The Development of Hindlimb Postural Asymmetry Induced by Focal Traumatic Brain Injury Is Not Related to Serotonin 2A/C Receptor Expression in the Spinal Cord
Abstract
:1. Introduction
2. Results
2.1. Time Course of Hindlimb Postural Asymmetry (HL-PA) Development over 4 Weeks
2.2. Gait Pattern after Traumatic Brain Injury (TBI) and Sham Surgery
2.2.1. TBI Rats Had a Longer Stride Length Than Sham Rats
2.2.2. Both TBI and Sham Surgery Changed the Hind-Paw Placement Angle
2.3. No Significant Changes in 5-HT2AR/2CR Expression in Lumbar Spinal Cord after TBI
2.4. No Changes in the Expression of the 5-HT Fibers in the Spinal Cord after TBI
3. Discussion
4. Materials and Methods
4.1. TBI and Sham Surgery
4.2. Postural Analysis
4.2.1. Analysis of HL-PA
4.2.2. Analysis of Gait Pattern during Walking
4.3. Tissue Preparation
4.4. Histological Processing of the Brain Injury Sites
4.5. Immunohistochemistry
4.5.1. 5-HT2AR
4.5.2. 5-HT2CR
4.5.3. 5-HT
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degani, A.M.; Santos, M.M.; Leonard, C.T.; Rau, T.F.; Patel, S.A.; Mohapatra, S.; Danna-Dos-Santos, A. The effects of mild traumatic brain injury on postural control. Brain Inj. 2017, 31, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Drijkoningen, D.; Caeyenberghs, K.; Leunissen, I.; Vander Linden, C.; Leemans, A.; Sunaert, S.; Duysens, J.; Swinnen, S.P. Training-induced improvements in postural control are accompanied by alterations in cerebellar white matter in brain injured patients. Neuroimage Clin. 2015, 7, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Klit, H.; Finnerup, N.B.; Jensen, T.S. Central post-stroke pain: Clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009, 8, 857–868. [Google Scholar] [CrossRef]
- Ward, N.S. Restoring brain function after stroke—Bridging the gap between animals and humans. Nat. Rev. Neurol. 2017, 13, 244–255. [Google Scholar] [CrossRef]
- DiGiorgio, A. Persistence of postural and motor asymmetries of cerebellar origin in spinal animals: I, II, III. Arch. Fisiol. 1929, 27, 518–580. [Google Scholar]
- DiGiorgio, A. Influences of the cerebellum-neocerebellum on the postural tone of the limbs and cerebellar somatotopy in the rhomboencephalic animal. Arch. Fisiol. 1942, 42, 25–79. [Google Scholar]
