Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging
Abstract
:1. Quantitative Changes in the Population of Dermal Fibroblasts
2. Age-Related Changes in the Dermal Stem Cell Population
2.1. Depletion of the SCs Pool
- A decrease in the ability of SCs to self-renew and the lowered proliferative potential [12,15,24], in particular, are due to a reduction in the number of receptors for growth factors located on the SCs’ surface membranes [25]. As a result, there is a decrease in the number of SCs capable of responding to signals stimulating their proliferation [26].
- Excessive SC proliferation causing the depletion of the cell niche [18,19]. It has been revealed that the SCs’ regenerative potential is limited by a certain number of cell divisions during the life of the organism [27]; therefore, the prolonged activation of a stress factor stimulating the proliferation of SCs inevitably leads to depletion of their pool or to aberrant differentiation of these cells [28,29].
2.2. Changes in the SC Genome
- Reactive oxygen species (ROS) produced by metabolic intermediates and dysfunctional Mt;
- DNA replication and DNA repair errors;
- Glycation end products;
- Dysfunction and shortening of telomeres (the end regions of chromosomes that ensure stable cell replication and protect chromosomes from fusion);
- Inflammation of the surrounding tissue.
3. Cellular Aging or Senescence
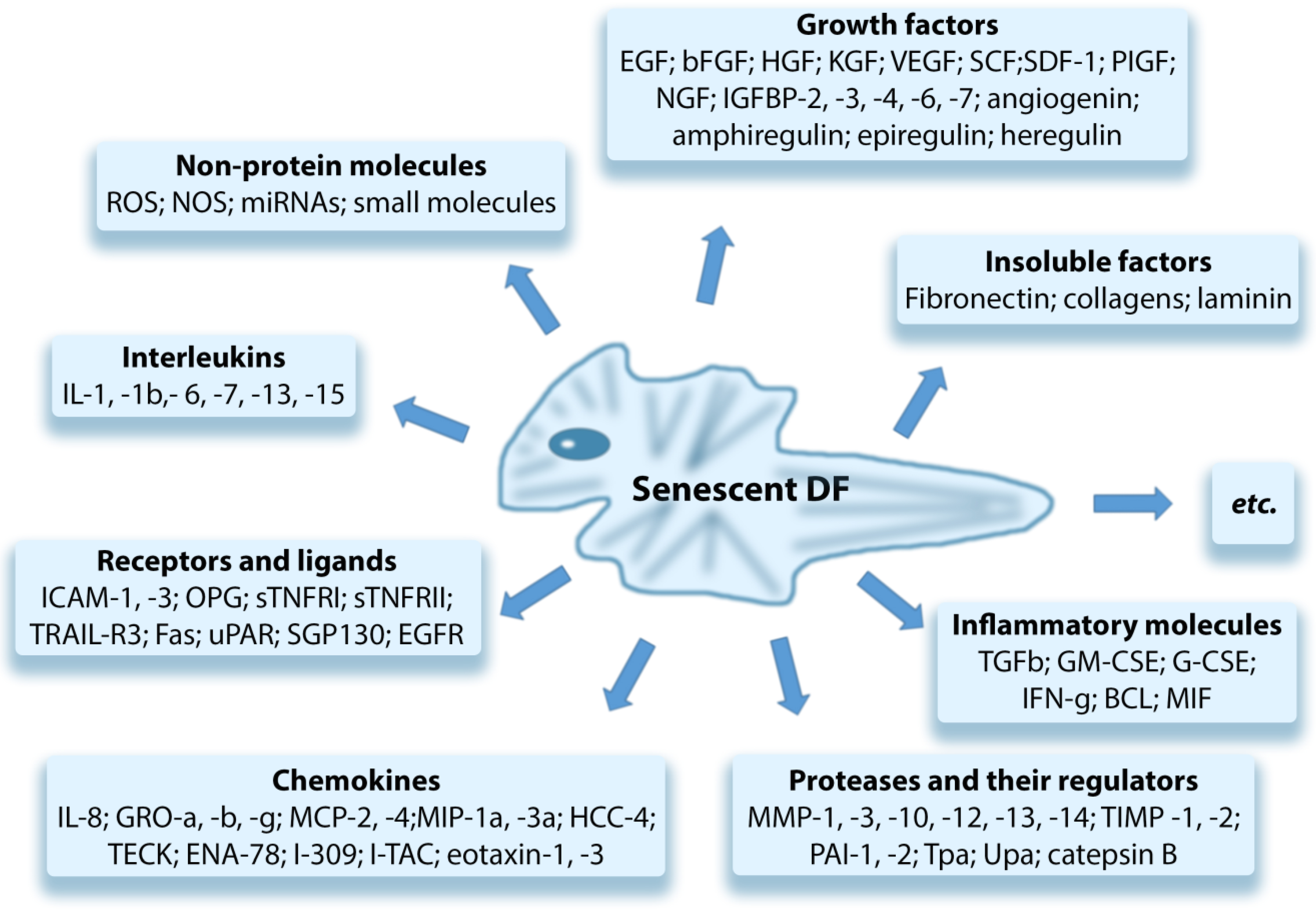
4. Senescent Fibroblasts and “Paracrine” Skin Aging
4.1. Identification of Senescent Fibroblasts
- Morphological changes: the increase in size and flattening of the shape [111];
- Increased activity of senescence-associated lysosomal enzyme β-galactosidase (SA-b-gal) which is the “gold standard” for the identification of senCs both in vitro and in vivo (in tissue samples) [112];
