Beta-Site Amyloid Precursor Protein-Cleaving Enzyme Inhibition Partly Restores Sevoflurane-Induced Deficits on Synaptic Plasticity and Spine Loss
Abstract
:1. Introduction
2. Results
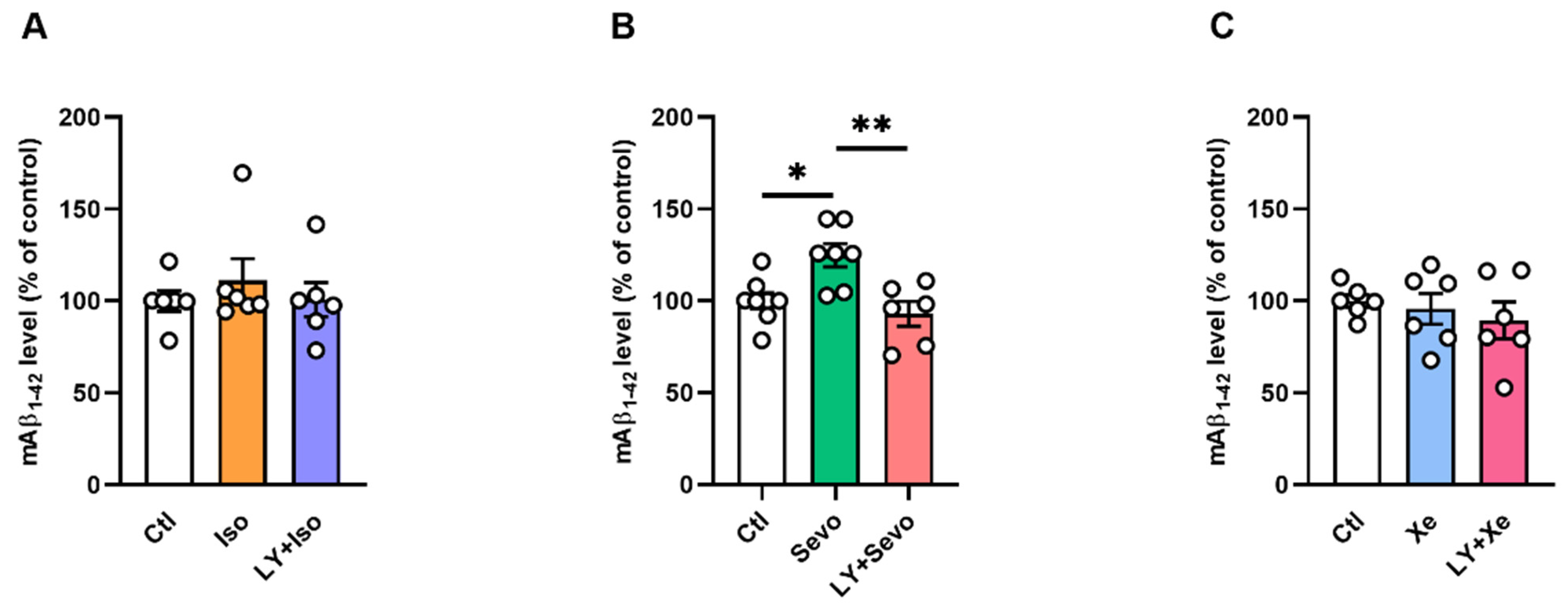
2.1. BACE Inhibition Restored the Sevo-Induced Upregulation of Mouse Aβ1–42 (mAβ1–42) Levels
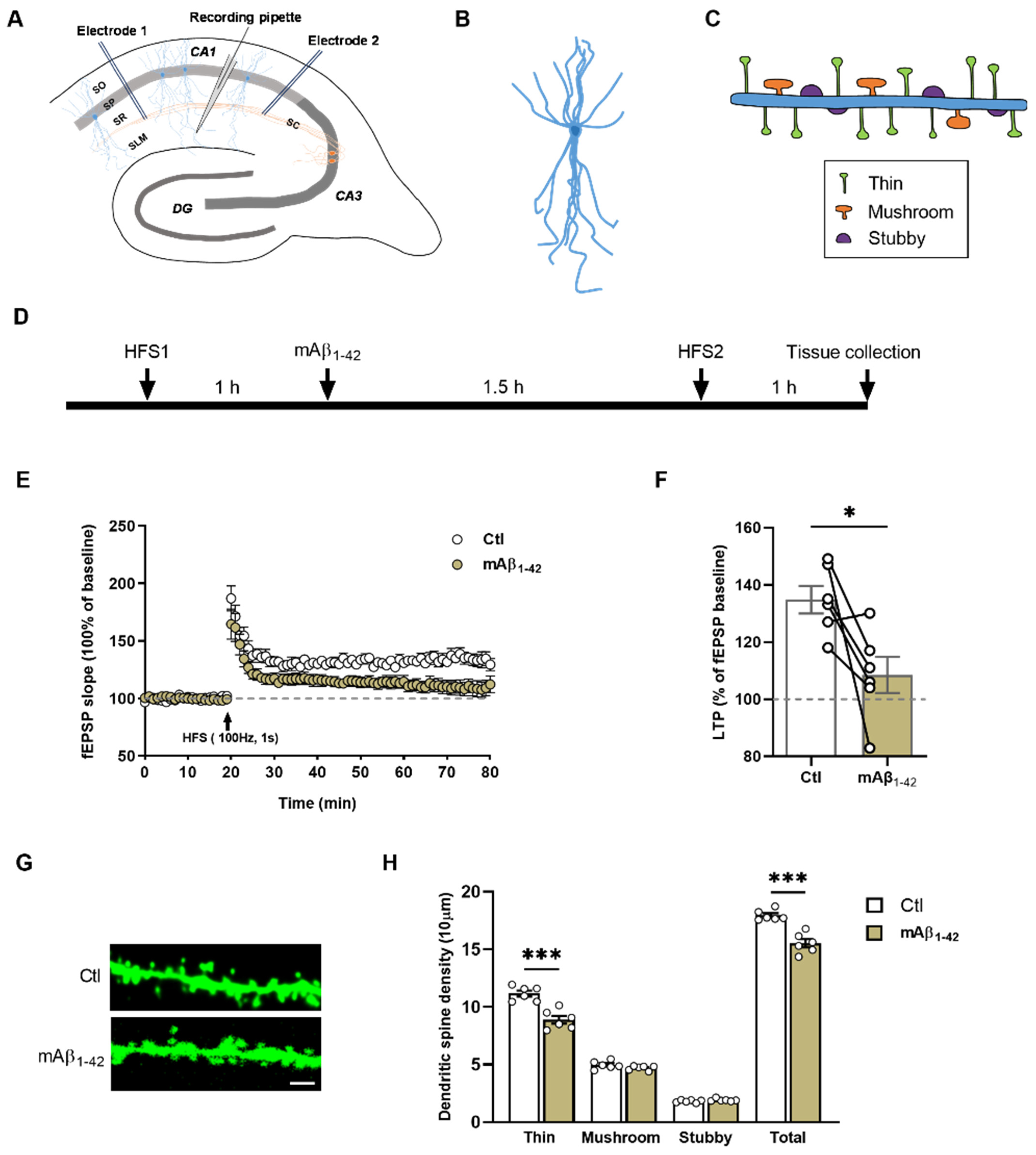
2.2. mAβ1–42 Blocked LTP and Reduced Dendritic Spine Density of CA1 Pyramidal Neurons
2.3. BACE Inhibition Partly Reversed the Sevo-Induced Dendritic Spines Loss
2.4. BACE Inhibition Reversed the Sevo-Induced Reduction in Nectin-3 Levels in CA1
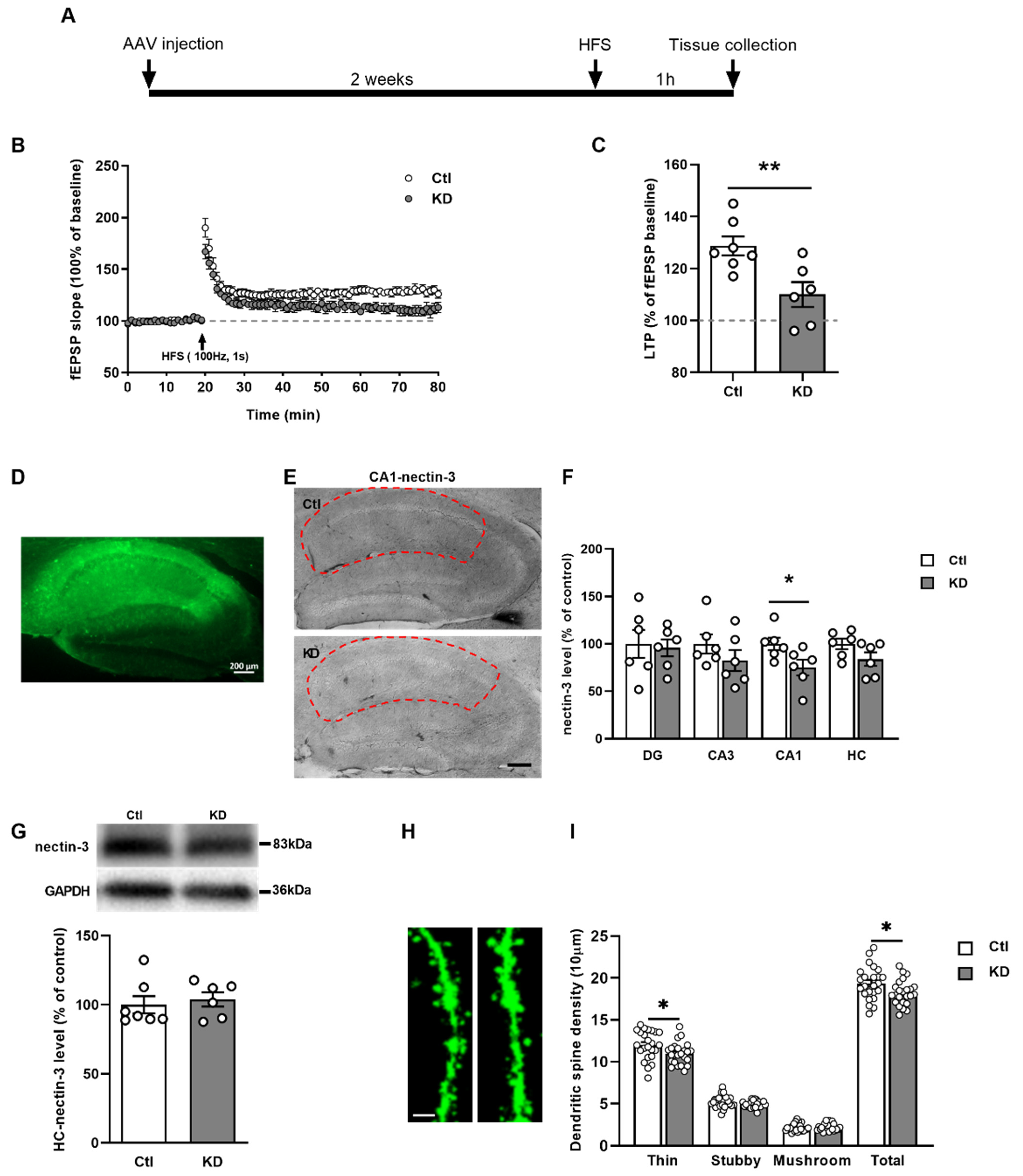
2.5. Nectin-3 Knockdown in CA1 Region Attenuated LTP and Evoked Spine Loss
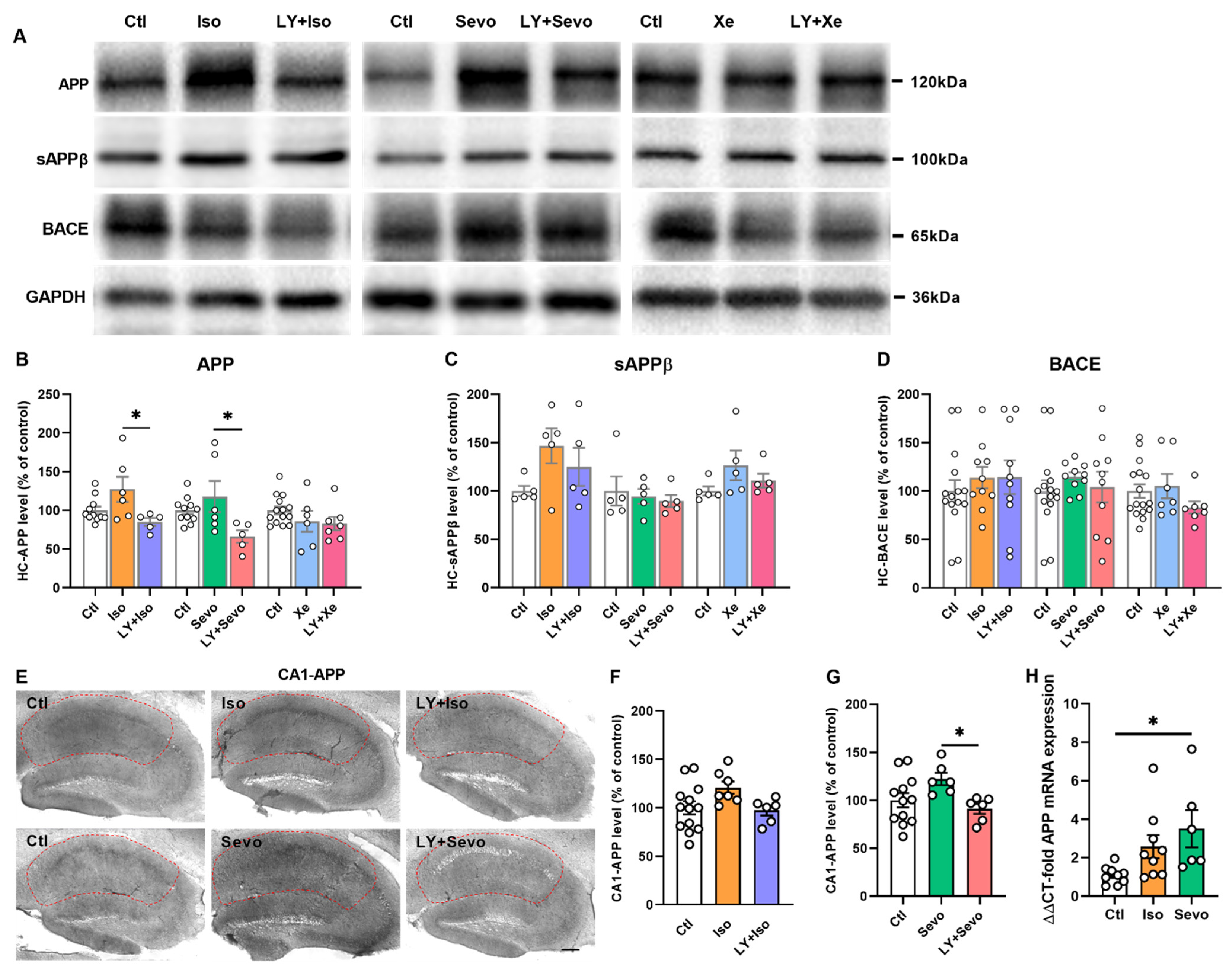
2.6. Regulation of APP Processing
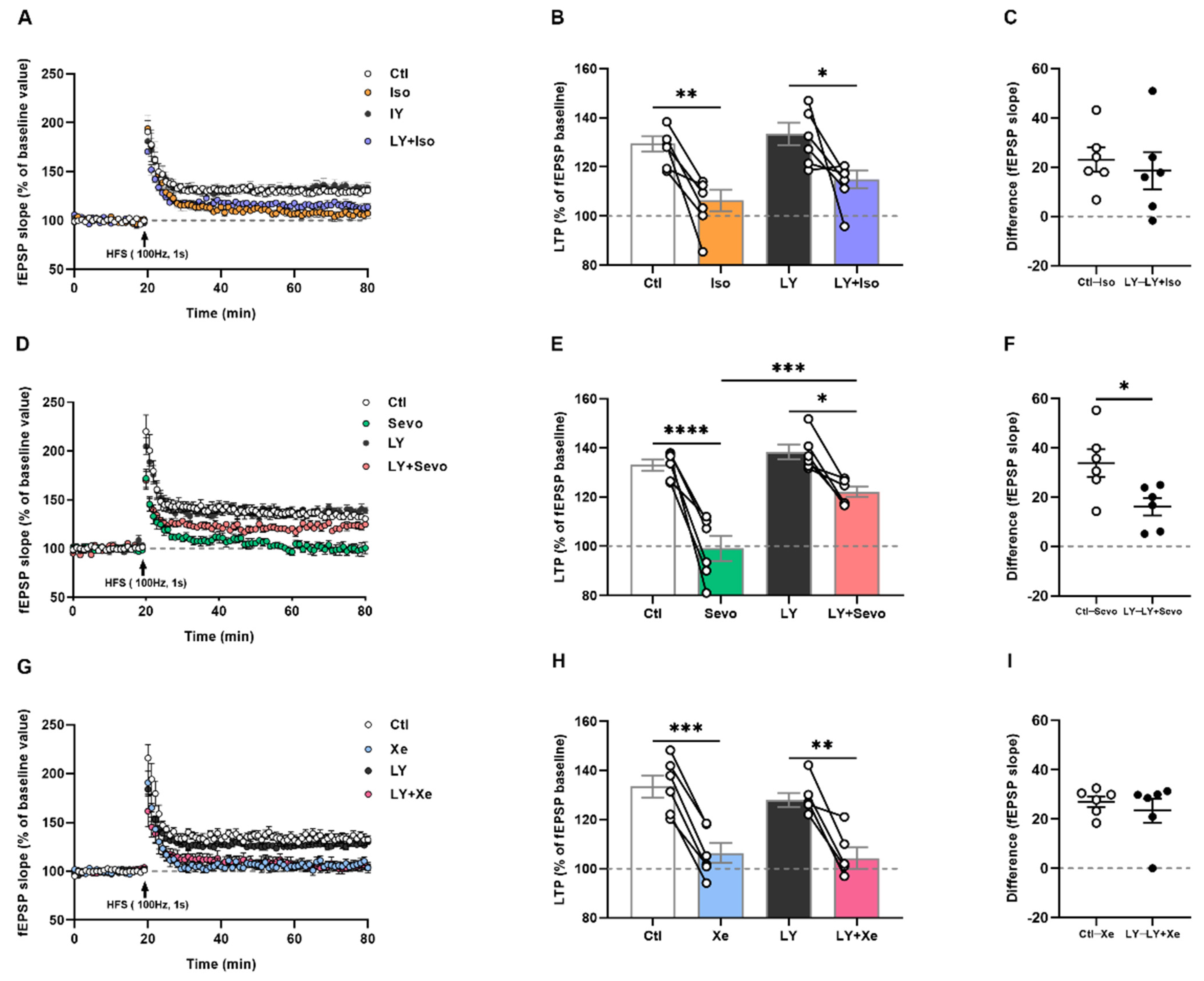
2.7. Iso, Sevo, and Xe Markedly Abolished LTP Respectively
2.8. Only Sevo-Induced LTP Deficits Were at Least Partly Restored by BACE Inhibition
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Compounds
4.3. Slices Preparation
4.4. Field Excitatory Postsynaptic Potentials (fEPSPs)
4.5. Stereotaxic Virus Microinjection
4.6. Immunostaining and Image Analysis
4.7. ELISA
4.8. Western Blot
4.9. Quantitative Real-Time PCR (RT-PCR)
4.10. Classification of Dendritic Spines
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campagna, J.A.; Miller, K.W.; Forman, S.A. Mechanisms of Actions of Inhaled Anesthetics. N. Engl. J. Med. 2003, 348, 2110–2124. [Google Scholar] [CrossRef] [PubMed]
- Haseneder, R.; Kratzer, S.; von Meyer, L.; Eder, M.; Kochs, E.; Rammes, G. Isoflurane and sevoflurane dose-dependently impair hippocampal long-term potentiation. Eur. J. Pharmacol. 2009, 623, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S. The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen. Res. 2017, 12, 1780–1785. [Google Scholar] [PubMed]