- Zhang, M.; Watanabe, H.; Sarkisyan, D.; Andersen, M.S.; Nosova, O.; Galatenko, V.; Carvalho, L.; Lukoyanov, N.; Thelin, J.; Schouenborg, J.; et al. Hindlimb motor responses to unilateral brain injury: Spinal cord encoding and left-right asymmetry. Brain Commun. 2020, 2, fcaa055. [Google Scholar] [CrossRef]
- Pin-Barre, C.; Laurin, J. Physical Exercise as a Diagnostic, Rehabilitation, and Preventive Tool: Influence on Neuroplasticity and Motor Recovery after Stroke. Neural Plast. 2015, 2015, 608581. [Google Scholar] [CrossRef] [Green Version]
- Sist, B.; Fouad, K.; Winship, I.R. Plasticity beyond peri-infarct cortex: Spinal up regulation of structural plasticity, neurotrophins, and inflammatory cytokines during recovery from cortical stroke. Exp. Neurol. 2014, 252, 47–56. [Google Scholar] [CrossRef]
- Tennant, K.A. Thinking outside the brain: Structural plasticity in the spinal cord promotes recovery from cortical stroke. Exp. Neurol. 2014, 254, 195–199. [Google Scholar] [CrossRef]
- Wolpaw, J.R. Harnessing neuroplasticity for clinical applications. Brain 2012, 135, e216. [Google Scholar] [CrossRef] [Green Version]
- Frigon, A.; Barriere, G.; Leblond, H.; Rossignol, S. Asymmetric changes in cutaneous reflexes after a partial spinal lesion and retention following spinalization during locomotion in the cat. J. Neurophysiol. 2009, 102, 2667–2680. [Google Scholar] [CrossRef] [Green Version]
- Hultborn, H.; Malmsten, J. Changes in segmental reflexes following chronic spinal cord hemisection in the cat. II. Conditioned monosynaptic test reflexes. Acta Physiol. Scand. 1983, 119, 423–433. [Google Scholar] [CrossRef]
- Hultborn, H.; Malmsten, J. Changes in segmental reflexes following chronic spinal cord hemisection in the cat. I. Increased monosynaptic and polysynaptic ventral root discharges. Acta Physiol. Scand. 1983, 119, 405–422. [Google Scholar] [CrossRef]
- Malmsten, J. Time course of segmental reflex changes after chronic spinal cord hemisection in the rat. Acta Physiol. Scand. 1983, 119, 435–443. [Google Scholar] [CrossRef]
- Rossignol, S.; Frigon, A. Recovery of locomotion after spinal cord injury: Some facts and mechanisms. Annu. Rev. Neurosci. 2011, 34, 413–440. [Google Scholar] [CrossRef] [Green Version]
- Antri, M.; Barthe, J.Y.; Mouffle, C.; Orsal, D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci. Lett. 2005, 384, 162–167. [Google Scholar] [CrossRef]
