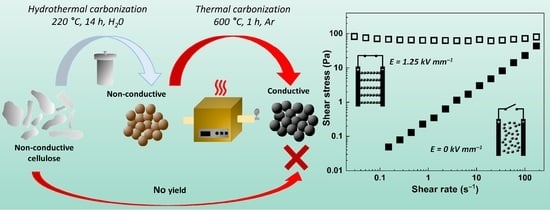
Transformation of Cellulose via Two-Step Carbonization to Conducting Carbonaceous Particles and Their Outstanding Electrorheological Performance
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Cellulose Carbonization
3.2. Particle Characterization
3.3. Preparation of Electrorheological Fluids and Their Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bober, P.; Kovarova, J.; Pfleger, J.; Stejskal, J.; Trchova, M.; Novak, I.; Berek, D. Twin carbons: The carbonization of cellulose or carbonized cellulose coated with a conducting polymer, polyaniline. Carbon 2016, 109, 836–842. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional Cellulose Beads: Preparation, Characterization, and Applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.X.; Odelius, K.; Rajarao, G.K.; Hakkarainen, M. Microwave carbonized cellulose for trace pharmaceutical adsorption. Chem. Eng. J. 2018, 346, 557–566. [Google Scholar] [CrossRef]
- Sheng, K.C.; Zhang, S.; Liu, J.L.; Shuang, E.; Jin, C.D.; Xu, Z.H.; Zhang, X.M. Hydrothermal carbonization of cellulose and xylan into hydrochars and application on glucose isomerization. J. Clean. Prod. 2019, 237, 117831. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Li, X.H.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Polprasert, C. Effects of hydrolysis and carbonization reactions on hydrochar production. Bioresour. Technol. 2015, 192, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Rejon, L.; Ramirez, A.; Paz, F.; Goycoolea, F.M.; Valdez, M.A. Response time and electrorheology of semidiluted gellan, xanthan and cellulose suspensions. Carbohydr. Polym. 2002, 48, 413–421. [Google Scholar] [CrossRef]
- Ramos-Tejada, M.M.; Arroyo, F.J.; Delgado, A.V. Negative electrorheological behavior in suspensions of inorganic particles. Langmuir 2010, 26, 16833–16840. [Google Scholar] [CrossRef]
- Tilki, T.; Yavuz, M.; Karabacak, C.; Cabuk, M.; Uluturk, M. Investigation of electrorheological properties of biodegradable modified cellulose/corn oil suspensions. Carbohydr. Res. 2010, 345, 672–679. [Google Scholar] [CrossRef]
- Kuznetsov, N.M.; Zagoskin, Y.D.; Bakirov, A.V.; Vdovichenko, A.Y.; Malakhov, S.N.; Istomina, A.P.; Chvalun, S.N. Is chitosan the promising candidate for filler in nature-friendly electrorheological fluids? Acs Sustain. Chem. Eng. 2021, 9, 3795–3803. [Google Scholar] [CrossRef]
- Gao, C.Y.; Kim, M.H.; Jin, H.J.; Choi, H.J. Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles. Polymers 2020, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Canto, M.A.; Fernandez-Silva, S.D.; Roman, C.; Garcia-Morales, M. On the electro-active control of nanocellulose-based functional biolubricants. ACS Appl. Mater. Interfaces 2020, 12, 46490–46500. [Google Scholar] [CrossRef] [PubMed]
- Kordonsky, V.I.; Korobko, E.V.; Lazareva, T.G. Electrorheological polymer-based suspensions. J. Rheol. 1991, 35, 1427–1439. [Google Scholar] [CrossRef]
- Ahn, B.G.; Choi, U.S.; Kwon, O.K. Electro-rheological properties of anhydrous ER suspensions based on phosphoric ester cellulose particles. Polym. Int. 2000, 49, 567–573. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.W.; Jang, W.H.; Choi, H.J.; Jhon, M.S. Electrorheological characteristics of phosphate cellulose-based suspensions. Polymer 2001, 42, 5005–5012. [Google Scholar] [CrossRef]
- Gan, S.; Piao, S.H.; Choi, H.J.; Zakaria, S.; Chia, C.H. Synthesis of kenaf cellulose carbamate and its smart electric stimuli-response. Carbohydr. Polym. 2016, 137, 693–700. [Google Scholar] [CrossRef]
- Choi, K.; Gao, C.Y.; Nam, J.D.; Choi, H.J. Cellulose-based smart fluids under applied electric fields. Materials 2017, 10, 1060. [Google Scholar] [CrossRef] [Green Version]
- Kawai, A.; Uchida, K.; Kamiya, K.; Gotoh, A.; Yoda, S.; Urabe, K.; Ikazaki, F. Effect of dielectric property of hydrous dispersoid on electrorheology. Int. J. Mod. Phys. B 1996, 10, 2849–2855. [Google Scholar] [CrossRef]
- Ikazaki, F.; Kawai, A.; Uchida, K.; Kawakami, T.; Edamura, K.; Sakurai, K.; Anzai, H.; Asako, Y. Mechanisms of electrorheology: The effect of the dielectric property. J. Phys. D-Appl. Phys. 1998, 31, 336–347. [Google Scholar] [CrossRef]
