Exposure of Mice to Thirdhand Smoke Modulates In Vitro and In Vivo Platelet Responses
Abstract
:1. Introduction
2. Results
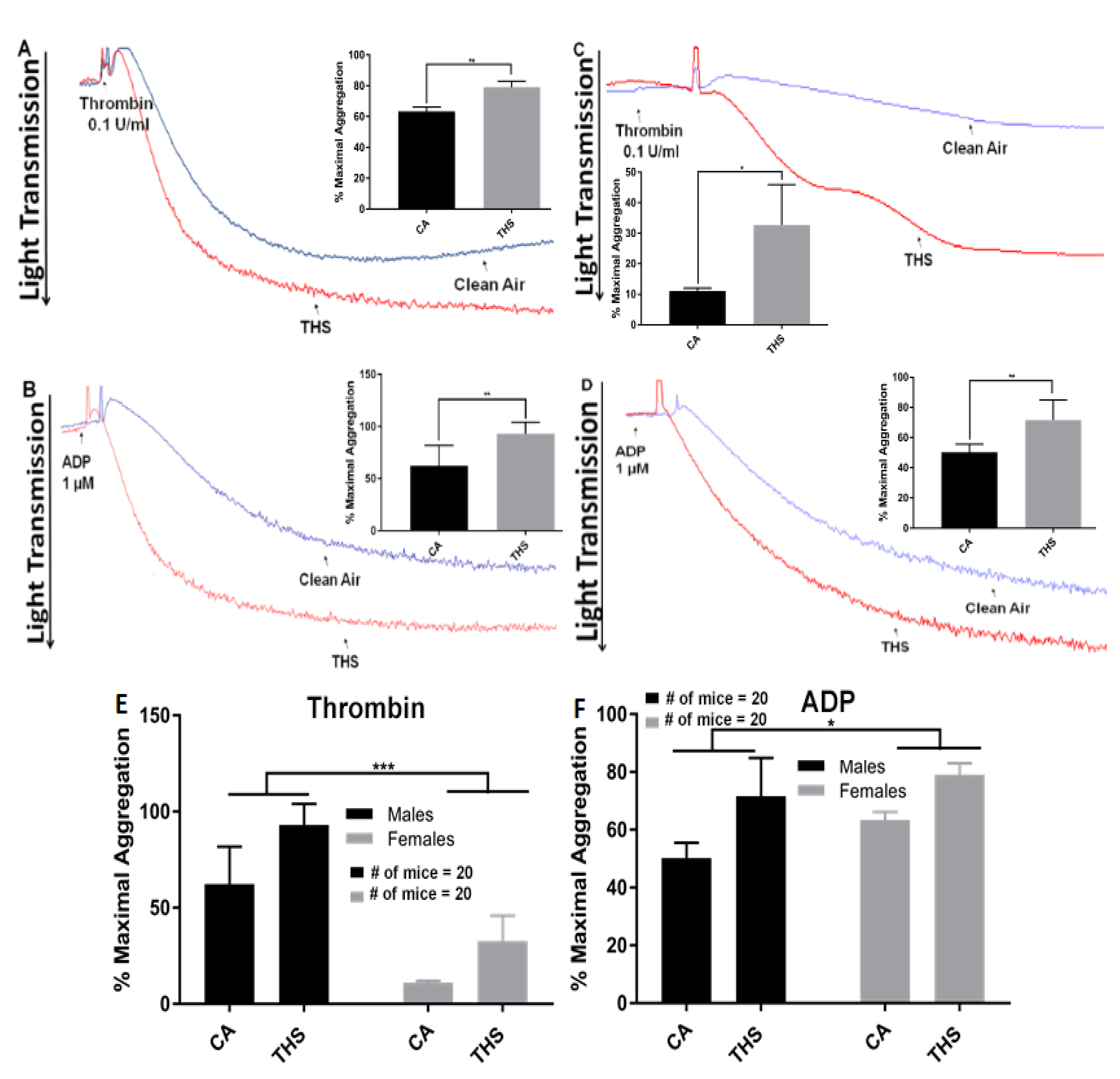
2.1. Platelets from THS-Exposed Mice Exhibit Increased Aggregation and Dense Granule Secretion
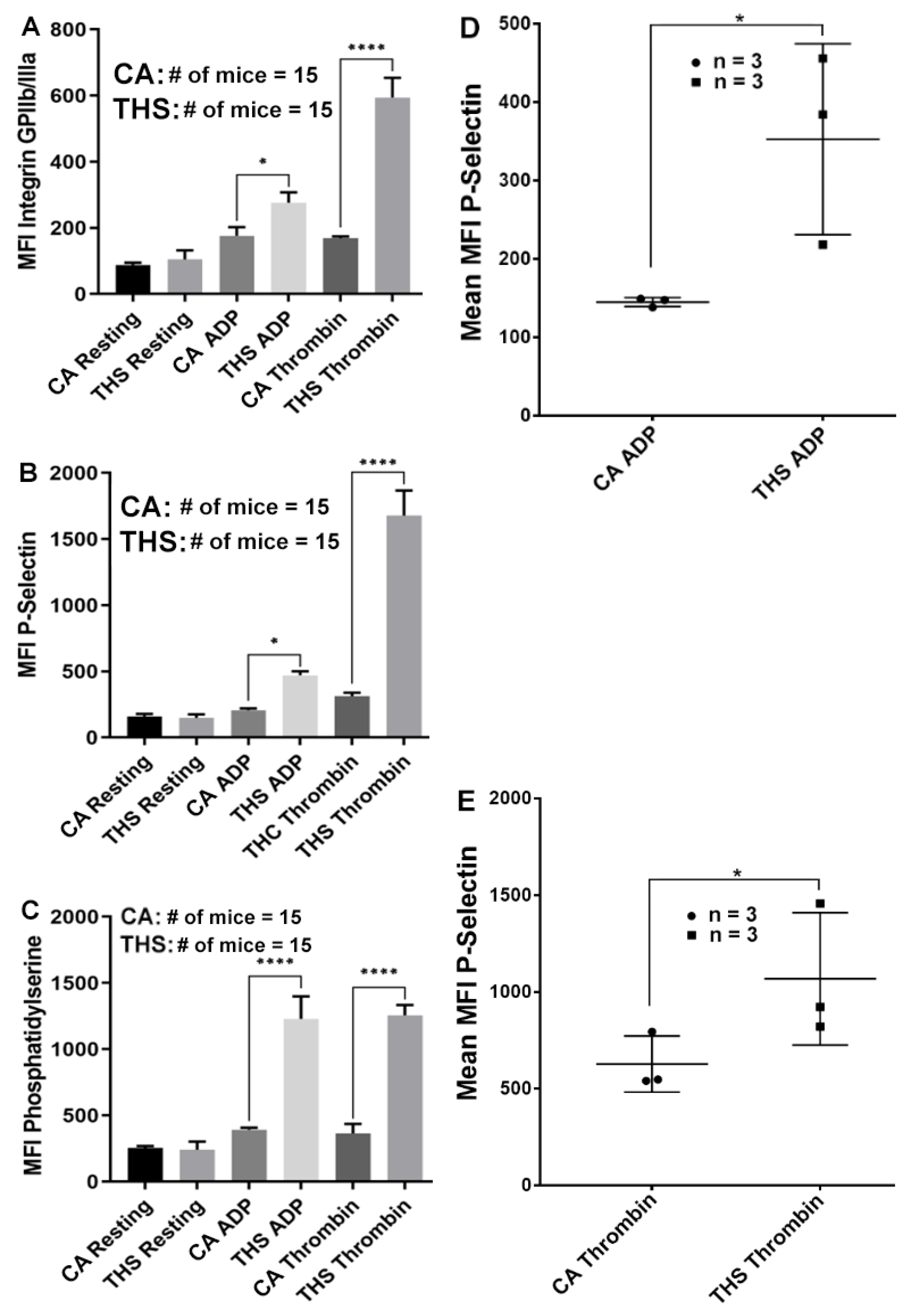
2.2. Platelets from THS-Exposed Mice Exhibit Increased Integrin αIIbβ3 Activation, α-Granule Secretion and Phosphatidylserine Expression
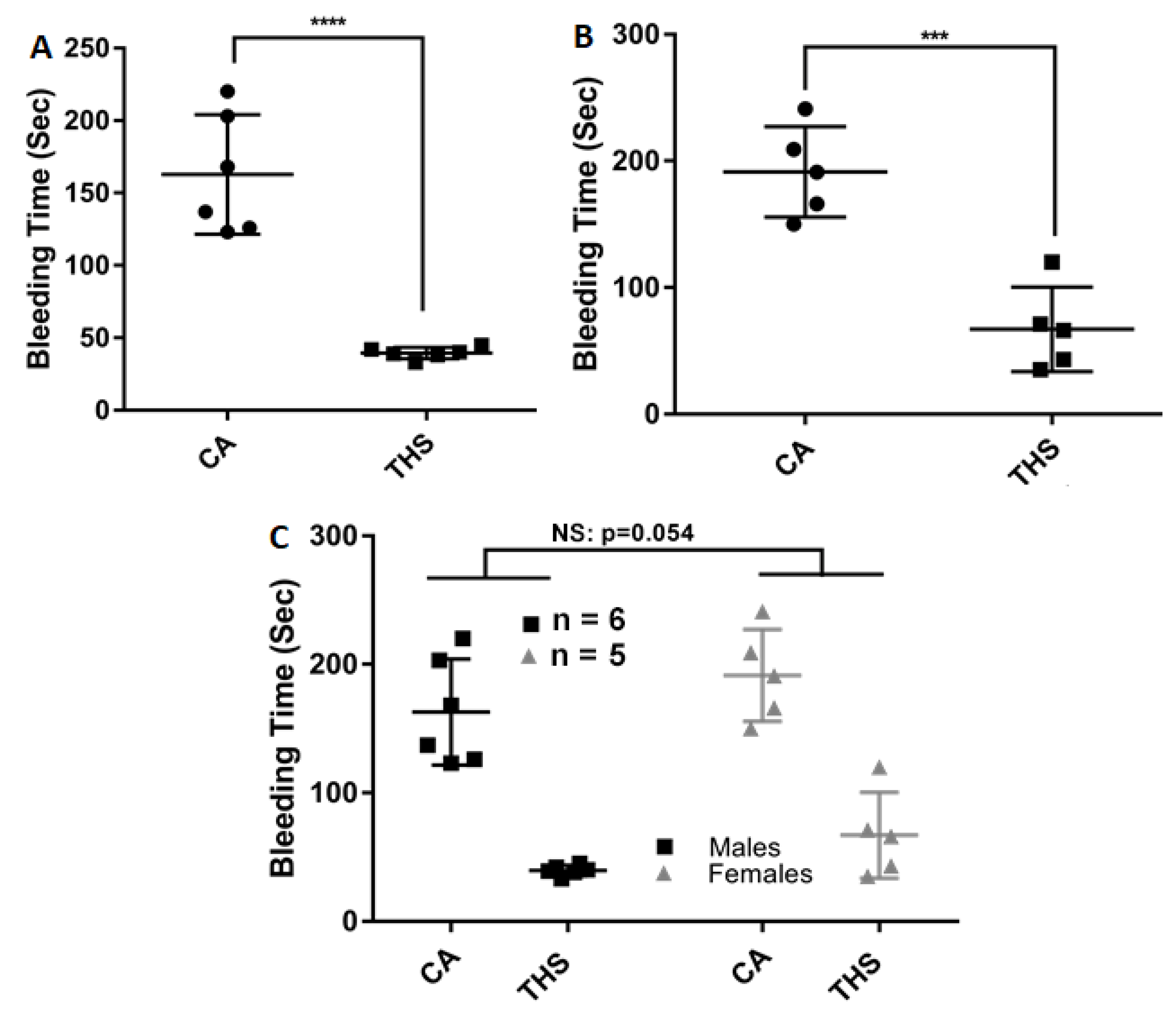
2.3. THS-Exposed Mice Have a Shortened Tail Bleeding Time
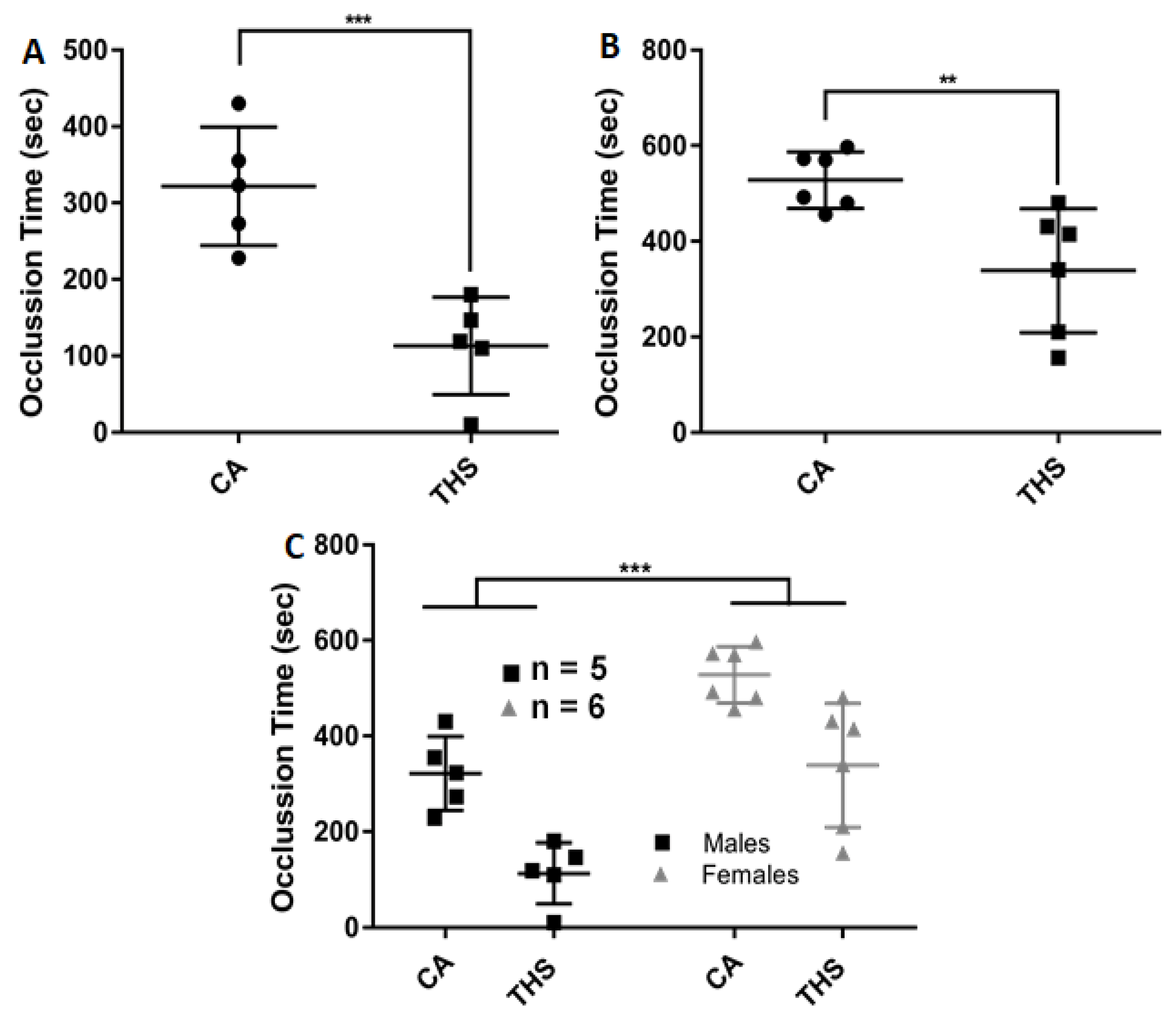
2.4. THS-Exposed Mice Have a Shortened Thrombosis Occlusion Time
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Animals
4.3. THS Exposure
4.4. Platelet Preparation
4.5. Platelet Aggregation
4.6. Flow Cytometry
4.7. Tail Bleeding Time Assay
4.8. Carotid Artery Injury–Induced Thrombosis Model
4.9. Platelet Count
4.10. Statistical analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committee on the Public Health Implications of Raising the Minimum Age for Purchasing Tobacco Products; Board on Population Health and Public Health Practice; Institute of Medicine. The Effects of Tobacco Use on Health. In Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products; Bonnie, R.J., Stratton, K., Kwan, L.Y., Eds.; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010. [Google Scholar]