- Antri, M.; Orsal, D.; Barthe, J.Y. Locomotor recovery in the chronic spinal rat: Effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 2002, 16, 467–476. [Google Scholar] [CrossRef]
- Fuller, D.D.; Baker-Herman, T.L.; Golder, F.J.; Doperalski, N.J.; Watters, J.J.; Mitchell, G.S. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J. Neurotrauma 2005, 22, 203–213. [Google Scholar] [CrossRef]
- Schmidt, B.J.; Jordan, L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000, 53, 689–710. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Basura, G.J.; Goshgarian, H.G. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J. Appl. Physiol. 2001, 91, 2665–2673. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M. Normal Distribution and Plasticity of Serotonin Receptors after Spinal Cord Injury and Their Impacts on Motor Outputs. In Recovery of Motor Function Following Spinal Cord Injury; Fuller, H., Gates, M., Eds.; Intech Open: London, UK, 2016; pp. 95–135. [Google Scholar] [CrossRef] [Green Version]
- Barbeau, H.; Bedard, P. Denervation supersensitivity to 5-hydroxytryptophan in rats following spinal transection and 5,7-dihydroxytryptamine injection. Neuropharmacology 1981, 20, 611–616. [Google Scholar] [CrossRef]
- Barber, R.P.; Phelps, P.E.; Houser, C.R.; Crawford, G.D.; Salvaterra, P.M.; Vaughn, J.E. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: An immunocytochemical study. J. Comp. Neurol. 1984, 229, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.J.; Li, X.; Li, Y.; Bennett, D.J. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 2006, 96, 1158–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.Y.; Wienecke, J.; Chen, M.; Hultborn, H.; Zhang, M. The time course of serotonin 2A receptor expression after spinal transection of rats: An immunohistochemical study. Neuroscience 2011, 177, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Wienecke, J.; Hultborn, H.; Zhang, M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: An immunohistochemical study. Brain Res. 2010, 1320, 60–68. [Google Scholar] [CrossRef]
- Ren, L.Q.; Wienecke, J.; Chen, M.; Moller, M.; Hultborn, H.; Zhang, M. The time course of serotonin 2C receptor expression after spinal transection of rats: An immunohistochemical study. Neuroscience 2013, 236, 31–46. [Google Scholar] [CrossRef]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Toda, T.; Ishida, K.; Kiyama, H.; Yamashita, T.; Lee, S. Down-regulation of KCC2 expression and phosphorylation in motoneurons, and increases the number of in primary afferent projections to motoneurons in mice with post-stroke spasticity. PLoS ONE 2014, 9, e114328. [Google Scholar] [CrossRef]