- Visualization of cytoplasmic granularity under a light microscope: it indicates an increase in the number and size of lysosomes (while this does not mean an increase in the activity of these organelles since there is a marked decrease in the level of autophagy associated with lysosomes during aging) [88,93,113];
- Accumulation of lipofuscin [113];
- Increase in the frequency of γH2AX (a marker of double-stranded DNA breaks that occur during persistent DNA damage and DDR activation [76]);
- Presence of telomere-associated foci of DNA damage (TAF) [114];
- Decrease in the level of nuclear intermediate plate protein and epigenetic modulator of lamin B1 [115,116,117,118,119] (the level of lamin B1 decreases in vitro in senDFs regardless of the stress factor [117] and in vivo in DFs isolated from skin samples with signs of premature and chronological aging [120]);
- Presence of senescence-associated heterochromatin foci (SAHF, special heterochromatin structures formed in the nuclei of senCs) [121];
- Presence of DNA foci with chromatin changes that enhance cell aging (DNA-SCARS, DNA Segments with Chromatin Alterations Reinforcing Senescence) [69];
- Presence of HMGB1 (a protein from the group of nuclear non-histone proteins; in senCs it leaves the nucleus and moves to the cytoplasm and ECM; a decrease in its level in the nucleus leads to a decrease in gene expression) [122];
- Mt dysfunction [84].
- High level of SA-β-gal activity;
- Change in the production of ECM components;
- Increased level of cyclin-dependent kinases p21 and p16INK4a (among the other markers detected in vivo, it has the highest correlation with markers revealed in vitro [31]);
- Depletion of lamin B1 [117];
- Increased level of SASP proinflammatory cytokines [124];
- Presence of telomere-associated foci of DNA damage (TAF) used as the quantitative marker of skin tissue aging in situ [114].