- Ghahremanfard, F.; Semnani, V.; Ghorbani, R.; Malek, F.; Behzadfar, A.; Zahmatkesh, M. Effects of cigarette smoking on morphological features of platelets in healthy men. Saudi Med. J. 2015, 36, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Malý, J.; Pecka, M.; Vodicková, L.; Pidrman, V.; Malá, H.; Bláha, M.; Jebavý, L. Changes in the activity of thrombocytes by smoking. Sbornik Vedeckych Praci Lekarske Fakulty Karlovy University v Hradci Kralove 1990, 33, 325–333. [Google Scholar] [PubMed]
- Barua, R.S.; Ambrose, J.A. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1460–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renaud, S.; Blache, D.; Dumont, E.; Thevenon, C.; Wissendanger, T. Platelet function after cigarette smoking in relation to nicotine carbon monoxide. Clin. Pharmacol. Ther. 1984, 36, 389–395. [Google Scholar] [CrossRef]
- Levine, P.H. An acute effect of cigarette smoking on platelet function A possible link between smoking arterial thrombosis. Circulation 1973, 48, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Hung, J.; Lam, J.Y.; Lacoste, L.; Letchacovski, G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation 1995, 92, 2432–2436. [Google Scholar] [CrossRef]
- Csordas, A.; Bernhard, D. The biology behind the atherothrombotic effects of cigarette smoke. Nat. Rev. Cardiol. 2013, 10, 219–230. [Google Scholar] [CrossRef]
- Alarabi, A.B.; Karim, Z.A.; Ramirez, J.E.M.; Hernandez, K.R.; Lozano, P.A.; Rivera, J.O.; Alshbool, F.Z.; Khasawneh, F.T. Short-Term Exposure to Waterpipe/Hookah Smoke Triggers a Hyperactive Platelet Activation State Increases the Risk of Thrombogenesis Arteriosclerosis thrombosis. Vasc. Biol. 2020, 40, 335–349. [Google Scholar] [CrossRef]