- Watanabe, H.; Nosova, O.; Sarkisyan, D.; Andersen, M.S.; Zhang, M.; Rorick-Kehn, L.; Clausen, F.; Gawel, K.; Kehr, J.; Hallberg, M.; et al. Ipsilesional versus contralesional postural deficits induced by unilateral brain trauma: A side reversal by opioid mechanism. Brain Commun. 2020, 2, fcaa208. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Duhaime, A.C.; Bullock, R.; Maas, A.I.; Valadka, A.; Manley, G.T.; Workshop Scientific, T.; Advisory Panel, M. Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 2008, 25, 719–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausser, N.; Johnson, K.; Parsley, M.A.; Guptarak, J.; Spratt, H.; Sell, S.L. Detecting Behavioral Deficits in Rats After Traumatic Brain Injury. J. Vis. Exp. 2018, 131, e56044. [Google Scholar] [CrossRef] [Green Version]
- Lukoyanov, N.; Watanabe, H.; Carvalho, L.S.; Kononenko, O.; Sarkisyan, D.; Zhang, M.; Andersen, M.S.; Lukoyanova, E.A.; Galatenko, V.; Tonevitsky, A.; et al. Left-right side-specific endocrine signaling complements neural pathways to mediate acute asymmetric effects of brain injury. eLife 2021, 10, e65247. [Google Scholar] [CrossRef]
- Kollen, B.; van de Port, I.; Lindeman, E.; Twisk, J.; Kwakkel, G. Predicting improvement in gait after stroke: A longitudinal prospective study. Stroke 2005, 36, 2676–2680. [Google Scholar] [CrossRef]
- Chow, J.W.; Yablon, S.A.; Horn, T.S.; Stokic, D.S. Temporospatial characteristics of gait in patients with lower limb muscle hypertonia after traumatic brain injury. Brain Inj. 2010, 24, 1575–1584. [Google Scholar] [CrossRef]
- Williams, G.; Galna, B.; Morris, M.E.; Olver, J. Spatiotemporal deficits and kinematic classification of gait following a traumatic brain injury: A systematic review. J. Head Trauma Rehabil. 2010, 25, 366–374. [Google Scholar] [CrossRef]
- Chow, J.W.; Stokic, D.S. Longitudinal Changes in Temporospatial Gait Characteristics during the First Year Post-Stroke. Brain Sci. 2021, 11, 1648. [Google Scholar] [CrossRef]
