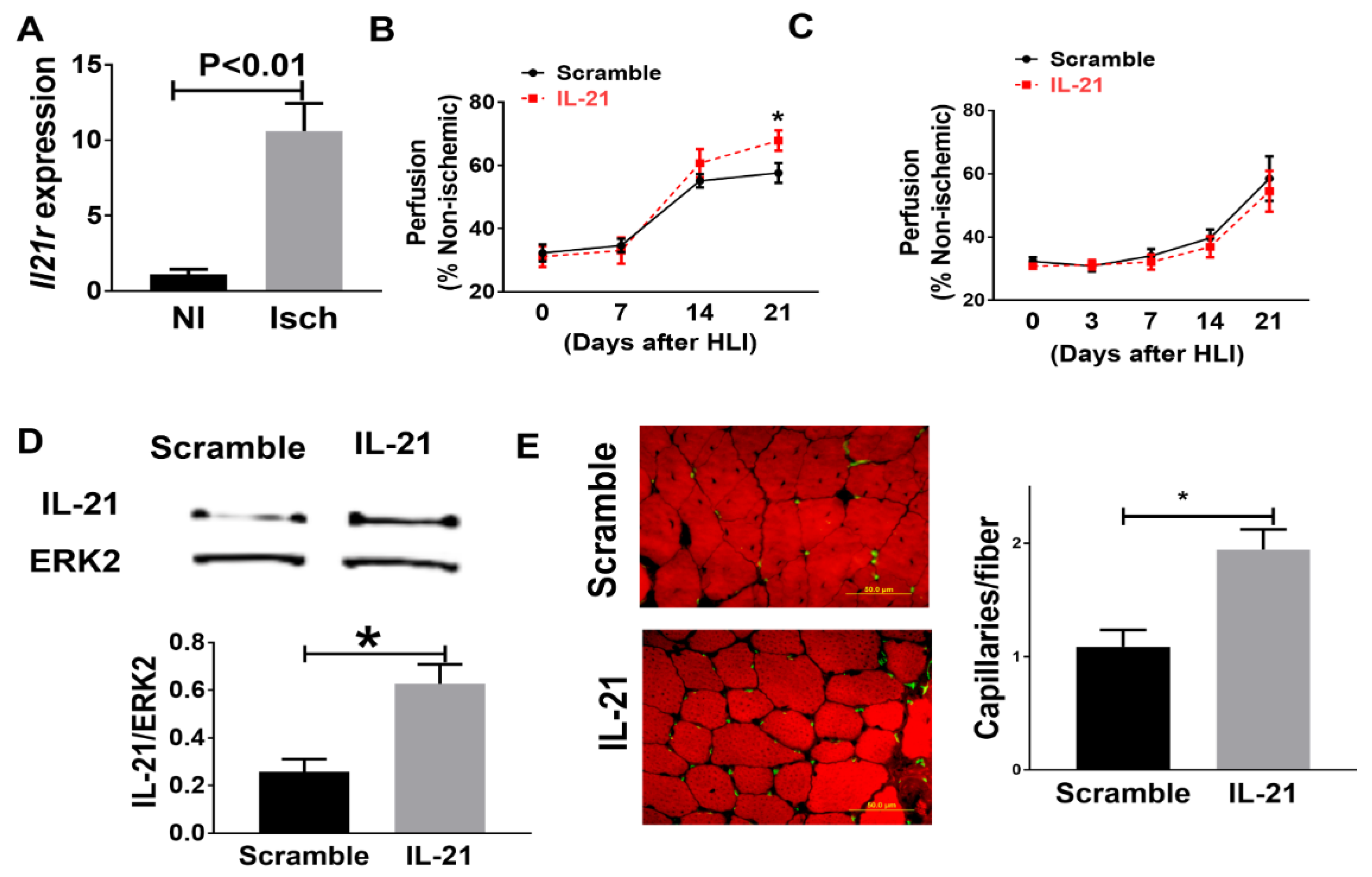
2.1. Identification of IL-21R Pathway Transcripts following Experimental PAD
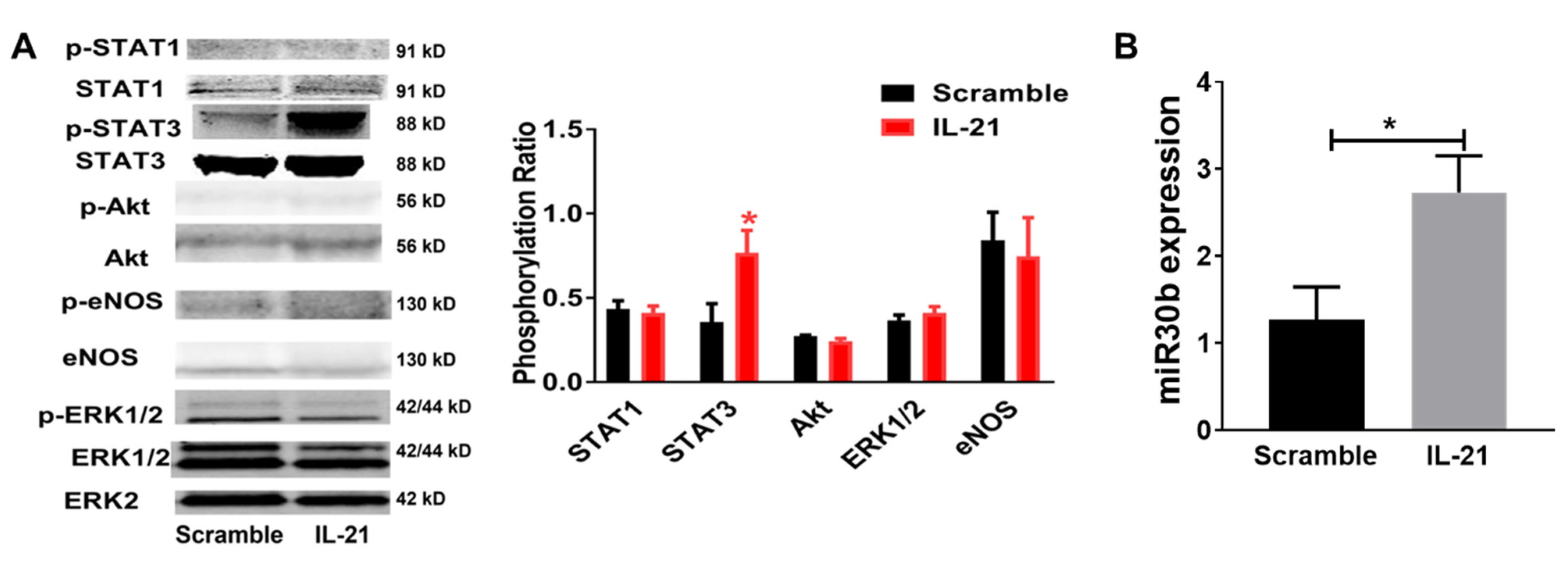
2.2. IL-21 Overexpression Improves Perfusion Recovery, Increases STAT3 Phosphorylation, and Upregulates miR-30b in the Ischemic Muscle after Hind Limb Ischemia (HLI) in Myoglobin Transgenic Mice
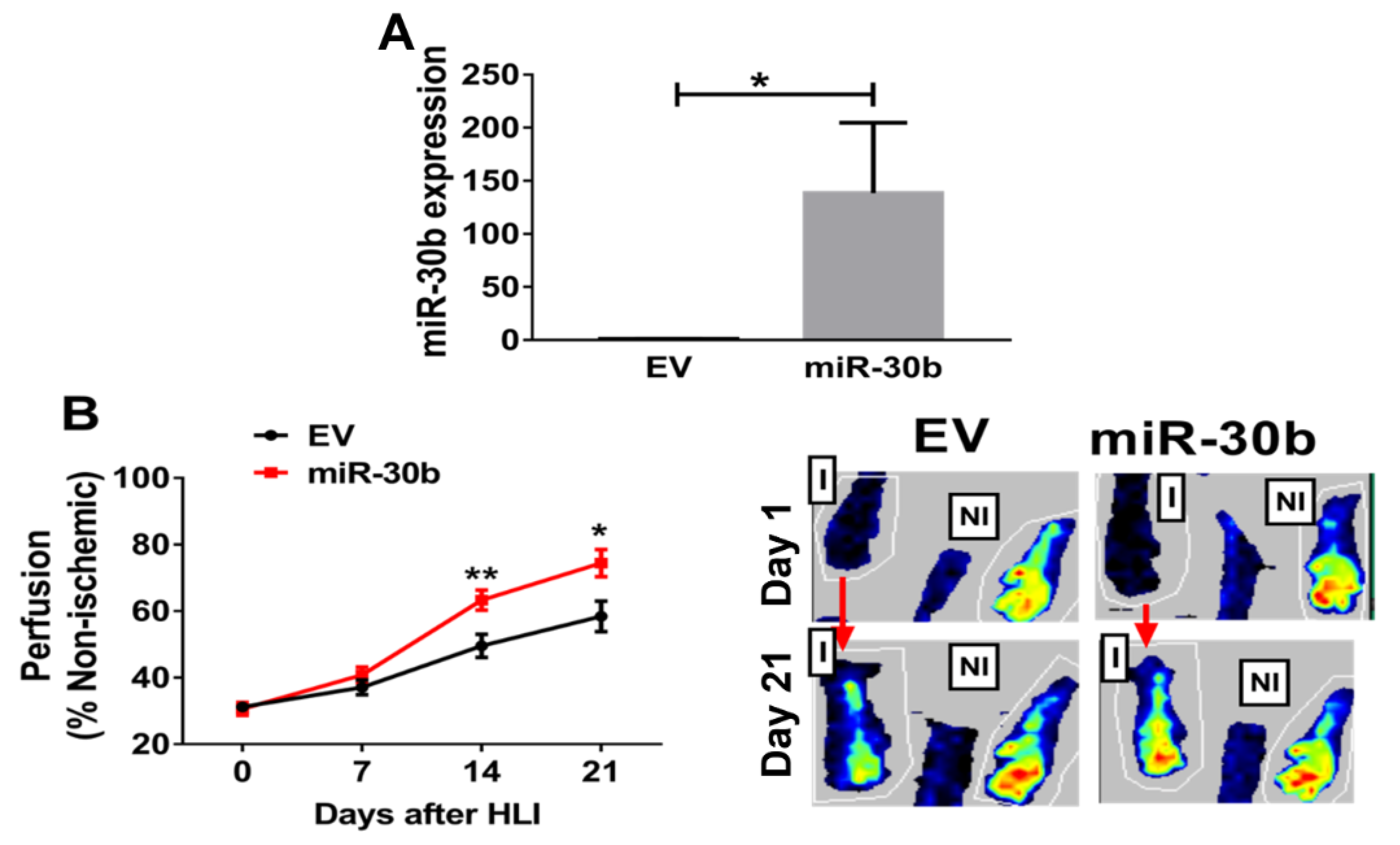
2.3. Overexpression of miR-30b Improves Perfusion Recovery in Il21r−/− Mice
2.4. MiR-30b Is Required for IL-21-Mediated Angiogenesis In Vitro
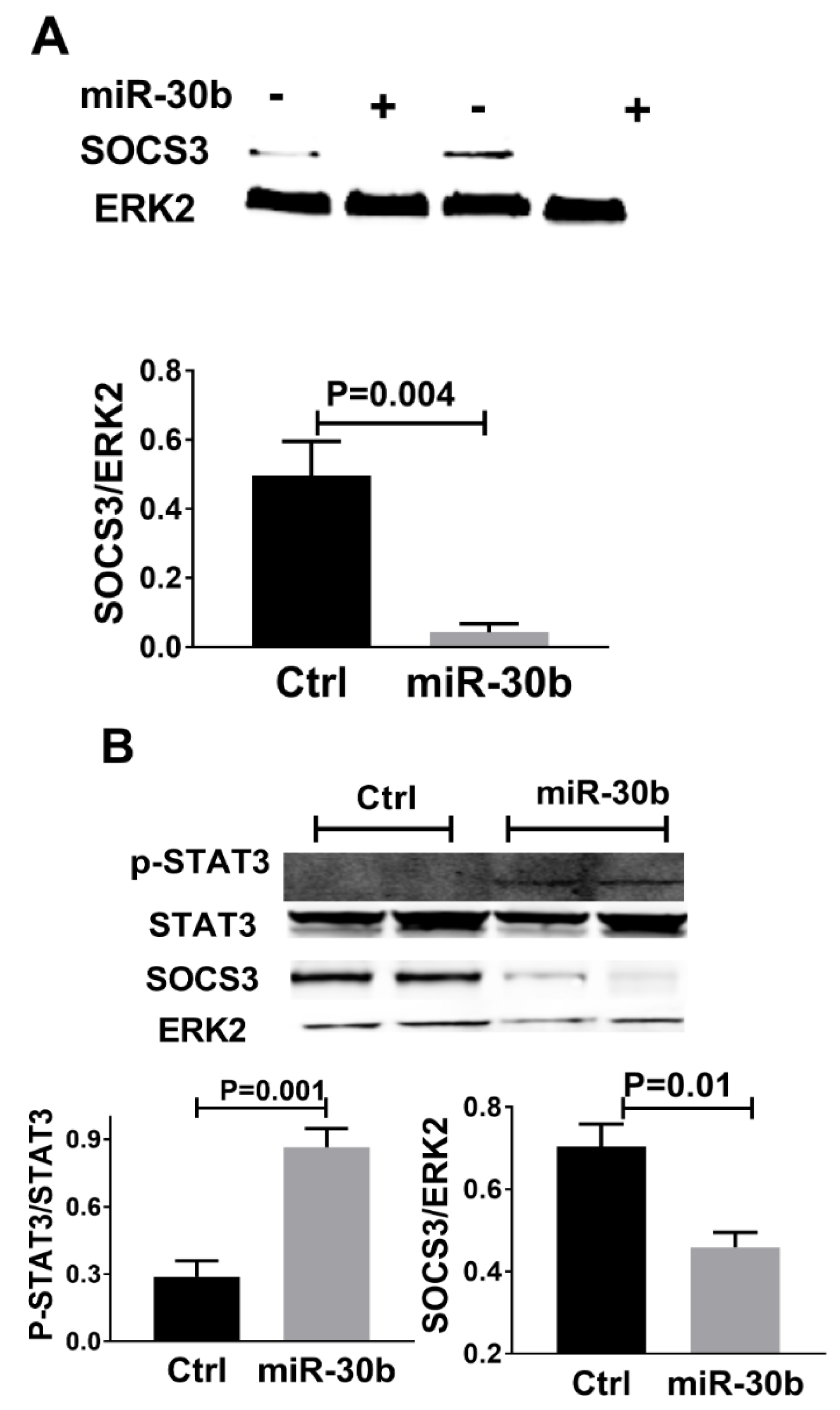
2.5. MiR-30b Increased STAT3 Phosphorylation and Reduced SOCS3 Expression
3. Discussion
4. Material and Methods
4.1. Hind-Limb Ischemia (HLI) Model, Perfusion Recovery, and In Vitro Transfection
4.2. Identification of Transcripts Regulated by IL-21R following HLI
4.3. RNA Isolation and Sequencing
4.4. Quantitative RT-PCR (qPCR)
4.5. Protein Analysis
4.6. In Vitro Transfection, Cellular Viability and Angiogenesis Assay
4.7. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
References
- Annex, B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef]
- Tanaka, M.; Taketomi, K.; Yonemitsu, Y. Therapeutic angiogenesis: Recent and future prospects of gene therapy in peripheral artery disease. Curr. Gene. Ther. 2014, 14, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Troidl, K.; Schaper, W. Arteriogenesis versus angiogenesis in peripheral artery disease. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. S1), 27–29. [Google Scholar] [CrossRef]
- Niiyama, H.; Huang, N.F.; Rollins, M.D.; Cooke, J.P. Murine model of hindlimb ischemia. J. Vis. Exp. 2009, 23, 1035. [Google Scholar] [CrossRef]