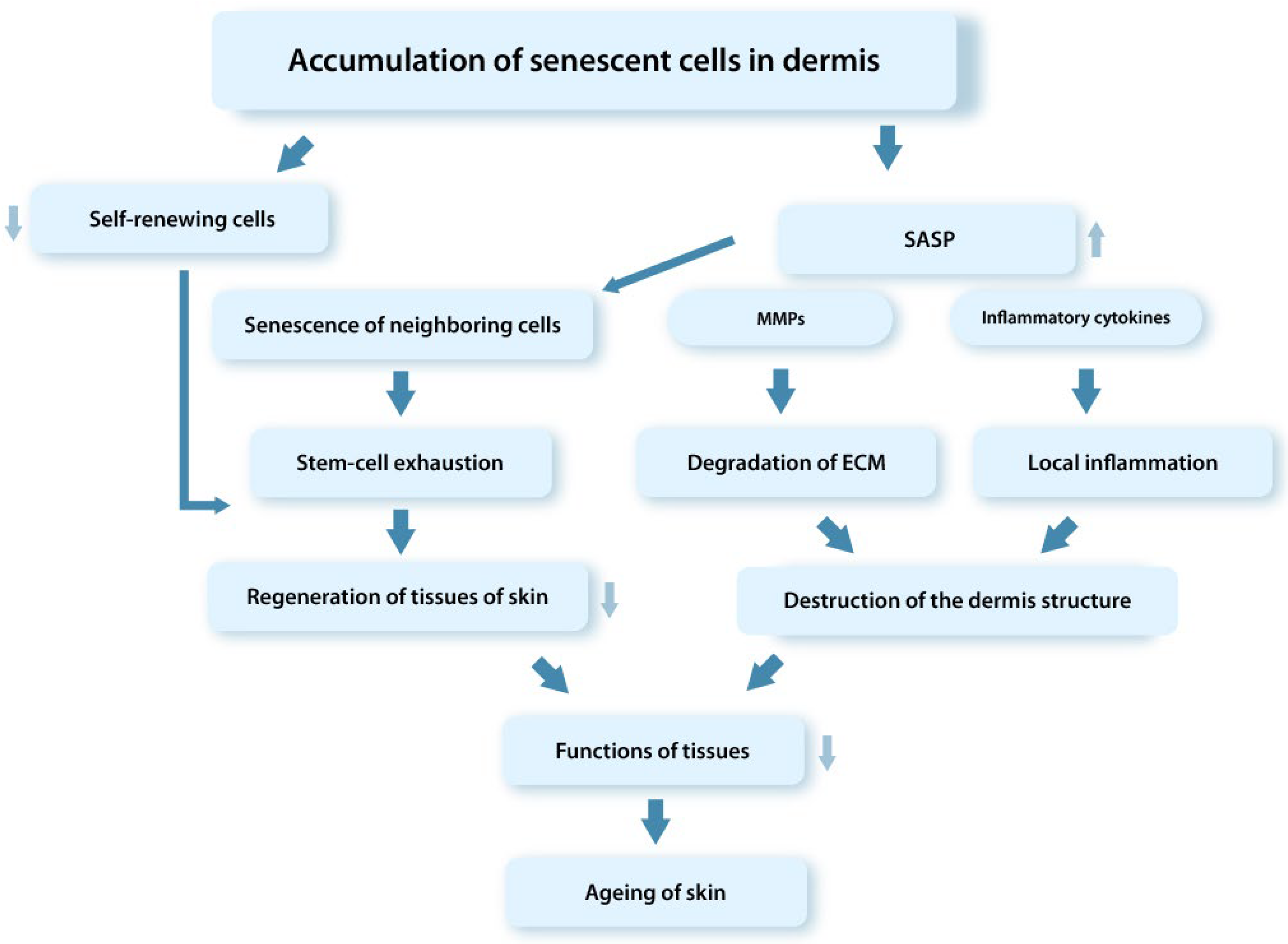
4.2. The Role of senDFs in Skin Aging
- They do not proliferate, which leads to violation of the SCs’ self-renewing process and depletion of the SCs pool;
- Cause aging of neighboring SCs;
- Promote an increase in the level of ROS and cause Mt dysfunction;
- Induce DNA damage and aging of “witness cells” through the paracrine mechanism and ROS overproduction;
- Cause the chronic aseptic inflammation in tissues due to the effect of proinflammatory SASP factors secreted by senCs;
- Enhance the ECM degradation in the dermis by producing the high MMPs level;
- Disrupt cellular and tissue homeostasis.