- Palanca, B.J.A.; Avidan, M.S.; Mashour, G.A. Human neural correlates of sevoflurane-induced unconsciousness. Br. J. Anaesth. 2017, 119, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Simon, W.; Hapfelmeier, G.; Kochs, E.; Zieglgaensberger, W.; Rammes, G. Isoflurane Blocks Synaptic Plasticity in the Mouse Hippocampus. Anesthesiology 2001, 94, 1058–1065. [Google Scholar] [CrossRef]
- Kratzer, S.; Mattusch, C.; Kochs, E.; Eder, M.; Haseneder, R.; Rammes, G. Xenon attenuates hippocampal long-term potentiation by diminishing synaptic and extrasynaptic N-methyl-D-aspartate receptor currents. Anesthesiology 2012, 116, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Sonner, J.M.; Cantor, R.S. Molecular mechanisms of drug action: An emerging view. Annu. Rev. Biophy. 2013, 42, 143–167. [Google Scholar] [CrossRef]
- Johnson, S.C.; Pan, A.; Li, L.; Sedensky, M.; Morgan, P. Neurotoxicity of anesthetics: Mechanisms and meaning from mouse intervention studies. Neurotoxicol. Teratol. 2019, 71, 22–31. [Google Scholar] [CrossRef]
- Hering, H.; Sheng, M. Dentritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef]
- Granak, S.; Hoschl, C.; Ovsepian, S.V. Dendritic spine remodeling and plasticity under general anesthesia. Brain Struct. Funct. 2021, 226, 2001–2017. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, H. Effect of the inhaled anesthetics isoflurane, sevoflurane and desflurane on the neuropathogenesis of Alzheimer’s disease (review). Mol. Med. Rep. 2015, 12, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westmark, C.J. What’s hAPPening at synapses? The role of amyloid β-protein precursor and β-amyloid in neurological disorders. Mol. Psychiatry 2013, 18, 425–434. [Google Scholar] [PubMed] [Green Version]
- Mueller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.L.; Krafft, G.A.; Finch, C.E. Targeting small Abeta oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001, 24, 219–224. [Google Scholar] [CrossRef]