- Zhang, S.; Winter, W.T.; Stipanovic, A.J. Water-activated cellulose-based electrorheological fluids. Cellulose 2005, 12, 135–144. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.J.; Jin, X.; Wang, L.M.; Liu, Y.D. Preparation of cellulose/laponite composite particles and their enhanced electrorheological responses. Molecules 2021, 26, 1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, P.P.; Jin, X.; Wang, L.M.; Liu, Y.D.; Choi, H.J. Enhanced electrorheological response of cellulose: A double effect of modification by urea-terminated silane. Polymers 2018, 10, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.; Nam, J.D.; Kwon, S.H.; Choi, H.J.; Islam, M.S.; Kao, N. Microfibrillated Cellulose Suspension and Its Electrorheology. Polymers 2019, 11, 2119. [Google Scholar] [CrossRef] [Green Version]
- Sim, B.; Bae, D.H.; Choi, H.J.; Choi, K.; Islam, M.S.; Kao, N. Fabrication and stimuli response of rice husk-based microcrystalline cellulose particle suspension under electric fields. Cellulose 2016, 23, 185–197. [Google Scholar] [CrossRef]
- Chun, Y.; Ko, Y.G.; Do, T.; Jung, Y.; Kim, S.W.; Choi, U.S. Spent coffee grounds: Massively supplied carbohydrate polymer applicable to electrorheology. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 562, 392–401. [Google Scholar] [CrossRef]
- Bae, D.H.; Choi, H.J.; Choi, K.; Nam, D.J.; Islam, M.S.; Kao, N. Fabrication of phosphate microcrystalline rice husk based cellulose particles and their electrorheological response. Carbohydr. Polym. 2017, 165, 247–254. [Google Scholar] [CrossRef]
- Yin, J.B.; Wang, X.X.; Zhao, X.P. Silicone-grafted carbonaceous nanotubes with enhanced dispersion stability and electrorheological efficiency. Nanotechnology 2015, 26, 065704. [Google Scholar] [CrossRef]
- Lin, C.; Shan, J.W. Electrically tunable viscosity of dilute suspensions of carbon nanotubes. Phys. Fluids 2007, 19, 121702. [Google Scholar] [CrossRef]
- Yin, J.B.; Shui, Y.J.; Chang, R.T.; Zhao, X.P. Graphene-supported carbonaceous dielectric sheets and their electrorheology. Carbon 2012, 50, 5247–5255. [Google Scholar] [CrossRef]
- Yin, J.B.; Xia, X.A.; Xiang, L.Q.; Zhao, X.P. Conductivity and polarization of carbonaceous nanotubes derived from polyaniline nanotubes and their electrorheology when dispersed in silicone oil. Carbon 2010, 48, 2958–2967. [Google Scholar] [CrossRef]
- Sedlacik, M.; Pavlinek, V.; Mrlik, M.; Moravkova, Z.; Hajna, M.; Trchova, M.; Stejskal, J. Electrorheology of polyaniline, carbonized polyaniline, and their core-shell composites. Mater. Lett. 2013, 101, 90–92. [Google Scholar] [CrossRef]
- Plachy, T.; Sedlacik, M.; Pavlinek, V.; Trchová, M.; Morávková, Z.; Stejskal, J. Carbonization of aniline oligomers to electrically polarizable particles and their use in electrorheology. Chem. Eng. J. 2014, 256, 398–406. [Google Scholar] [CrossRef]
- Plachy, T.; Sedlacik, M.; Pavlinek, V.; Moravkova, Z.; Hajna, M.; Stejskal, J. An effect of carbonization on the electrorheology of poly(p-phenylenediamine). Carbon 2013, 63, 187–195. [Google Scholar] [CrossRef]
- Yin, J.B.; Xia, X.A.; Xiang, L.Q.; Zhao, X.P. Temperature effect of electrorheological fluids based on polyaniline derived carbonaceous nanotubes. Smart Mater. Struct. 2011, 20, 015002. [Google Scholar] [CrossRef]
- Qiao, Y.P.; Zhao, X. Electrorheological effect of carbonaceous materials with hierarchical porous structures. Colloid Surf. A-Physicochem. Eng. Asp. 2009, 340, 33–39. [Google Scholar] [CrossRef]
- Prekob, A.; Hajdu, V.; Muranszky, G.; Fiser, B.; Sycheva, A.; Ferenczi, T.; Viskolcz, B.; Vanyorek, L. Application of carbonized cellulose-based catalyst in nitrobenzene hydrogenation. Mater. Today Chem. 2020, 17, 100337. [Google Scholar] [CrossRef]
- Ruan, C.Q.; Wang, Z.H.; Lindh, J.; Stromme, M. Carbonized cellulose beads for efficient capacitive energy storage. Cellulose 2018, 25, 3545–3556. [Google Scholar] [CrossRef] [Green Version]