- Qasim, H.; Karim, Z.A.; Silva-Espinoza, J.C.; Khasawneh, F.T.; Rivera, J.O.; Ellis, C.C.; Bauer, S.L.; Almeida, I.C. Alshbool FZShort-Term E-Cigarette Exposure Increases the Risk of Thrombogenesis Enhances Platelet Function in Mice. J. Am. Heart Assoc. 2018, 7, e009264. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Biondi-Zoccai, G.; Sciarretta, S.; Peruzzi, M.; Pagano, F.; Loffredo, L.; Pignatelli, P.; Bullen, C.; Frati, G.; Carnevale, R. Impact of Tobacco Versus Electronic Cigarette Smoking on Platelet Function. Am. J. Cardiol. 2018, 122, 1477–1481. [Google Scholar] [CrossRef]
- Committee on the Public Health Implications of Raising the Minimum Age for Purchasing Tobacco Products; Board on Population Health and Public Health Practice; Institute of Medicine. Public Health Implications of Raising the Minimum Age of Legal Access to Tobacco Products; Bonnie, R.J., Stratton, K., Kwan, L.Y., Eds.; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Jacob, P., 3rd; Benowitz, N.L.; Destaillats, H.; Gundel, L.; Bo Hang, M.M.; Matt, G.E.; Quintana, P.J.E.; Samet, J.M.; Schick, S.F.; Talbot, P.; et al. Thirdhand Smoke: New Evidence Challenges, and Future Directions. Chem. Res. Toxicol. 2017, 30, 270–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, Z.A.; Alshbool, F.Z.; Vemana, H.P.; Adhami, N.; Dhall, S.; Espinosa, E.V.; Martins-Green, M.; Khasawneh, F.T. Third-hand Smoke: Impact on Hemostasis Thrombogenesis. J. Cardiovasc. Pharmacol. 