- Dever, A.; Powell, D.; Graham, L.; Mason, R.; Das, J.; Marshall, S.J.; Vitorio, R.; Godfrey, A.; Stuart, S. Gait Impairment in Traumatic Brain Injury: A Systematic Review. Sensors 2022, 22, 1480. [Google Scholar] [CrossRef]
- Katz-Leurer, M.; Rotem, H.; Keren, O.; Meyer, S. The relationship between step variability, muscle strength and functional walking performance in children with post-traumatic brain injury. Gait Posture 2009, 29, 154–157. [Google Scholar] [CrossRef]
- Brandstater, M.E.; de Bruin, H.; Gowland, C.; Clark, B.M. Hemiplegic gait: Analysis of temporal variables. Arch. Phys. Med. Rehabil. 1983, 64, 583–587. [Google Scholar]
- Chow, J.W.; Stokic, D.S. Gait Impairments in Patients Without Lower Limb Hypertonia Early Poststroke Are Related to Weakness of Paretic Knee Flexors. Arch. Phys. Med. Rehabil. 2019, 100, 1091–1101. [Google Scholar] [CrossRef]
- De Quervain, I.A.; Simon, S.R.; Leurgans, S.; Pease, W.S.; McAllister, D. Gait pattern in the early recovery period after stroke. J. Bone Jt. Surg. Am. 1996, 78, 1506–1514. [Google Scholar] [CrossRef] [Green Version]
- Alingh, J.F.; Groen, B.E.; Kamphuis, J.F.; Geurts, A.C.H.; Weerdesteyn, V. Task-specific training for improving propulsion symmetry and gait speed in people in the chronic phase after stroke: A proof-of-concept study. J. Neuroeng. Rehabil. 2021, 18, 69. [Google Scholar] [CrossRef]
- Cleland, B.T.; Schindler-Ivens, S. Symmetry Is Associated With Interlimb Coordination During Walking and Pedaling After Stroke. J. Neurol. Phys. Ther. 2022, 46, 81–87. [Google Scholar] [CrossRef]
- Yang, Y.R.; Yen, J.G.; Wang, R.Y.; Yen, L.L.; Lieu, F.K. Gait outcomes after additional backward walking training in patients with stroke: A randomized controlled trial. Clin. Rehabil. 2005, 19, 264–273. [Google Scholar] [CrossRef]
- Ji, S.G.; Kim, M.K. The effects of mirror therapy on the gait of subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2015, 29, 348–354. [Google Scholar] [CrossRef]
- Noh, H.J.; Lee, S.H.; Bang, D.H. Three-Dimensional Balance Training Using Visual Feedback on Balance and Walking Ability in Subacute Stroke Patients: A Single-Blinded Randomized Controlled Pilot Trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 994–1000. [Google Scholar] [CrossRef]