- Kotia, A.; Yadav, A.; Raj, T.R.; Keischgens, M.G.; Rathore, H.; Sarris, I.E. Carbon nanoparticles as sources for a cost-effective water purification method: A comprehensive review. Fluids 2020, 5, 230. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.H.; Wang, Z.P.; Zhao, L.Y.; Li, P.H.; Yang, Y.; Yang, C.; Huang, H.X.; Guo, S.J. Short-range order in mesoporous carbon boosts potassium-ion battery performance. Adv. Energy Mater. 2018, 8, 1701648. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.C. Pore Structure Characteristics of Foam Composite with Active Carbon. Materials 2020, 13, 4038. [Google Scholar] [CrossRef] [PubMed]
- Plachy, T.; Sedlacik, M.; Pavlinek, V.; Stejskal, J.; Graca, M.P.; Costa, L.C. Temperature-dependent electrorheological effect and its description with respect to dielectric spectra. J. Intell. Mater. Syst. Struct. 2016, 27, 880–886. [Google Scholar] [CrossRef]
- Saabome, S.M.; Park, Y.S.; Ko, Y.G. Designing particle size of aminated polyacrylonitrile spheres to enhance electrorheological performances of their suspensions. Powder Technol. 2021, 394, 986–995. [Google Scholar] [CrossRef]
- Li, L.Z.; Gao, S.J. Polyaniline (PANI) and BaTiO3 composite nanotube with high suspension performance in electrorheological fluid. Mater. Today Commun. 2020, 24, 100993. [Google Scholar] [CrossRef]
- Davis, L.C. Polarization forces and conductivity effects in electrorheological fluids. J. Appl. Phys. 1992, 72, 1334–1340. [Google Scholar] [CrossRef]
- Mrlik, M.; Sedlacik, M.; Pavlinek, V.; Bober, P.; Trchova, M.; Stejskal, J.; Saha, P. Electrorheology of aniline oligomers. Colloid Polym. Sci. 2013, 291, 2079–2086. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Liu, Y.; Zhao, X.P.; Yin, J.B. Interfacial polarization and electroresponsive electrorheological effect of anionic and cationic poly(ionic liquids). Acs Appl. Polym. Mater. 2019, 1, 2862–2874. [Google Scholar] [CrossRef]
- Stenicka, M.; Pavlinek, V.; Saha, P.; Blinova, N.V.; Stejskal, J.; Quadrat, O. The electrorheological efficiency of polyaniline particles with various conductivities suspended in silicone oil. Colloid Polym. Sci. 2009, 287, 403–412. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Kraev, A.S.; Gajtko, O.M.; Kusova, T.V.; Baranchikov, A.E.; Agafonov, A.V.; Ivriov, V.K. High electrorheological effect in Bi1.8Fe1.2SbO7 suspensions. Powder Technol. 2020, 360, 96–103. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of alpha-dispersions in some polymer systems. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Hao, T. Electrorheological suspensions. Adv. Colloid Interface Sci. 2002, 97, 1–35. [Google Scholar] [CrossRef]
- He, L.; Xin, L.P.; Chai, X.S.; Li, J. A novel method for rapid determination of alpha-cellulose content in dissolving pulps by visible spectroscopy. Cellulose 2015, 22, 2149–2156. [Google Scholar] [CrossRef]
- Bu, D.Q.; Hu, X.Z.; Yang, Z.J.; Yang, X.; Wei, W.; Jiang, M.; Zhou, Z.W.; Zaman, A. Elucidation of the Relationship between Intrinsic Viscosity and Molecular Weight of Cellulose Dissolved in Tetra-N-Butyl Ammonium Hydroxide/Dimethyl Sulfoxide. Polymers 2019, 11, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Electric Field Strength (kV mm−1) | Leaking Current Density (µA cm−2) | ||
|---|---|---|---|
| Cellulose | HTC-Cellulose | HTC-TC600-CelluLose | |
| 0.50 | 0 | 0 | 63.03 |
| 0.75 | 0 | 0 | 176.03 |
| 1.00 | 0 | 0 | 319.90 |
| 1.25 | 0 | 0 | × * |
| 1.50 | 0 | 0 | × * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plachy, T.; Kutalkova, E.; Skoda, D.; Holcapkova, P. Transformation of Cellulose via Two-Step Carbonization to Conducting Carbonaceous Particles and Their Outstanding Electrorheological Performance. Int. J. Mol. Sci. 2022, 23, 5477. https://doi.org/10.3390/ijms23105477
Plachy T, Kutalkova E, Skoda D, Holcapkova P. Transformation of Cellulose via Two-Step Carbonization to Conducting Carbonaceous Particles and Their Outstanding Electrorheological Performance. International Journal of Molecular Sciences. 2022; 23(10):5477. https://doi.org/10.3390/ijms23105477
Chicago/Turabian StylePlachy, Tomas, Erika Kutalkova, David Skoda, and Pavlina Holcapkova. 2022. "Transformation of Cellulose via Two-Step Carbonization to Conducting Carbonaceous Particles and Their Outstanding Electrorheological Performance" International Journal of Molecular Sciences 23, no. 10: 5477. https://doi.org/10.3390/ijms23105477