- Dokun, A.O.; Keum, S.; Hazarika, S.; Li, Y.; Lamonte, G.M.; Wheeler, F.; Marchuk, D.A.; Annex, B.H. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 2008, 117, 1207–1215. [Google Scholar] [CrossRef]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1746. [Google Scholar] [CrossRef]
- Wang, T.; Cunningham, A.; Dokun, A.O.; Hazarika, S.; Houston, K.; Chen, L.; Lye, R.J.; Spolski, R.; Leonard, W.J.; Annex, B.H. Loss of interleukin-21 receptor activation in hypoxic endothelial cells impairs perfusion recovery after hindlimb ischemia. Arter. Thromb. Vasc. Biol. 2015, 35, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Cunningham, A.; Houston, K.; Sharma, A.M.; Chen, L.; Dokun, A.O.; Lye, R.J.; Spolski, R.; Leonard, W.J.; Annex, B.H. Endothelial interleukin-21 receptor up-regulation in peripheral artery disease. Vasc. Med. 2016, 21, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008, 26, 57–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booty, M.G.; Barreira-Silva, P.; Carpenter, S.M.; Nunes-Alves, C.; Jacques, M.K.; Stowell, B.L.; Jayaraman, P.; Beamer, G.; Behar, S.M. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci. Rep. 2016, 6, 36720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Que, R.; Liu, B.; Li, M.; Cai, J.; Shou, D.; Wen, L.; Liu, D.; Chen, L.; Liang, T.; et al. Role of IL-21 signaling pathway in transplant-related biology. Transpl. Rev. 2016, 30, 27–30. [Google Scholar] [CrossRef]