- van Bloemendaal, M.; Bus, S.A.; Nollet, F.; Geurts, A.C.H.; Beelen, A. Feasibility and Preliminary Efficacy of Gait Training Assisted by Multichannel Functional Electrical Stimulation in Early Stroke Rehabilitation: A Pilot Randomized Controlled Trial. Neurorehabil. Neural Repair. 2021, 35, 131–144. [Google Scholar] [CrossRef]
- Day, K.V.; Kautz, S.A.; Wu, S.S.; Suter, S.P.; Behrman, A.L. Foot placement variability as a walking balance mechanism post-spinal cord injury. Clin. Biomech. 2012, 27, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Niechwiej-Szwedo, E.; Inness, E.L.; Howe, J.A.; Jaglal, S.; McIlroy, W.E.; Verrier, M.C. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture 2007, 25, 70–77. [Google Scholar] [CrossRef]
- Stimpson, K.H.; Embry, A.E.; Dean, J.C. Post-stroke deficits in mediolateral foot placement accuracy depend on the prescribed walking task. J. Biomech. 2021, 128, 110738. [Google Scholar] [CrossRef]
- Drew, T.; Jiang, W.; Widajewicz, W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res. Brain Res. Rev. 2002, 40, 178–191. [Google Scholar] [CrossRef]
- Carmel, J.B.; Berrol, L.J.; Brus-Ramer, M.; Martin, J.H. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J. Neurosci. 2010, 30, 10918–10926. [Google Scholar] [CrossRef]
- Neumann, M.; Wang, Y.; Kim, S.; Hong, S.M.; Jeng, L.; Bilgen, M.; Liu, J. Assessing gait impairment following experimental traumatic brain injury in mice. J. Neurosci. Methods 2009, 176, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Haarman, J.A.M.; Vlutters, M.; Olde Keizer, R.; van Asseldonk, E.H.F.; Buurke, J.H.; Reenalda, J.; Rietman, J.S.; van der Kooij, H. Paretic versus non-paretic stepping responses following pelvis perturbations in walking chronic-stage stroke survivors. J. Neuroeng. Rehabil. 2017, 14, 106. [Google Scholar] [CrossRef] [Green Version]
- Ung, R.V.; Landry, E.S.; Rouleau, P.; Lapointe, N.P.; Rouillard, C.; Guertin, P.A. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur. J. Neurosci. 2008, 28, 2231–2242. [Google Scholar] [CrossRef]
- Basura, G.J.; Zhou, S.Y.; Walker, P.D.; Goshgarian, H.G. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp. Neurol. 2001, 169, 255–263. [Google Scholar] [CrossRef]
- Tan, A.M.; Chakrabarty, S.; Kimura, H.; Martin, J.H. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J. Neurosci. 2012, 32, 12896–12908. [Google Scholar] [CrossRef] [Green Version]
