Pressure Loading Induces DNA Damage in Human Hepatocyte Line L02 Cells via the ERK1/2–Dicer Signaling Pathway
Abstract
:1. Introduction
2. Results
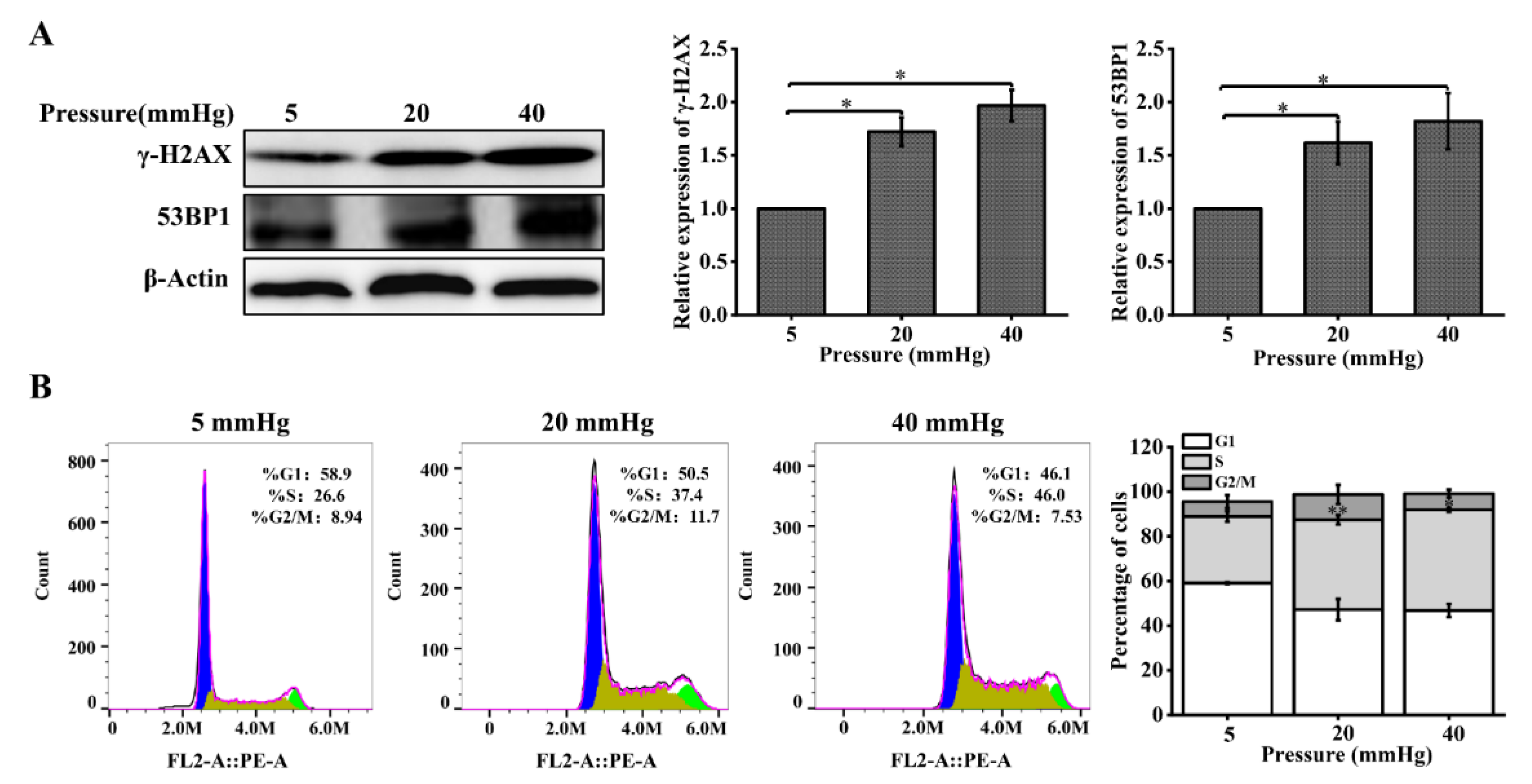
2.1. Pressure Aggravates DNA Damage in Hepatocytes and Activates the DDR Pathway
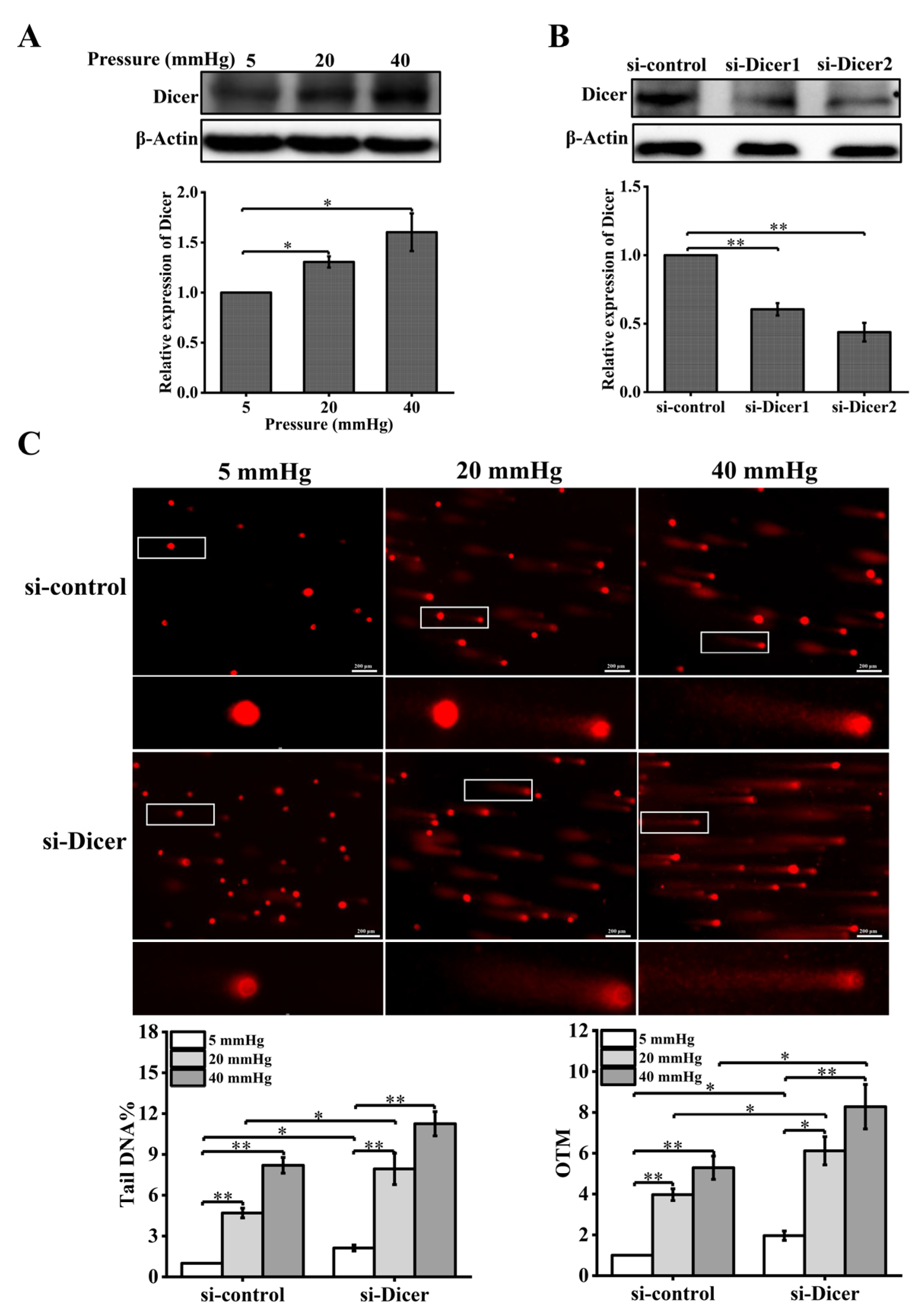
2.2. Downregulation of Dicer Expression Exacerbates Stress-Induced DNA Damage by Attenuating the DDR Pathway Response
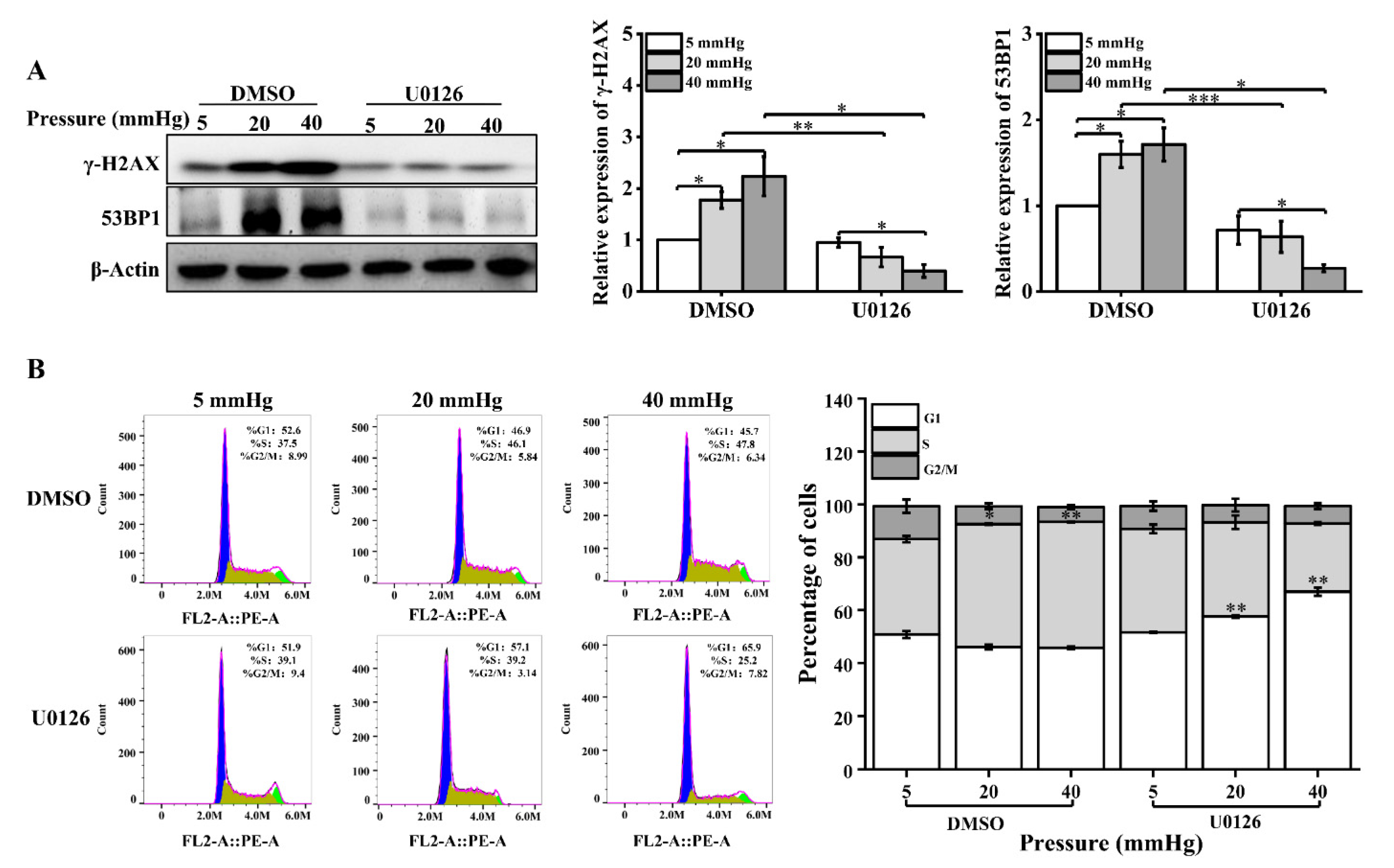
2.3. ERK1/2 Signaling Molecules Regulate Dicer Expression Levels and DNA Damage in Hepatocytes under Pressure Loading
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Pressure-Loading Apparatus and Pressure Loading on Cells
4.3. Comet Assay
4.4. Cell-Cycle Analysis
4.5. siRNA Transfection
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Zhang, Y.; Wang, X.; Li, S.; Tang, L. Substrate stiffness drives epithelial to mesenchymal transition and proliferation through the NEAT1-Wnt/β-Catenin pathway in liver cancer. Int. J. Mol. Sci. 2021, 22, 12066. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.S.; Tung, J.C.; Zhou, V.X.; Grenert, J.P.; Malato, Y.; Rezvani, M.; Español-Suñer, R.; Willenbring, H.; Weaver, V.M.; Chang, T.T. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology 2016, 64, 261–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, A.; Djouder, N. Cirrhosis: A questioned risk factor for hepatocellular carcinoma. Trends Cancer 2021, 7, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev. Cell 2019, 49, 920–935.e925. [Google Scholar] [CrossRef]
- dos Santos, Á.; Cook, A.W.; Gough, R.E.; Schilling, M.; Olszok, N.A.; Nora, A.; Brown, I.; Wang, L.; Aaron, J.; Martin-Fernandez, M.L.; et al. DNA damage alters nuclear mechanics through chromatin reorganization. Nucleic Acids Res. 2021, 49, 340–353. [Google Scholar] [CrossRef]