- An increase in the level of p16INK4a-positive DFs in the dermis correlating with the formation of wrinkles and the appearance of typical signs of elastic fiber aging;
- During chronological aging, DFs have a proteomic profile in situ identical to the senDFs profile;
- An increase in the ROS level leads to an increase in the number of p16INK4a-positive DFs in the skin and correlates with the progression of skin aging;
- The spread of senescence to neighboring DFs with the expression of characteristic markers of cellular aging was recorded during transplantation of human senDFs into the skin of young immunodeficient mice;
- Organ cultures obtained on the basis of human senDFs have signs of aging typical for chronological aging of the skin, including impairment of epidermal morphogenesis;
- DFs isolated from the skin of elderly people are characterized by a gene expression pattern similar to that of senDFs;
- Studies using a model of perforin-deficient mice (characterized by reduced functions of NK cells) have demonstrated the suppressed ability of the immune system to eliminate senDFs which by accumulating in the dermis lead to structural changes and the progression of aging processes in the dermis;
- The results of a clinical study conducted using the local application of rapamycin on the skin of elderly people (with chronological aging) showed a decrease in the level of p16INK4a-positive DFs, as well as a decrease in the number of fine wrinkles and an increase in the thickness and elasticity of the skin.
- Rapamycin is an inhibitor of mTOR (protein regulating the cell cycle and participating in the aging of DFs through the regulation of SASP) suppressing the translation of membrane-bound cytokine IL-1a and thereby inhibiting the secretion of pro-inflammatory SASP factors induced by IL-1a.
5. Conclusions
Funding
Conflicts of Interest
References
- Varani, J.; Dame, M.K. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solé-Boldo, L.; Raddatz, G. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun. Biol. 2020, 3, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrell, J.M.; Caplan, A.I. Fibroblasts—A diverse population at the center of it all. Int. Rev. Cell. Molec. Biol. 2009, 276, 161–214. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhao, R. Dermal Fibroblast Heterogeneity and Its Contribution to the Skin Repair and Regeneration. Adv. Wound Care 2022, 11, 87–107. [Google Scholar] [CrossRef]
- Woodley, D.T. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatol. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef]
- Fisher, G.; Kang, S. Mechanism of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1467. [Google Scholar] [CrossRef]
- Gunin, A.G.; Petrov, V.V. Age-related changes in angiogenesis in human dermis. Exp. Gerontol. 2014, 55, 143–151. [Google Scholar] [CrossRef]
- Mine, S.; Fortunel, N.O. Aging Alters Functionally Human Dermal Papillary Fibroblasts but Not Reticular Fibroblasts: A New View of Skin Morphogenesis and Aging. PLoS ONE 2008, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Goodell, M.A.; Rando, T.A. Stem cells and healthy aging. Science 2015, 350, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Ocampo, A.; Liu, G.H.; Belmonte, J.C.I. Regulation of stem cell aging by metabolism and epigenetics. Cell. Metab. 2017, 26, 460–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sameri, S.; Samadi, P. Stem cell aging in lifespan and disease: A state-of-the-art review. Curr. Stem Cell Res. Ther. 2020, 15, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Cinat, D.; Coppes, R.P. DNA Damage-Induced Inflammatory Microenvironment and Adult Stem Cell Response. Front. Cell. Dev. Biol. 2021, 9, 729136. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Brack, A.S. Cellular mechanisms of somatic stem cell aging. Curr. Topics Dev. Biol. 2014, 107, 405–438. [Google Scholar] [CrossRef] [Green Version]
- Stolzing, A.; Jones, E. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. Cell. Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.J.; Jamieson, C.H. Stems cells and the pathways to aging and cancer. Cell 2008, 132, 681–696. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Kerr, C. The Epigenetics of Stem Cell Aging Comes of Age. Trends Cell Biol. 2019, 29, 563–568. [Google Scholar] [CrossRef]
- Oh, J.; Lee, Y.D. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.Y.; Solis, M.A. Molecular mechanism of extrinsic factors affecting antiaging of stem cells. World J. Stem Cells 2015, 7, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Tümpel, S.; Rudolph, K.L. Quiescence: Good and Bad of Stem Cell Aging. Trends Cell Biol. 2019, 29, 672–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, G. Molecular mechanisms of skin ageing. Mechanisms of Ageing and Development. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Wang, J.; Nirmala, X. Perspectives on Improving the Efficacy of PRP Treatment for Tendinopathy. J. Musculoskelet. Disord. Treat. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Zouboulis, C.; Adjaye, J. Human skin stem cells and the ageing process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef] [Green Version]