- Ganta, V.C.; Choi, M.; Kutateladze, A.; Annex, B.H. VEGF165b Modulates Endothelial VEGFR1-STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circ. Res. 2017, 120, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, S.; Farber, C.R.; Dokun, A.O.; Pitsillides, A.N.; Wang, T.; Lye, R.J.; Annex, B.H. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation 2013, 127, 1818–1828. [Google Scholar] [CrossRef] [Green Version]
- Ganta, V.C.; Choi, M.H.; Kutateladze, A.; Fox, T.E.; Farber, C.R.; Annex, B.H. A MicroRNA93-IRF9-IRG1-Itaconic Acid Pathway Modulates M2-like-Macrophage Polarization to Revascularize Ischemic Muscle. Circulation 2017, 135, 2403. [Google Scholar] [CrossRef] [PubMed]
- Dokun, A.O.; Chen, L.; Lanjewar, S.S.; Lye, R.J.; Annex, B.H. Glycaemic control improves perfusion recovery and VEGFR2 protein expression in diabetic mice following experimental PAD. Cardiovasc. Res. 2014, 101, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Dokun, A.O.; Chen, L.; Okutsu, M.; Farber, C.R.; Hazarika, S.; Jones, W.S.; Craig, D.; Marchuk, D.A.; Lye, R.J.; Shah, S.H.; et al. ADAM12: A genetic modifier of preclinical peripheral arterial disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H790–H803. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Hazarika, S.; Xie, D.H.; Pippen, A.M.; Kontos, C.D.; Annex, B.H. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes 2007, 56, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Spolski, R.; Wang, L.; Wang, C.K.; Bonville, C.; Domachowske, J.; Kim, H.P.; Yu, Z.X.; Leonard, W. IL-21 promotes the pathologic immune response to Pneumovirus infection. J. Immunol. 2012, 188, 1924–1932. [Google Scholar] [CrossRef]