- Kononenko, O.; Galatenko, V.; Andersson, M.; Bazov, I.; Watanabe, H.; Zhou, X.W.; Iatsyshyna, A.; Mityakina, I.; Yakovleva, T.; Sarkisyan, D.; et al. Intra- and interregional coregulation of opioid genes: Broken symmetry in spinal circuits. FASEB J. 2017, 31, 1953–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, E.M.; Su, D.; Lopez-Velazquez, L.; Haus, D.L.; Perez, H.; Lacuesta, G.A.; Anderson, A.J.; Cummings, B.J. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen. Med. 2013, 8, 483–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, A.M.; Islam, R.; Cuellar, C.A.; Silvernail, J.L.; Knudsen, B.; Curley, D.E.; Strickland, T.; Manske, E.; Suwan, P.T.; Latypov, T.; et al. Newly regenerated axons via scaffolds promote sub-lesional reorganization and motor recovery with epidural electrical stimulation. NPJ Regen. Med. 2021, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, J.; Swiadek, N.; Ashmwe, M.; Ruhrnossl, A.; Oberhauser, V.; Kolbenschlag, J.; Hercher, D. Automated Gait Analysis to Assess Functional Recovery in Rodents with Peripheral Nerve or Spinal Cord Contusion Injury. J. Vis. Exp. 2020, 164, e61852. [Google Scholar] [CrossRef]
- Pingel, J.; Nielsen, M.S.; Lauridsen, T.; Rix, K.; Bech, M.; Alkjaer, T.; Andersen, I.T.; Nielsen, J.B.; Feidenhansl, R. Injection of high dose botulinum-toxin A leads to impaired skeletal muscle function and damage of the fibrilar and non-fibrilar structures. Sci. Rep. 2017, 7, 14746. [Google Scholar] [CrossRef] [PubMed]
- Aad, G.; Abbott, B.; Abdallah, J.; Abdinov, O.; Aben, R.; Abolins, M.; AbouZeid, O.S.; Abramowicz, H.; Abreu, H.; Abreu, R.; et al. Combined Measurement of the Higgs Boson Mass in pp Collisions at sqrt[s]=7 and 8 TeV with the ATLAS and CMS Experiments. Phys. Rev. Lett. 2015, 114, 191803. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Nosova, O.; Sarkisyan, D.; Storm Andersen, M.; Carvalho, L.; Galatenko, V.; Bazov, I.; Lukoyanov, N.; Maia, G.H.; Hallberg, M.; et al. Left-Right Side-Specific Neuropeptide Mechanism Mediates Contralateral Responses to a Unilateral Brain Injury. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Hall RD, L.E. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974, 66, 23–38. [Google Scholar] [CrossRef]

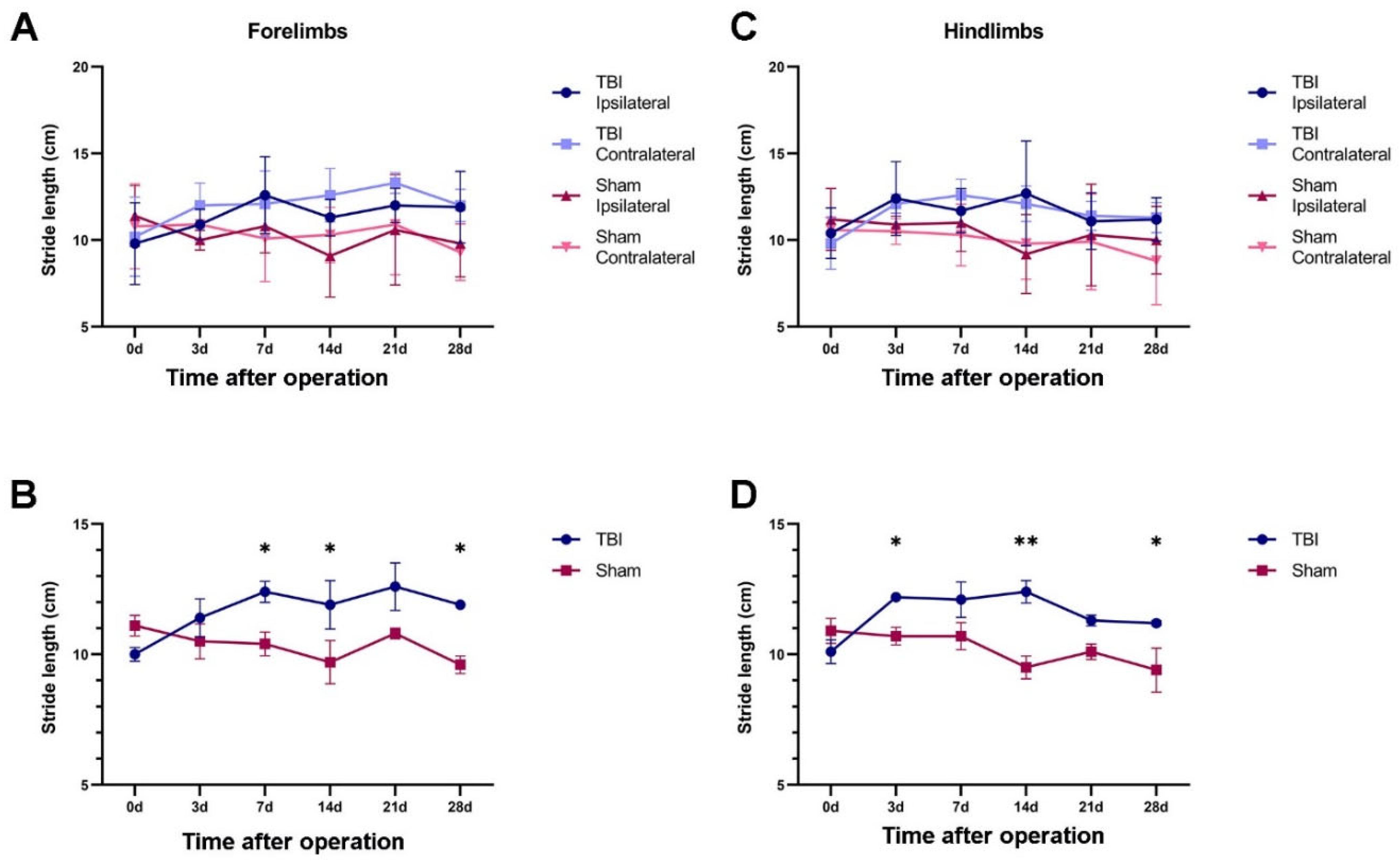

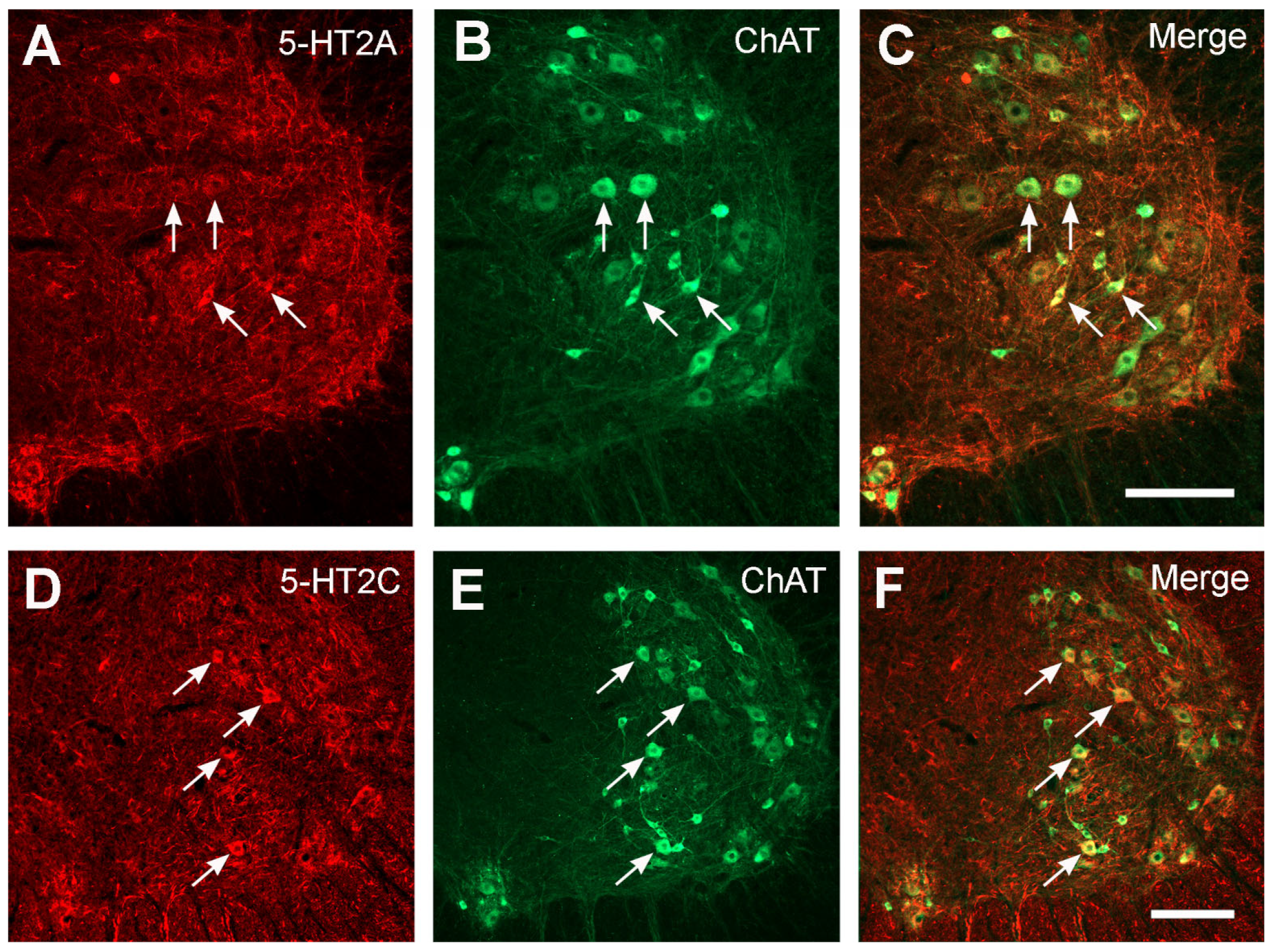
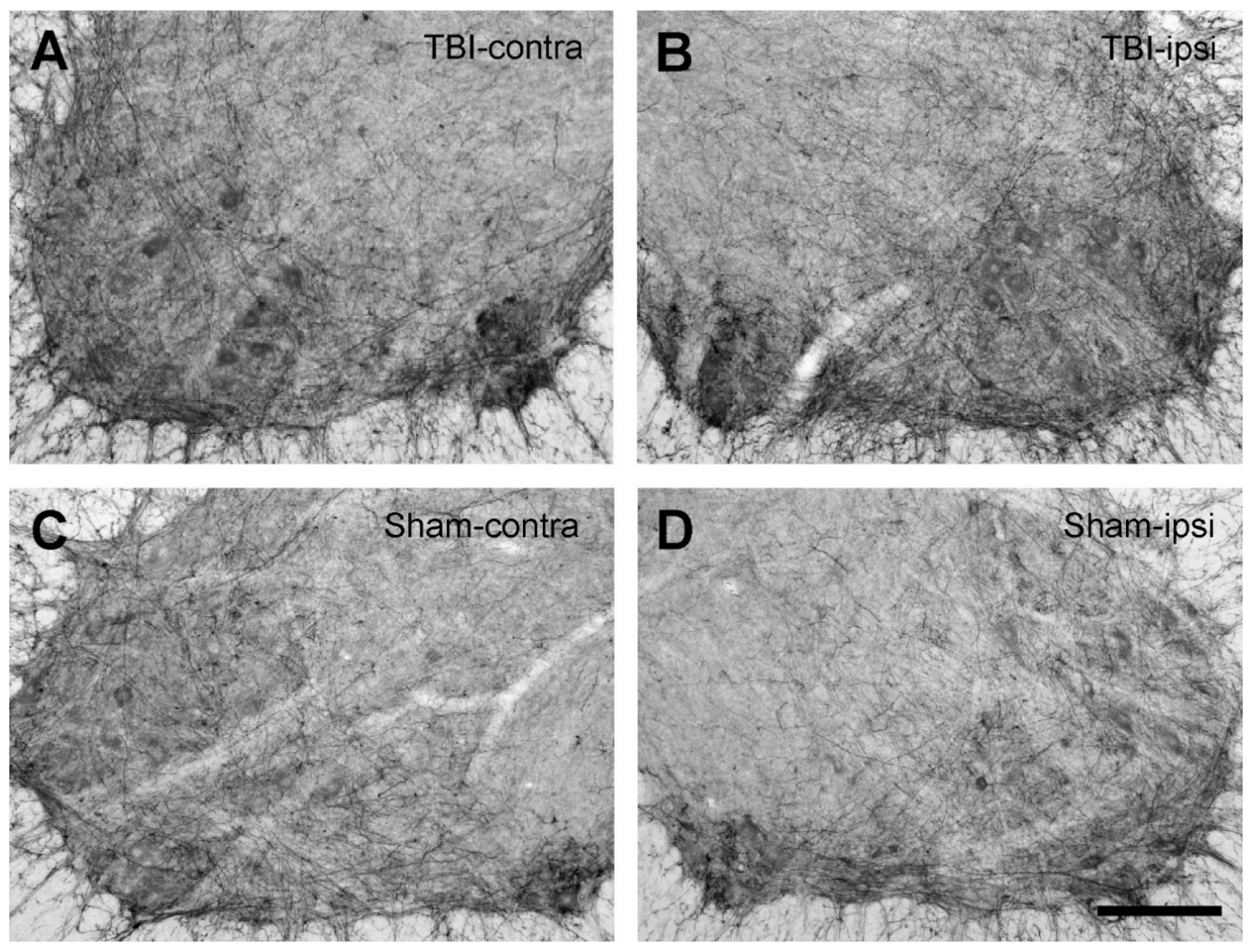

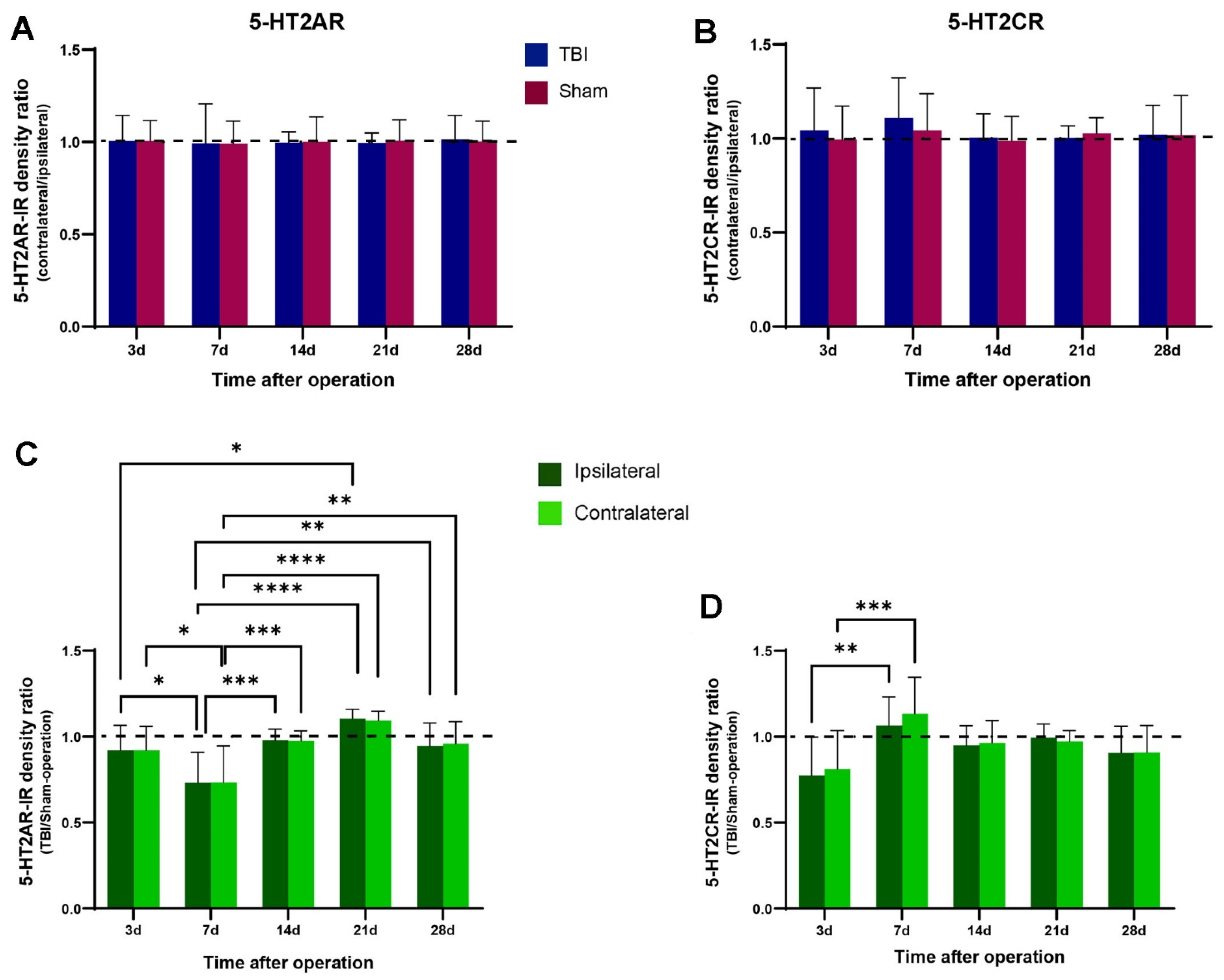