- Rammes, G.; Hasenjaeger, A.; Sroka-Saidi, K.; Deussing, J.M.; Parsons, C.G. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology 2011, 60, 982–990. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, D. Amyloid-β Impairs Synaptic Inhibition via GABA(A) Receptor Endocytosis. J. Neurosci. 2015, 35, 9205–9210. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Dong, Y.; Maeda, U.; Moir, R.D.; Xia, W.; Culley, D.J.; Crosby, G.; Tanzi, R.E. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J. Neurosci. 2007, 27, 1247–1254. [Google Scholar] [CrossRef]
- Xie, Z.; Culley, D.J.; Dong, Y.; Zhang, G.; Zhang, B.; Moir, R.D.; Frosch, M.P.; Crosby, G.; Tanzi, R.E. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann. Neurol. 2008, 64, 618–627. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Guan, W.; Luan, L.; Li, B.; Tang, Q.; Fan, H. Isoflurane anesthesia promotes cognitive impairment by inducing expression of β-amyloid protein-related factors in the hippocampus of aged rats. PLoS ONE 2017, 12, e0175654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckenhoff, R.G.; Johansson, J.S.; Wei, H.; Carnini, A.; Kang, B.; Wei, W.; Pidikiti, R.; Keller, J.M.; Eckenhoff, M.F. Inhaled Anesthetic Enhancement of Amyloid-β Oligomerization and Cytotoxicity. Anesthesiology 2004, 101, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Dong, Y.; Maeda, U.; Moir, R.; Inouye, S.K.; Culley, D.J.; Crosby, G.; Tanzi, R.E. Isoflurane-induced apoptosis: A potential pathogenic link between delirium and dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1300–1306. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhang, G.; Zhang, B.; Moir, R.D.; Xia, W.; Marcantonio, E.R.; Culley, D.J.; Crosby, G.; Tanzi, R.E.; Xie, Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch. Neurol. 2009, 66, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xia, J.; Chen, Y.; Zhang, J. Sevoflurane-Induced Endoplasmic Reticulum Stress Contributes to Neuroapoptosis and BACE-1 Expression in the Developing Brain: The Role of eIF2α. Neurotox. Res. 2017, 31, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, Z.; Zhang, Y.; McAuliffe, S.; Wang, H.; Shen, X.; Yue, Y.; Xie, Z. RNA