- Sáez, G.T. DNA injury and repair systems. Int. J. Mol. Sci. 2018, 19, 1902. [Google Scholar] [CrossRef] [Green Version]
- Ribezzo, F.; Shiloh, Y.; Schumacher, B. Systemic DNA damage responses in aging and diseases. Semin. Cancer Biol. 2016, 37, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The trinity at the heart of the DNA damage response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [Green Version]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000, 10, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef] [Green Version]
- Chitale, S.; Richly, H. DICER- and MMSET-catalyzed H4K20me2 recruits the nucleotide excision repair factor XPA to DNA damage sites. J. Cell Biol. 2017, 217, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Swahari, V.; Nakamura, A.; Baran-Gale, J.; Garcia, I.; Crowther, A.J.; Sons, R.; Gershon, T.R.; Hammond, S.; Sethupathy, P.; Deshmukh, M. Essential function of Dicer in resolving DNA damage in the rapidly dividing cells of the developing and malignant cerebellum. Cell Rep. 2016, 14, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelini, F.; Pitchiaya, S.; Vitelli, V.; Sharma, S.; Gioia, U.; Pessina, F.; Cabrini, M.; Wang, Y.; Capozzo, I.; Iannelli, F.; et al. Damage-induced lncRNAs control the DNA damage response through interaction with DDRNAs at individual double-strand breaks. Nat. Cell Biol. 2017, 19, 1400–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.H.; Hsu, T.W.; Chen, H.A.; Su, C.M.; Huang, M.T.; Chuang, T.H.; Leo Su, J.; Hsieh, C.L.; Chiu, C.F. ERK-mediated transcriptional activation of Dicer is involved in gemcitabine resistance of pancreatic cancer. J. Cell Physiol. 2021, 236, 4420–4434. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yang, X.G.; Wang, F.; Ma, X.Y. IL-1α induces apoptosis and inhibits the osteoblast differentiation of MC3T3-E1 cells through the JNK and p38 MAPK pathways. Int. J. Mol. Med. 2016, 38, 319–327. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Zhang, Y.; Xu, H.; Xu, D.; Wei, Z.; Gao, X.; Cai, W.; Mao, N.; Zhang, L.; et al. Matrix stiffness regulates α-TAT1-mediated acetylation of α-tubulin and promotes silica-induced epithelial–mesenchymal transition via DNA damage. J. Cell Sci. 2021, 134, jcs243394. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Kontek, R. Cyclin-dependent kinases in DNA damage response. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188716. [Google Scholar] [CrossRef]
- Cardano, M.; Buscemi, G.; Zannini, L. Sex disparities in DNA damage response pathways: Novel determinants in cancer formation and therapy. iScience 2022, 25, 103875. [Google Scholar] [CrossRef]
- Le, J.; Perez, E.; Nemzow, L.; Gong, F. Role of deubiquitinases in DNA damage response. DNA Repair 2019, 76, 89–98. [Google Scholar] [CrossRef]
- Francia, S.; Cabrini, M.; Matti, V.; Oldani, A.; d’Adda di Fagagna, F. DICER, DROSHA and DNA damage response RNAs are necessary for the secondary recruitment of DNA damage response factors. J. Cell Sci. 2016, 129, 1468–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskoski, R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Breton, S. The MAPK/ERK signaling pathway regulates the expression and localization of Cx43 in mouse proximal epididymis. Biol. Reprod. 2022, ioac034. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.P.; Luo, Q.; Deng, B.; Ju, Y.; Song, G.B. Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic glycolysis via the MAPK-YAP signaling. Cancers 2020, 12, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, A.K.; Andring, B.; Patel, A.; Trimmer, C.; Kalva, S.P. Portal hypertension: A review of portosystemic collateral pathways and endovascular interventions. Clin. Liver Dis. 2015, 70, 1047–1059. [Google Scholar] [CrossRef]