| Limbs | Group | TBI | Sham | ||
|---|---|---|---|---|---|
| Contra | Ipsi | Contra | Ipsi | ||
| Forelimbs | 3 days | 12.0 ± 1.3 | 10.9 ± 0.9 | 10.9 ± 1.0 | 10.0 ± 0.6 |
| 7 days | 12.1 ± 1.9 | 12.6 ± 2.2 | 10.1 ± 2.5 | 10.8 ± 1.5 | |
| 14 days | 12.6 ± 1.5 | 11.3 ± 1.1 | 10.3 ± 1.6 | 9.1 ± 2.4 | |
| 21 days | 13.3 ± 0.6 | 12.0 ± 1.0 | 10.9 ± 2.9 | 10.6 ± 3.2 | |
| 28 days | 12.0 ± 0.9 | 11.9 ± 2.1 | 9.3 ± 1.6 | 9.8 ± 1.9 | |
| Hindlimbs | 3 days | 12.1 ± 0.5 | 12.4 ± 2.1 | 10.5 ± 0.7 | 10.9 ± 0.5 |
| 7 days | 12.6 ± 0.9 | 11.7 ± 1.3 | 10.3 ± 1.8 | 11.0 ± 1.6 | |
| 14 days | 12.1 ± 1.0 | 12.7 ± 3.0 | 9.8 ± 2.1 | 9.2 ± 2.3 | |
| 21 days | 11.4 ± 0.8 | 11.1 ± 1.6 | 9.9 ± 2.8 | 10.3 ± 2.9 | |
| 28 days | 11.3 ± 0.9 | 11.2 ± 1.2 | 8.8 ± 2.5 | 10.0 ± 1.9 | |
| Survival Time | Total No. of Rats | HL-PA Analysis | Gait Pattern Analysis | 5-HT2AR IHC | 5-HT2CR IHC | 5-HT IHC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBI | Sham | TBI | Sham | TBI | Sham | TBI | Sham | TBI | Sham | TBI | Sham | |
| 3 days | 6 | 6 | 5 | 6 | - | - | 6 | 6 | 6 | 6 | - | - |
| 7 days | 9 | 9 | 5 | 4 | - | - | 6 | 6 | 5 | 5 | 3 | 3 |
| 14 days | 6 | 6 | 4 | 5 | - | - | 6 | 6 | 6 | 4 | - | - |
| 21 days | 6 | 6 | 5 | 6 | - | - | 5 | 5 | 5 | 5 | - | - |
| 28 days | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, M.S.; Güler, D.B.; Larsen, J.; Rich, K.K.; Svenningsen, Å.F.; Zhang, M. The Development of Hindlimb Postural Asymmetry Induced by Focal Traumatic Brain Injury Is Not Related to Serotonin 2A/C Receptor Expression in the Spinal Cord. Int. J. Mol. Sci. 2022, 23, 5358. https://doi.org/10.3390/ijms23105358
Andersen MS, Güler DB, Larsen J, Rich KK, Svenningsen ÅF, Zhang M. The Development of Hindlimb Postural Asymmetry Induced by Focal Traumatic Brain Injury Is Not Related to Serotonin 2A/C Receptor Expression in the Spinal Cord. International Journal of Molecular Sciences. 2022; 23(10):5358. https://doi.org/10.3390/ijms23105358
Chicago/Turabian StyleAndersen, Marlene Storm, Dilârâ Bedriye Güler, Jonas Larsen, Karen Kalhøj Rich, Åsa Fex Svenningsen, and Mengliang Zhang. 2022. "The Development of Hindlimb Postural Asymmetry Induced by Focal Traumatic Brain Injury Is Not Related to Serotonin 2A/C Receptor Expression in the Spinal Cord" International Journal of Molecular Sciences 23, no. 10: 5358. https://doi.org/10.3390/ijms23105358