interference-mediated silencing of BACE and APP attenuates the isoflurane-induced caspase activation. Med. Gas. Res. 2011, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilling, S.; Mehr, A.; Ludewig, S.; Stephan, J.; Zimmermann, M.; August, A.; Strecker, P.; Korte, M.; Koo, E.H.; Mueller, U.C.; et al. APLP1 Is a Synaptic Cell Adhesion Molecule, Supporting Maintenance of Dendritic Spines and Basal Synaptic Transmission. J. Neurosci. 2017, 37, 5345–5365. [Google Scholar] [CrossRef]
- Wang, X.D.; Su, Y.A.; Wagner, K.V.; Avrabos, C.; Scharf, S.H.; Hartmann, J.; Wolf, M.; Liebl, C.; Kuehne, C.; Wurst, W.; et al. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat. Neurosci. 2013, 16, 706–713. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, J.T.; Xie, X.M.; Gu, Y.; Si, T.M.; Schmidt, M.V.; Wang, X.D. Nectin-3 modulates the structural plasticity of dentate granule cells and long-term memory. Transl. Psychiatry 2017, 7, e1228. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Wang, H.L.; Sun, Y.X.; Su, Y.A.; Wang, X.D.; Li, J.T.; Si, T.M. Postnatal nectin-3 knockdown induces structural abnormalities of hippocampal principal neurons and memory deficits in adult mice. Hippocampus 2019, 29, 1063–1074. [Google Scholar] [CrossRef] [Green Version]
- Platholi, J.; Herold, K.F.; Hemmings, H.C., Jr.; Halpain, S. Isoflurane reversibly destabilizes hippocampal dendritic spines by an actin-dependent mechanism. PLoS ONE 2014, 9, e102978. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Y.; Ao, J.; Ding, L.; Liu, Y.; Yuan, Y.; Wang, Z.; Wang, G. Role of α7nAChR-NMDAR in sevoflurane-induced memory deficits in the developing rat hippocampus. PLoS ONE 2018, 13, e0192498. [Google Scholar] [CrossRef] [PubMed]