2015, 66, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.A.; Alarabi, A.B.; Karim, Z.A.; Rodriguez, V.; Hernandez, K.R.; Lozano, P.A.; El-Halawany, M.S.; Alshbool, F.Z.; Khasawneh, F.T. In utero thirdhand smoke exposure modulates platelet function in a sex-dependent manner. Haematologica 2022, 107, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Vinholt, P.J.; Frederiksen, H.; Hvas, A.M.; Sprogøe, U.; Nielsen, C. Measurement of platelet aggregation independently of patient platelet count: A flow-cytometric approach. J. Thromb. Haemost. 2017, 15, 1191–1202. [Google Scholar] [CrossRef] [Green Version]
- Matt, G.E.; Quintana, P.J.E.; Hoh, E.; Zakarian, J.M.; Chowdhury, Z.; Hovell, M.F.; Jacob, P.; Watanabe, K.; Theweny, T.S.; Flores, V.; et al. A Casino goes smoke free: A longitudinal study of secondhand thirdhand smoke pollution exposure. Tob. Control. 2018, 27, 643–649. [Google Scholar] [CrossRef]
- Dhall, S.; Alamat, R.; Castro, A.; Sarker, A.H.; Mao, J.H.; Chan, A.; Hang, B.; Martins-Green, M. Tobacco toxins deposited on surfaces (third hand smoke) impair wound healing. Clin. Sci. 2016, 130, 1269–1284. [Google Scholar] [CrossRef] [Green Version]
- Adhami, N.; Starck, S.R.; Flores, C.; Martins Green, M. A Health Threat to Bystanders Living in the Homes of Smokers: How Smoke Toxins Deposited on Surfaces Can Cause Insulin Resistance. PLoS ONE 2016, 11, e0149510. [Google Scholar]