- Balog, S.; Li, Y.; Ogawa, T.; Miki, T.; Saito, T.; French, S.W.; Asahina, K. Development of capsular fibrosis beneath the liver surface in humans and mice. Hepatology 2020, 71, 291–305. [Google Scholar] [CrossRef]
- Guixé-Muntet, S.; Ortega-Ribera, M.; Wang, C.; Selicean, S.; Andreu, I.; Kechagia, J.Z.; Fondevila, C.; Roca-Cusachs, P.; Dufour, J.F.; Bosch, J.; et al. Nuclear deformation mediates liver cell mechanosensing in cirrhosis. JHEP Rep. 2020, 2, 100145. [Google Scholar] [CrossRef]
- Shah, P.; Hobson, C.M.; Cheng, S.; Colville, M.J.; Paszek, M.J.; Superfine, R.; Lammerding, J. Nuclear Deformation Causes DNA damage by increasing replication stress. Curr. Biol. 2021, 31, 753–765.e756. [Google Scholar] [CrossRef]
- Mammel, A.E.; Hatch, E.M. Genome instability from nuclear catastrophe and DNA damage. Semin. Cell Dev. Biol. 2022, 123, 131–139. [Google Scholar] [CrossRef]
- Chitale, S.; Richly, H. DICER and ZRF1 contribute to chromatin decondensation during nucleotide excision repair. Nucleic Acids Res. 2017, 45, 5901–5912. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.Y.; Li, G.; Deng, Z.J.; Liu, L.Y.; Chen, L.; Tang, J.Z.; Wang, Y.Q.; Cao, S.T.; Fang, Y.X.; Wen, F.; et al. Dicer interacts with SIRT7 and regulates H3K18 deacetylation in response to DNA damaging agents. Nucleic Acids Res. 2016, 44, 3629–3642. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.F.; Wu, X.; Zhang, H.C.; Chen, L.; Zhang, P.Y.; Liu, L.Y.; Ma, D.; Chen, T.; Zhou, L.; et al. Dicer regulates non-homologous end joining and is associated with chemosensitivity in colon cancer patients. Carcinogenesis 2017, 38, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Francia, S.; Michelini, F.; Saxena, A.; Tang, D.; de Hoon, M.; Anelli, V.; Mione, M.; Carninci, P.; d’Adda di Fagagna, F. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 2012, 488, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, M.; Wang, J.; Zhang, X.; Wang, X.; Wang, P.; Wang, H.; Li, W.; Wang, Y. DICER-dependent biogenesis of let-7 miRNAs affects human cell response to DNA damage via targeting p21/p27. Nucleic Acids Res. 2015, 43, 1626–1636. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.K.; Becker, A.; Park, J.I. Growth inhibitory signaling of the Raf/MEK/ERK pathway. Int. J. Mol. Sci. 2020, 21, 5436. [Google Scholar] [CrossRef]
- Wei, F.; Yan, J.; Tang, D. Extracellular signal-regulated kinases modulate DNA damage response—a contributing factor to using MEK inhibitors in cancer therapy. Curr. Med. Chem. 2011, 18, 5476–5482. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Xie, Y.; Tao, L.; Tang, D. Both ERK1 and ERK2 kinases promote G2/M arrest in etoposide-treated MCF7 cells by facilitating ATM activation. Cell. Signal. 2010, 22, 1783–1789. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Fan, Y.; Luo, Q.; Song, G. Pressure Loading Induces DNA Damage in Human Hepatocyte Line L02 Cells via the ERK1/2–Dicer Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 5342. https://doi.org/10.3390/ijms23105342
Tang Y, Fan Y, Luo Q, Song G. Pressure Loading Induces DNA Damage in Human Hepatocyte Line L02 Cells via the ERK1/2–Dicer Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(10):5342. https://doi.org/10.3390/ijms23105342
Chicago/Turabian StyleTang, Yanping, Yanan Fan, Qing Luo, and Guanbin Song. 2022. "Pressure Loading Induces DNA Damage in Human Hepatocyte Line L02 Cells via the ERK1/2–Dicer Signaling Pathway" International Journal of Molecular Sciences 23, no. 10: 5342. https://doi.org/10.3390/ijms23105342