- Esencan, E.; Yuksel, S.; Tosun, Y.B.; Robinot, A.; Solaroglu, I.; Zhang, J.H. XENON in medical area: Emphasis on neuroprotection in hypoxia and anesthesia. Med. Gas. Res. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuer, T.; Emontzpohl, C.; Coburn, M.; Benstoem, C.; Rossaint, R.; Marx, G.; Schaelte, G.; Bernhagen, J.; Bruells, C.S.; Goetzenich, A.; et al. Xenon triggers pro-inflammatory effects and suppresses the anti-inflammatory response compared to sevoflurane in patients undergoing cardiac surgery. Crit. Care 2015, 19, 365. [Google Scholar] [CrossRef] [Green Version]
- Mattusch, C.; Kratzer, S.; Buerge, M.; Kreuzer, M.; Engel, T.; Kopp, C.; Biel, M.; Hammelmann, V.; Ying, S.W.; Goldstein, P.A.; et al. Impact of Hyperpolarization-activated, Cyclic Nucleotide-gated Cation Channel Type 2 for the Xenon-mediated Anesthetic Effect: Evidence from In Vitro and In Vivo Experiments. Anesthesiology 2015, 122, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, L.; Love, J.; Colman, D.R. Adhesion Molecules in the Nervous System: Structural Insights into Function and Diversity. Annu. Rev. Neurosci. 2007, 30, 451–474. [Google Scholar] [CrossRef] [PubMed]
- Giagtzoglou, N.; Ly, C.V.; Bellen, H.J. Cell adhesion, the backbone of the synapse: “vertebrate” and “invertebrate” perspectives. Cold Spring Harb. Perspect. Biol. 2009, 1, a003079. [Google Scholar] [CrossRef] [Green Version]
- Haseneder, R.; Kratzer, S.; Kochs, E.; Eckle, V.S.; Zieglgaensberger, W.; Rammes, G. Xenon reduces N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology 2008, 109, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Basu, J.; Siegelbaum, S.A. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb. Perspect. Biol. 2015, 7, a021733. [Google Scholar] [CrossRef]
- Buerge, M.; Kratzer, S.; Mattusch, C.; Hofmann, C.; Kreuzer, M.; Parsons, C.G.; Rammes, G. The anaesthetic xenon partially restores an amyloid beta-induced impairment in murine hippocampal synaptic plasticity. Neuropharmacology 2019, 151, 21–32. [Google Scholar] [CrossRef]
- Volfovsky, N.; Parnas, H.; Segal, M.; Korkotian, E. Geometry of dendritic spines affects calcium dynamics in hippocampal neurons: Theory and experiments. J. Neurophysiol. 1999, 82, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Korkotian, E.; Segal, M. Structure-function relations in dendritic spines: Is size important? Hippocampus 2000, 10, 587–595. [Google Scholar] [CrossRef]