- Pazhanisamy, S.K. Stem cells, DNA damage, ageing and cancer. Hematol. Oncol. Stem Cell Ther. 2009, 2, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Barazzuol, L.; Ju, L. A coordinated DNA damage response promotes adult quiescent neural stem cell activation. PLoS Biol. 2017, 15, e2001264. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Wilson, D.M., III. DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.E.; Dollé, M.E.T. Deficiency in the DNA repair protein ERCC1 triggers a link between senescence and apoptosis in human fibroblasts and mouse skin. Aging Cell 2020, 19, e13072. [Google Scholar] [CrossRef]
- Sharpless, N.E.; DePinho, R.A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, V.J.; Pignolo, R.J. Replicative senescence of human fibroblast-like cells in culture. Physiol. Rev. 1993, 73, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Price, F.D.; von Maltzahn, J. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 2014, 20, 1174–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Luo, M. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 2014, 14, 673–688. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L. No place like home: Anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008, 9, 11–21. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [Green Version]
- Scadden, D.T. Nice neighborhood: Emerging concepts of the stem cell niche. Cell 2014, 157, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, W. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011, 25, 1474–1485. [Google Scholar] [CrossRef] [Green Version]
- Shakouri-Motlagh, A.; O’Connor, A.J. Native and solubilized decellularized extracellular matrix: A critical assessment of their potential for improving the expansion of mesenchymal stem cells. Acta Biomater. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Koester, J.; Miroshnikova, Y.A. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. Nat. Cell Biol. 2021, 23, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, R.; Pan, L. DNA damage and its links to neurodegeneration. Neuron 2014, 83, 266–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskalev, A.A.; Shaposhnikov, M.V. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Gorgoulis, V.; Adams, P.D. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 814–827. [Google Scholar] [CrossRef]
- Cavinato, M. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, S. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxidative Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [Green Version]
- Moskalev, A.A.; Proshkina, E.N. Genetics of Aging and Longevity. Vavilov J. Genet. Breed. 2016, 20, 426–440. [Google Scholar] [CrossRef] [Green Version]
- Beerman, I.; Seita, J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell 2014, 15, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garagnani, P.; Marquis, J. Whole-genome sequencing analysis of semi-supercentenarians. eLife 2021, 10, e57849. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Genever, P. Non-epithelial oral mucosal progenitor cell populations. Oral Dis. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schworer, S.; Becker, F. Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals. Nature 2016, 540, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Victor, P.; Ayyaz, A. Piwi is required to limit exhaustion of aging somatic stem cells. Cell Rep. 2017, 20, 2527–2537. [Google Scholar] [CrossRef] [Green Version]
- Proshkina, E.N.; Solovev, I.A. Key molecular mechanisms of aging, biomarkers, and potential interventions. Mol. Biol. 2020, 54, 883–921. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Gurcar, A.U. Nuclear genomic instability and aging. Annu. Rev. Biochem. 2018, 87, 295–322. [Google Scholar] [CrossRef]
- Ben-Avraham, D. Epigenetics of aging. In Longevity Genes; Atzmon, G., Ed.; Springer: New York, NY, USA, 2015; Volume 847, pp. 179–191. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Scully, R.; Panday, A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J. Cellular senescence: Aging, cancer, and injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Beausejour, C.M.; Krtolica, A. Reversal of human cellular senescence: Roles of the p53 and p16 pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Schafera, M.J.; Millera, J.D. Cellular senescence: Implications for metabolic disease. Mol. Cell Endocrinol. 2017, 455, 93–102. [Google Scholar] [CrossRef]