- Martins-Green, M.; Adhami, N.; Frankos, M.; Valdez, M.; Goodwin, B.; Lyubovitsky, J.; Dhall, S.; Garcia, M.; Egiebor, I.; Martinez, B.; et al. Cigarette smoke toxins deposited on surfaces: Implications for human health. PLoS ONE 2014, 9, e86391. [Google Scholar] [CrossRef] [Green Version]
- Kool, M.J.; Hoeks, A.P.; Struijker Boudier, H.A.; Reneman, R.S.; Van Bortel, L.M. Short-, long-term effects of smoking on arterial wall properties in habitual smokers. J. Am. Coll. Cardiol. 1993, 22, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Schick, S.F.; Glantz, S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: Results from unpublished tobacco industry research. Cancer Epidemiol. Prev. Biomark. 2007, 16, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Sleiman, M.; Logue, J.M.; Luo, W.; Pankow, J.F.; Gundel, L.A.; Destaillats, H. Inhalable constituents of thirdhand tobacco smoke: Chemical characterization health impact considerations. Environ. Sci. Technol. 2014, 48, 13093–13101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitlatch, A.; Schick, S. Thirdhand Smoke at Philip Morris. Nicotine Tob. Res. 2019, 21, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Grindler, N.M.; Allsworth, J.E.; Macones, G.A.; Kannan, K.; Roehl, K.A.; Cooper, A.R. Persistent organic pollutants early menopause in US women. PLoS ONE 2015, 10, e0116057. [Google Scholar] [CrossRef] [PubMed]
- Evlampidou, I.; Bagkeris, M.; Vardavas, C.; Koutra, K.; Patelarou, E.; Koutis, A.; Chatzi, L.; Kogevinas, M. Prenatal Second-Hand Smoke Exposure Measured with Urine Cotinine May Reduce Gross Motor Development at 18 Months of