- Majewska, A.; Brown, E.; Ross, J.; Yuste, R. Mechanisms of calcium decay kinetics in hippocampal spines: Role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J. Neurosci. 2000, 20, 1722–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, P.C.; Willis, B.A.; Lowe, S.L.; Dean, R.A.; Monk, S.A.; Cocke, P.J.; Audia, J.E.; Boggs, L.N.; Borders, A.R.; Brier, R.A.; et al. The potent BACE1 inhibitor LY2886721 elicits robust central Aβ pharmacodynamic responses in mice, dogs, and humans. J. Neurosci. 2015, 35, 1199–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satir, T.M.; Agholme, L.; Karlsson, A.; Karlsson, M.; Karila, P.; Illes, S.; Bergstrom, P.; Zetterberg, H. Partial reduction of amyloid beta production by beta-secretase inhibitors does not decrease synaptic transmission. Alzheimers Res. 2020, 12, 63. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef] [Green Version]
- Eger, E.I.I. Age, Minimum Alveolar Anesthetic Concentration, and Minimum Alveolar Anesthetic Concentration-Awake. Anesth. Analg. 2001, 93, 947–953. [Google Scholar] [CrossRef]
- Jones, M.V.; Brooks, P.A.; Harrison, N.L. Enhancement of gamma-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J. Physiol. 1992, 449, 279–293. [Google Scholar] [CrossRef]
- Zhang, L.; Oz, M.; Stewart, R.R.; Peoples, R.W.; Weight, F.F. Volatile general anaesthetic actions on recombinant nAChα7, 5-HT3 and chimeric nAChα7-5-HT3 receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 1997, 120, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.M.B.; Franks, N.P.; Lieb, W.R. Actions of general anaesthetics and arachidonic acid pathway inhibitors on K+ currents activated by volatile anaesthetics and FMRFamide in molluscan neurones. Br. J. Pharmacol. 