- D’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Chevet, E. Targeting the unfolded protein response in disease. Nat. Rev. Drug Discov. 2013, 12, 703–719. [Google Scholar] [CrossRef]
- Sturmlechner, I.; Zhang, C. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science 2021, 374, eabb3420. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Narita, M.; Nũnez, S. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Waaijer, M.; Parish, W.E. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Torrice, C. Ink4a/Arf expression is a biomarker of aging. J. Clin Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Wiggins, K.A.; Clarke, M.C. Senescence utilises inflammatory caspases to drive the SASP. Aging 2019, 11, 3891–3892. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, L.; Matmati, S. Solving the telomere replication problem. Genes 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Molecular signaling and genetic pathways of senescence: Its role in tumorigenesis and aging. J. Cell Physiol. 2007, 210, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Moehle, E.A.; Shen, K. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J. Biol. Chem. 2019, 294, 5396–5407. [Google Scholar] [CrossRef] [Green Version]
- Boyman, L.; Karbowski, M. Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol Med. 2020, 26, 21–39. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, J.C.; Bwiza, C.P. Mitonuclear genomics and aging. Hum. Genet. 2020, 139, 381–399. [Google Scholar] [CrossRef]
- Cao, K.; Feng, Z. Mitoepigenetics: An intriguing regulatory layer in aging and metabolic-related diseases. Free Radic. Biol. Med. 2021, 177, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.; Nemoto, S. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korolchuk, V.I.; Miwa, S. Mitochondria in cell senescence: Is mitophagy the weakest link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorgoulis, V.G.; Pefani, D.E. Integrating the DNA damage and protein stress responses during cancer development and treatment. J. Pathol. 2018, 246, 12–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koliada, A.K.; Krasnenkov, D.S. Telomeric aging: Mitotic clock or stress indicator? Front. Genet. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.B.; Larsson, N.G. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.T. Adjustment of the lysosomal-mitochondrial axis for control of cellular senescence. Ageing Res. Rev. 2018, 47, 176–182. [Google Scholar] [CrossRef]
- Huang, H.; Manton, K.G. The role of oxidative damage in mitochondria during aging: A review. Front. Biosci. 2004, 9, 1100–1117. [Google Scholar] [CrossRef] [Green Version]
- Treiber, N.; Maity, P. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell 2011, 10, 239–254. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Han, J. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Ott, C.; König, J. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biol. 2016, 10, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin: The defective powerhouse model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitsiou, E.; Pulido, T. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J. Investig. Dermatol. 2020, 141, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Choi, Y.J. Molecular insights into SIRT1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. J. Gerontol A Biol. Sci. Med. Sci. 2015, 70, 959e68. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Ott, C. Carbonylation of the cytoskeletal protein actin leads to aggregate formation. Free Radic. Biol. Med. 2012, 53, 916–925. [Google Scholar] [CrossRef]
- Grune, T. Oxidized protein aggregates: Formation and biological effects. Free Radic. Biol. Med. 2020, 150, 120–124. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.M. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.I.; Devine, O.P. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Desprez, P. Cell Autonomous and Non-autonomous Effects of Senescent Cells in the Skin. J. Investig. Dermatol. 2015, 35, 1722–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Rodier, F.; Coppe, J.-P. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Borghesan, M.; Fafián-Labora, J. Small Extracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM. Cell Rep. 2019, 27, 3956–3971. [Google Scholar] [CrossRef] [Green Version]
- Basisty, N.; Kale, A. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [Green Version]
- Waldera Lupa, D.M.; Kalfalah, F. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J. Investig. Dermatol. 2015, 135, 1954–1956. [Google Scholar] [CrossRef] [Green Version]