Age. J. Pediatrics 2015, 167, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.V.; O’Connor, R.J.; Stephens, W.E.; Cummings, K.M.; Fong, G.T. Toxic metal concentrations in cigarettes obtained from US smokers in 2009: Results from the International Tobacco Control. Int. J. Environ. Res. Public Health 2014, 11, 202–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregg, E.O.; Minet, E.; McEwan, M. Urinary biomarkers of smokers’ exposure to tobacco smoke constituents in tobacco products assessment: A fit for purpose approach. Biomarkers 2013, 18, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.H.; Reyes, L.R.; Bernert, J.T. Analysis of 4-aminobiphenyl hemoglobin adducts in smokers nonsmokers by pseudo capillary on-column gas chromatography- tandem mass spectrometry. J. Anal. Toxicol. 2010, 34, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Goniewicz, M.L.; Havel, C.M.; Peng, M.W.; Jacob, P., 3rd; Dempsey, D.; Yu, L.; Zielinska-Danch, W.; Koszowski, B.; Czogala, J.; Sobczak, A.; et al. Elimination kinetics of the tobacco-specific biomarker lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol. Prev. Biomark. 2009, 18, 3421–3425. [Google Scholar] [CrossRef] [Green Version]
- Carmella, S.G.; Chen, M.; Han, S.; Briggs, A.; Jensen, J.; Hatsukami, D.K.; Hecht, S.S. Effects of smoking cessation on eight urinary tobacco carcinogen toxicant biomarkers. Chem. Res. Toxicol. 2009, 22, 734–741. [Google Scholar] [CrossRef] [Green Version]
- The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2006.