1998, 125, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Au, J.D.; Zou, H.L.; Cotten, J.F.; Yost, C.S. Potent Activation of the Human Tandem Pore Domain K Channel TRESK with Clinical Concentrations of Volatile Anesthetics. Anesth. Analg. 2004, 99, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Wise-Faberowski, L.; Zhang, H.; Ing, R.; Pearlstein, R.D.; Warner, D.S. Isoflurane-induced neuronal degeneration: An evaluation in organotypic hippocampal slice cultures. Anesth. Analg. 2005, 101, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zuo, Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011, 61, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Liu, J.; Chen, X.D. General anesthesia mediated by effects on ion channels. World J. Crit. Care Med. 2012, 1, 80–93. [Google Scholar] [CrossRef]
- Istaphanous, G.K.; Howard, J.; Nan, X.; Hughes, E.A.; McCann, J.C.; McAuliffe, J.J.; Danzer, S.C.; Loepke, A.W. Comparison of the Neuroapoptotic Properties of Equipotent Anesthetic Concentrations of Desflurane, Isoflurane, or Sevoflurane in Neonatal Mice. Anesthesiology 2013, 114, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Aranake, A.; Mashour, G.A.; Avidan, M.S. Minimum alveolar concentration: Ongoing relevance and clinical utility. Anaesthesia 2013, 68, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Koblin, D.D.; Fang, Z.; Eger, E.I.; Laster, M.J.; Gong, D.; Ionescu, P.; Halsey, M.J.; Trudell, J.R. Minimum Alveolar Concentrations of Noble Gases, Nitrogen, and Sulfur Hexafluoride in Rats: Helium and Neon as Nonimmobilizers (Nonanesthetics). Anesth. Analg. 1998, 87, 419–424. [Google Scholar]
- Dwyer, R.; Bennett, H.L.; Eger, E.I.; Heilbron, D. Effects of Isoflurane and Nitrous Oxide in Subanesthetic Concentrations on Memory and Responsiveness in Volunteers. Anesthesiology 1992, 77, 888–898. [Google Scholar] [CrossRef]
- Chortkoff, B.S.; Bennett, H.L.; Eger, E.I., 2nd. Subanesthetic concentrations of isoflurane suppress learning as defined by the category-example task. Anesthesiology 1993, 79, 16–22. [Google Scholar]