- Gruber, F.; Kremslehner, C. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhart, L.; Tschachler, E. Autophagic control of skin aging. Front. Cell Dev. Biol. 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, G.; Jurk, D. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.C.; Gunn, D.A. Do senescence markers correlate in vitro and in situ within individual human donors? Aging 2018, 10, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Scaffidi, P.; Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 2006, 312, 1059–1163. [Google Scholar] [CrossRef] [Green Version]
- Freund, A.; Laberge, R.-M. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef]
- Taimen, P.; Pflegar, K. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc. Natl. Acad. Sci. USA 2009, 106, 20788–20793. [Google Scholar] [CrossRef] [Green Version]
- Dreesen, O.; Chojnowski, A. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J. Cell Biol. 2013, 200, 605–617. [Google Scholar] [CrossRef] [Green Version]
- Bernardes de Jesus, B.; Blasco, M.A. Assessing Cell and Organ Senescence Biomarkers. Circ. Res. 2012, 111, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Yu, R. Chromatin architectural changes during cellular senescence and aging. Genes 2018, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Davalos, A.R.; Kawahara, M. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Tilstra, J.S.; Robinson, A.R. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Investig. 2012, 122, 2601–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppe, J.P.; Desprez, P.Y. The senescence-associated secretory phenotype: The dark side of tumor suppression. Ann. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, J.C. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ressler, S.; Bartkova, J. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Ann. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debacq-Chainiaux, F.; Leduc, C. UV, stress and aging. Dermato-Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Aird, K.M.; Zhang, R. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol. Biol. 2013, 965, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Coppe, J.P.; Kauser, K. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568e74. [Google Scholar] [CrossRef] [Green Version]
- Velarde, M.C.; Demaria, M. Targeting senescent cells: Possible implications for delaying skin aging: A mini-review. Gerontology 2016, 62, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.-Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Age Dev. 2021, 198, 111525. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, P.F.L.; Schumacher, B. DNA damage responses in ageing. Open Biol. 2019, 9, 190168. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toutfaire, M.; Bauwens, E. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem. Pharmacol. 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G. Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184e9. [Google Scholar] [CrossRef] [Green Version]
- Demaria, M.; Ohtani, N. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Espín, D.; Cañamero, M. Programmed cell senescence during mammalian embryonic development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [Green Version]
- Fafián-Labora, J.A.; O’Loghlen, A. NF-κB/ IKK activation by small extracellular vesicles within the SASP. Aging Cell 2021, 20, e13426. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Inflammaging: Disturbed interplay between autophagy and inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, G.; Wordsworth, J. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, P.F.L.; Ogrodnik, M. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 2019, 18, e12848. [Google Scholar] [CrossRef]
- Nelson, G.; Kucheryavenko, O. The senescent bystander effect is caused by ROS-activated NF-kB signaling. Mech. Ageing Dev. 2018, 170, 30–36. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Tomaru, U.; Takahashi, S. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am. J. Pathol. 2012, 180, 963–972. [Google Scholar] [CrossRef]
- Meyer, P.; Maity, P. A model of the onset of the senescence associated secretory phenotype after DNA damage induced senescence. PLoS Comput. Biol. 2017, 13, e1005741. [Google Scholar] [CrossRef] [Green Version]
- Laberge, R.M.; Sun, Y. mTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting il1a translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. Int. J. Mol. Sci. 2022, 23, 6135. https://doi.org/10.3390/ijms23116135
Zorina A, Zorin V, Kudlay D, Kopnin P. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. International Journal of Molecular Sciences. 2022; 23(11):6135. https://doi.org/10.3390/ijms23116135
Chicago/Turabian StyleZorina, Alla, Vadim Zorin, Dmitry Kudlay, and Pavel Kopnin. 2022. "Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging" International Journal of Molecular Sciences 23, no. 11: 6135. https://doi.org/10.3390/ijms23116135