- Hecht, S.S.; Carmella, S.G.; Ye, M.; Le, K.A.; Jensen, J.A.; Zimmerman, C.L.; Hatsukami, D.K. Quantitation of metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone after cessation of smokeless tobacco use. Cancer Res. 2002, 62, 129–134. [Google Scholar]
- Dempsey, D.; Jacob, P., 3rd; Benowitz, N.L. Nicotine metabolism elimination kinetics in newborns. Clin. Pharmacol. Ther. 2000, 67, 458–465. [Google Scholar] [CrossRef]
- Hecht, S.S.; Carmella, S.G.; Chen, M.; Dor Koch, J.F.; Miller, A.T.; Murphy, S.E.; Jensen, J.A.; Zimmerman, C.L.; Hatsukami, D.K. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999, 59, 590–596. [Google Scholar] [PubMed]
- Kyerematen, G.A.; Damiano, M.D.; Dvorchik, B.H.; Vesell, E.S. Smoking-induced changes in nicotine disposition: Application of a new HPLC assay for nicotine its metabolites. Clin. Pharmacol. Ther. 1982, 32, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Q.; Sun, Y.P.; Sudhir, K.; Sievers, R.E.; Browne, A.E.; Gao, L.; Hutchison, S.J.; Chou, T.M.; Deedwania, P.C.; Chatterjee, K.; et al. Effects of second-hand smoke gender on infarct size of young rats exposed in utero in the neonatal to adolescent period. J. Am. Coll. Cardiol. 1997, 30, 1878–1885. [Google Scholar] [CrossRef] [Green Version]
- Soo Kim, B.; Auerbach, D.S.; Sadhra, H.; Godwin, M.; Bhandari, R.; Ling, F.S.; Mohan, A.; Yule, D.I.; Wagner, L., 2nd; Rich, D.Q.; et al. Sex-Specific Platelet Activation Through Protease-Activated Receptors Reverses in Myocardial Infarction Arterioscler. Thromb. Vasc. Biol. 2021, 41, 390–400. [Google Scholar]
- Women’s Health Research: Progress, Pitfalls, and Promise; National Academies Press: Washington, DC, USA, 2010.
- Rendon, A.D.; Unger, J.B.; Cruz, T.; Soto, D.W.; Baezconde-Garbanati, L. Perceptions of Secondhand Thirdhand Smoke Among Hispanic Residents of Multiunit Housing. J. Immigr. Minor. Health 2017, 19, 162–169. [Google Scholar] [CrossRef]
- Lewinson, T.; Bryant, L.O. “There’s no-fresh air there”: Narratives of smoke exposure among residents of extended-stay hotels. Health Soc. Work 2015, 40, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Matt, G.E.; Quintana, P.J.; Destaillats, H.; Gundel, L.A.; Sleiman, M.; Singer, B.C.; Jacob, P.; Benowitz, N.; Winickoff, J.P.; Rehan, V.; et al. Thirdhand tobacco smoke: Emerging evidence arguments for a multidisciplinary research agenda. Environ. Health Perspect. 2011, 119, 1218–1226. [Google Scholar] [CrossRef] [Green Version]
- Karim, Z.A.; Alshbool, F.Z.; Vemana, H.P.; Conlon, C.; Druey, K.M.; Khasawneh, F.T. CXCL12 regulates platelet activation via the regulator of G-protein signaling 16. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 314–321. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-García, D.; Ali, H.E.A.; Alarabi, A.B.; El-Halawany, M.S.; Alshbool, F.Z.; Khasawneh, F.T. Exposure of Mice to Thirdhand Smoke Modulates In Vitro and In Vivo Platelet Responses. Int. J. Mol. Sci. 2022, 23, 5595. https://doi.org/10.3390/ijms23105595
Villalobos-García D, Ali HEA, Alarabi AB, El-Halawany MS, Alshbool FZ, Khasawneh FT. Exposure of Mice to Thirdhand Smoke Modulates In Vitro and In Vivo Platelet Responses. International Journal of Molecular Sciences. 2022; 23(10):5595. https://doi.org/10.3390/ijms23105595
Chicago/Turabian StyleVillalobos-García, Daniel, Hamdy E. A. Ali, Ahmed B. Alarabi, Medhat S. El-Halawany, Fatima Z. Alshbool, and Fadi T. Khasawneh. 2022. "Exposure of Mice to Thirdhand Smoke Modulates In Vitro and In Vivo Platelet Responses" International Journal of Molecular Sciences 23, no. 10: 5595. https://doi.org/10.3390/ijms23105595