- Ye, F.; Zuo, Z.Y. Anesthetic effects on autophagy. Med. Gas. Res. 2017, 7, 204–211. [Google Scholar] [PubMed] [Green Version]
- Parsons, C.G.; Rammes, G. Preclinical to phase II amyloid beta (Aβ) peptide modulators under investigation for Alzheimer’s disease. Expert Opin. Investig. Drugs 2017, 26, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Xiang, X.; Filser, S.; Marinković, P.; Dorostkar, M.M.; Crux, S.; Neumann, U.; Shimshek, D.R.; Rammes, G.; Haass, C.; et al. Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibition Impairs Synaptic Plasticity via Seizure Protein 6. Biol. Psychiatry 2018, 83, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammes, G.; Mattusch, C.; Wulff, M.; Seeser, F.; Kreuzer, M.; Zhu, K.; Deussing, J.M.; Herms, J.; Parsons, C.G. Involvement of GluN2B subunit containing N-methyl-d-aspartate (NMDA) receptors in mediating the acute and chronic synaptotoxic effects of oligomeric amyloid-beta (Aβ) in murine models of Alzheimer’s disease (AD). Neuropharmacology 2017, 123, 100–115. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Shi, Q.; Pradhan, A.K.; Ziegon, L.; Schlegel, M.; Rammes, G. Beta-Site Amyloid Precursor Protein-Cleaving Enzyme Inhibition Partly Restores Sevoflurane-Induced Deficits on Synaptic Plasticity and Spine Loss. Int. J. Mol. Sci. 2022, 23, 6637. https://doi.org/10.3390/ijms23126637
Wang X, Shi Q, Pradhan AK, Ziegon L, Schlegel M, Rammes G. Beta-Site Amyloid Precursor Protein-Cleaving Enzyme Inhibition Partly Restores Sevoflurane-Induced Deficits on Synaptic Plasticity and Spine Loss. International Journal of Molecular Sciences. 2022; 23(12):6637. https://doi.org/10.3390/ijms23126637
Chicago/Turabian StyleWang, Xingxing, Qinfang Shi, Arpit Kumar Pradhan, Laura Ziegon, Martin Schlegel, and Gerhard Rammes. 2022. "Beta-Site Amyloid Precursor Protein-Cleaving Enzyme Inhibition Partly Restores Sevoflurane-Induced Deficits on Synaptic Plasticity and Spine Loss" International Journal of Molecular Sciences 23, no. 12: 6637. https://doi.org/10.3390/ijms23126637