- Calabrese, G.; Bennett, B.J.; Orozco, L.; Kang, H.M.; Eskin, E.; Dombret, C.; De Backer, O.; Lusis, A.J.; Farber, C.R. Systems Genetic Analysis of Osteoblast-Lineage Cells. PLoS Genet. 2012, 8, e1003150. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Cunningham, A.; Dokun, A.; Hazarika, S.; Chen, L.; Lye, R.; Leonard, W.; Annex, B. Angiogenic Properties of Interleukin-21 under Hypoxic Conditions. Blood 2014. e-Letter. [Google Scholar]
- Wang, T.; Satoh, F.; Morimoto, R.; Nakamura, Y.; Sasano, H.; Auchus, R.J.; Edwards, M.A.; Rainey, W.E. Gene expression profiles in aldosterone-producing adenomas and adjacent adrenal glands. Eur. J. Endocrinol. 2011, 164, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Rowland, J.G.; Parmar, J.; Nesterova, M.; Seki, T.; Rainey, W.E. Comparison of aldosterone production among human adrenocortical cell lines. Horm. Metab. Res. 2012, 44, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, S.; Dokun, A.O.; Li, Y.; Popel, A.S.; Kontos, C.D.; Annex, B.H. Impaired angiogenesis after Hindlimb ischemia in type 2 diabetes Mellitus—Differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ. Res. 2007, 101, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Hazarika, S.; Angelo, M.; Li, Y.J.; Aldrich, A.J.; Odronic, S.I.; Yan, Z.; Stamler, J.S.; Annex, B.H. Myocyte Specific Overexpression of Myoglobin Impairs Angiogenesis After Hind-Limb Ischemia. Arterioscl. Throm. Vas. 2008, 28, 2144–2175. [Google Scholar] [CrossRef] [Green Version]
- Meisner, J.K.; Song, J.; Annex, B.H.; Price, R.J. Myoglobin Overexpression Inhibits Reperfusion in the Ischemic Mouse Hindlimb through Impaired Angiogenesis but Not Arteriogenesis. Am. J. Pathol. 2013, 183, 1710–1718. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, F.; Niu, R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci. Rep. 2015, 5, 17663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adoro, S.; Cubillos-Ruiz, J.R.; Chen, X.; Deruaz, M.; Vrbanac, V.D.; Song, M.; Park, S.; Murooka, T.T.; Dudek, T.E.; Luster, A.D.; et al. IL-21 induces antiviral microRNA-29 in CD4 T cells to limit HIV-1 infection. Nat. Commun. 2015, 6, 7562. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.K.; Andersen, T.; Bak, R.O.; Yiu, G.; Sorensen, C.M.; Stengaard-Pedersen, K.; Mikkelsen, J.G.; Utz, P.J.; Holm, C.K.; Deleuran, B. Overexpression of microRNA-155 increases IL-21 mediated STAT3 signaling and IL-21 production in systemic lupus erythematosus. Arthritis. Res. Ther. 2015, 17, 154. [Google Scholar] [CrossRef] [Green Version]
- De Cecco, L.; Capaia, M.; Zupo, S.; Cutrona, G.; Matis, S.; Brizzolara, A.; Orengo, A.M.; Croce, M.; Marchesi, E.; Ferrarini, M.; et al. Interleukin 21 Controls mRNA and MicroRNA Expression in CD40-Activated Chronic Lymphocytic Leukemia Cells. PLoS ONE 2015, 10, e0134706. [Google Scholar] [CrossRef]
- Bridge, G.; Monteiro, R.; Henderson, S.; Emuss, V.; Lagos, D.; Georgopoulou, D.; Patient, R.; Boshoff, C. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood 2012, 120, 5063–5072. [Google Scholar] [CrossRef] [Green Version]
- Howe, G.A.; Kazda, K.; Addison, C.L. MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2. PLoS ONE 2017, 12, e0185619. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Wu, X.; Jiang, Z.; Kasman, I.; Yao, J.; Guan, Y.; Oeh, J.; Modrusan, Z.; Bais, C.; Sampath, D.; et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012, 31, 3513–3523. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Mi, S.; Liang, X.; Zhang, Z.; Su, X.; Liu, J.; Chen, Y.; Wang, M.; Zhang, Y.; et al. Both miR-17-5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression. J. Immunol. 2011, 186, 4716–4724. [Google Scholar] [CrossRef] [Green Version]
- Gaziel-Sovran, A.; Segura, M.F.; Di Micco, R.; Collins, M.K.; Hanniford, D.; Vega-Saenz de Miera, E.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011, 20, 104–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, K.N.; Nielsen, B.S.; Lindebjerg, J.; Hansen, T.F.; Holst, R.; Sorensen, F.B. microRNA-17 Is the Most Up-Regulated Member of the miR-17-92 Cluster during Early Colon Cancer Evolution. PLoS ONE 2015, 10, e0140503. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, S.; Zhao, Y.; Zhang, H.; Wu, Q.; Chen, F. Global analysis of miRNA gene clusters and gene families reveals dynamic and coordinated expression. BioMed Res. Int. 2014, 2014, 782490. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gibson, N.W. Engineered microRNA therapeutics. J. R. Coll. Physicians Edinb. 2014, 44, 196–200. [Google Scholar] [CrossRef]
- Barger, J.F.; Nana-Sinkam, S.P. MicroRNA as tools and therapeutics in lung cancer. Respir. Med. 2015, 109, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Sluijter, J.P.; van Mil, A. MicroRNA Therapeutics for Cardiac Regeneration. Mini Rev. Med. Chem. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E.; Qin, G.; Losordo, D.W.; Kishore, R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009, 104, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.J.; Harms, U.; Rex, A.; Szulzewsky, F.; Wolf, S.A.; Grittner, U.; Lattig-Tunnemann, G.; Sendtner, M.; Kettenmann, H.; Dirnagl, U.; et al. Vascular Signal Transducer and Activator of Transcription-3 Promotes Angiogenesis and Neuroplasticity Long-Term after Stroke. Circulation 2015, 131, 1772–1782. [Google Scholar] [CrossRef] [Green Version]




| miRs | Fold | q-Value |
|---|---|---|
| miR-343 | 0.03 | 0.01 |
| miR-5103 | 0.06 | 0.01 |
| miR-100, miR-let7a-2 | 0.17 | 0.01 |
| miR-30b, miR-30d | 0.20 | 0.01 |
| miR-322,miR-351,miR-503 | 0.31 | 0.01 |
| miR-3572 | 10.08 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yang, L.; Yuan, M.; Farber, C.R.; Spolski, R.; Leonard, W.J.; Ganta, V.C.; Annex, B.H. MicroRNA-30b Is Both Necessary and Sufficient for Interleukin-21 Receptor-Mediated Angiogenesis in Experimental Peripheral Arterial Disease. Int. J. Mol. Sci. 2022, 23, 271. https://doi.org/10.3390/ijms23010271
Wang T, Yang L, Yuan M, Farber CR, Spolski R, Leonard WJ, Ganta VC, Annex BH. MicroRNA-30b Is Both Necessary and Sufficient for Interleukin-21 Receptor-Mediated Angiogenesis in Experimental Peripheral Arterial Disease. International Journal of Molecular Sciences. 2022; 23(1):271. https://doi.org/10.3390/ijms23010271
Chicago/Turabian StyleWang, Tao, Liang Yang, Mingjie Yuan, Charles R. Farber, Rosanne Spolski, Warren J. Leonard, Vijay C. Ganta, and Brian H. Annex. 2022. "MicroRNA-30b Is Both Necessary and Sufficient for Interleukin-21 Receptor-Mediated Angiogenesis in Experimental Peripheral Arterial Disease" International Journal of Molecular Sciences 23, no. 1: 271. https://doi.org/10.3390/ijms23010271






