Preparation of Calcium Phosphate Compounds on Zirconia Surfaces for Dental Implant Applications
Abstract
:1. Introduction
1.1. Titanium
1.2. Zirconia
1.3. Surface Treatment
1.3.1. Alkaline Treatment
1.3.2. Calcium Phosphate Coatings
1.3.3. Sintering Treatment
Sintering Zirconia
Sintering Calcium Phosphate
2. Results
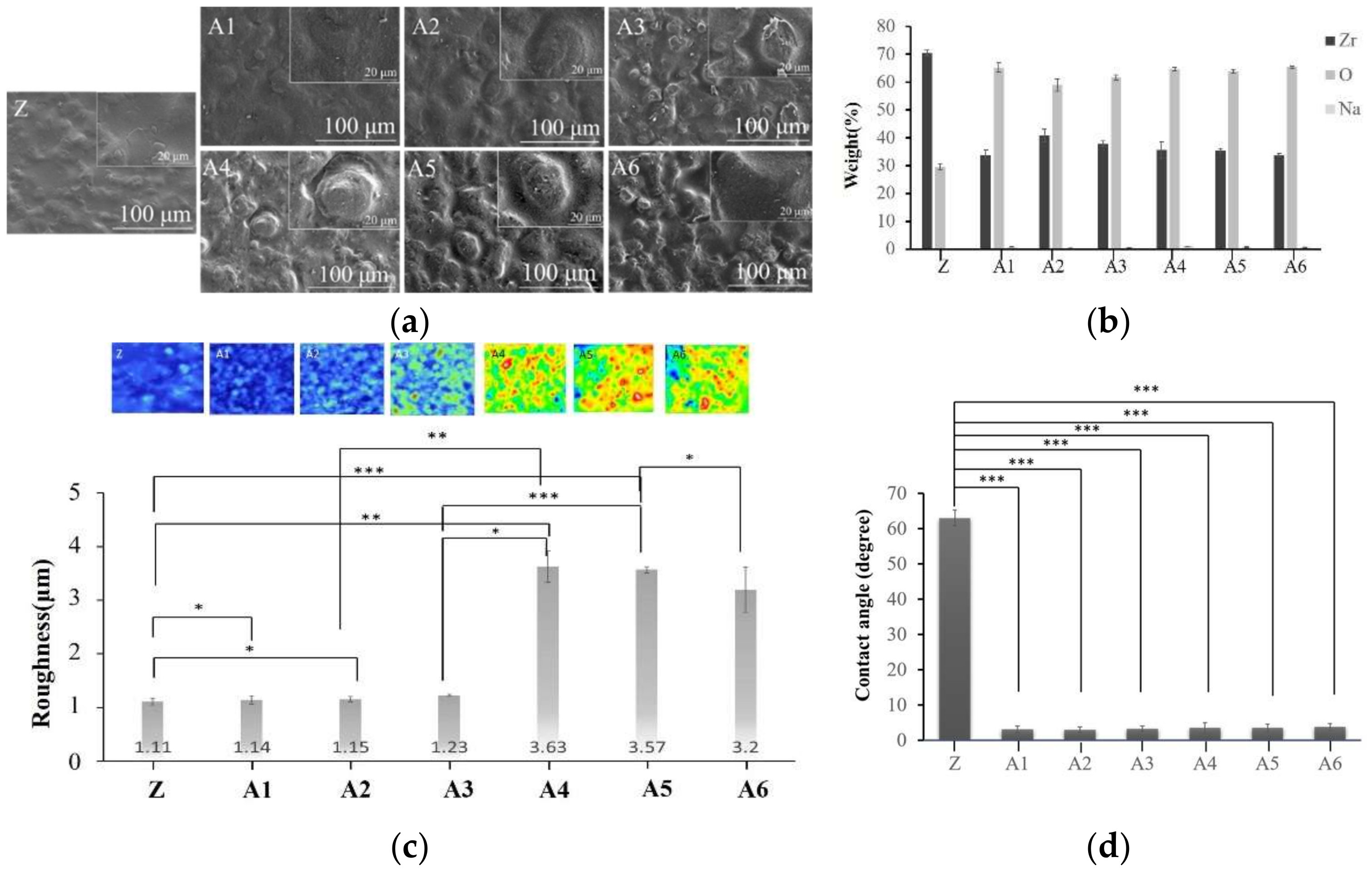
2.1. Optimal Sodium Hydroxide Concentration
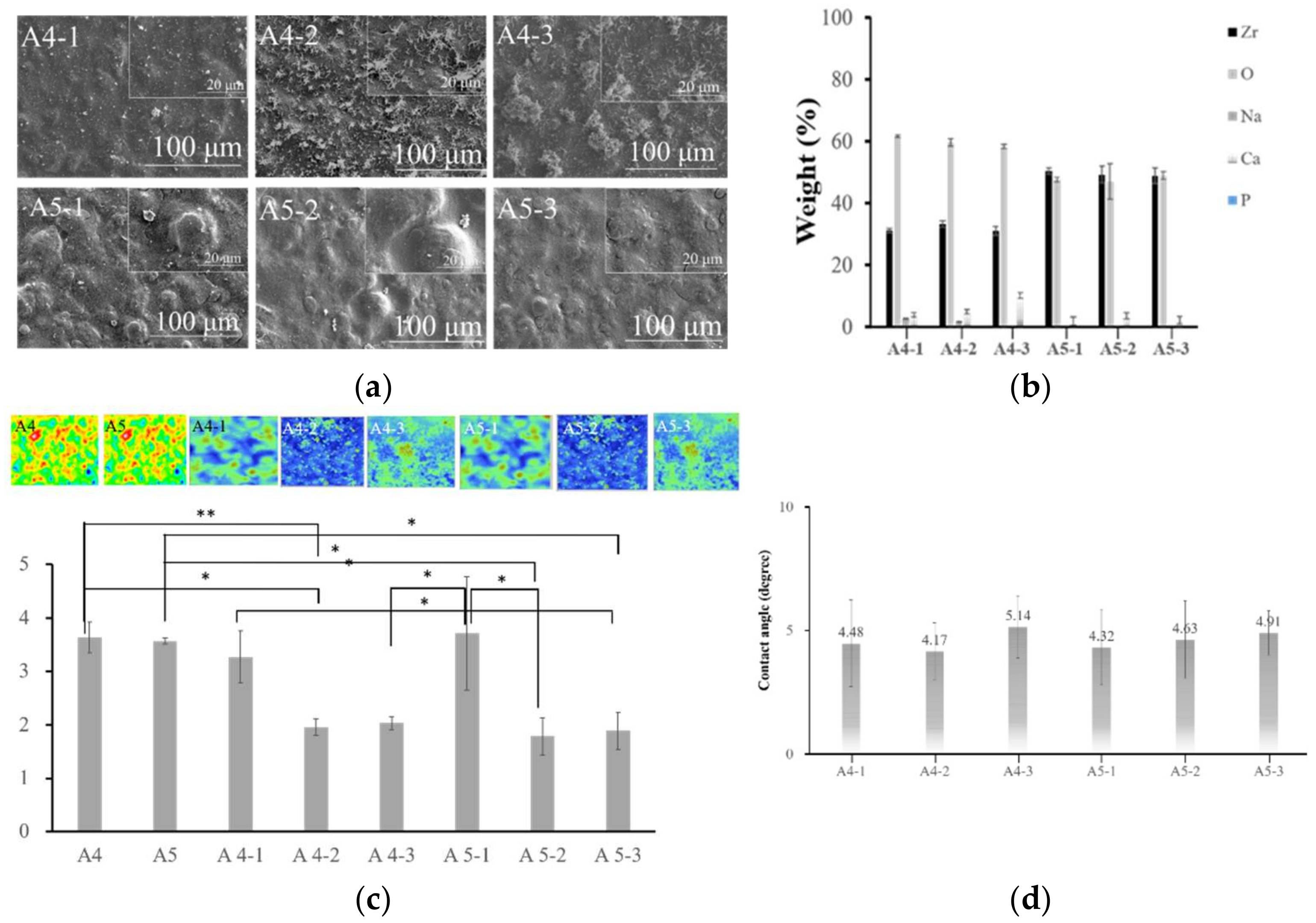
2.2. Optimal Concentration of Calcium Hydroxide
2.3. Optimal Concentration of Sodium Phosphate
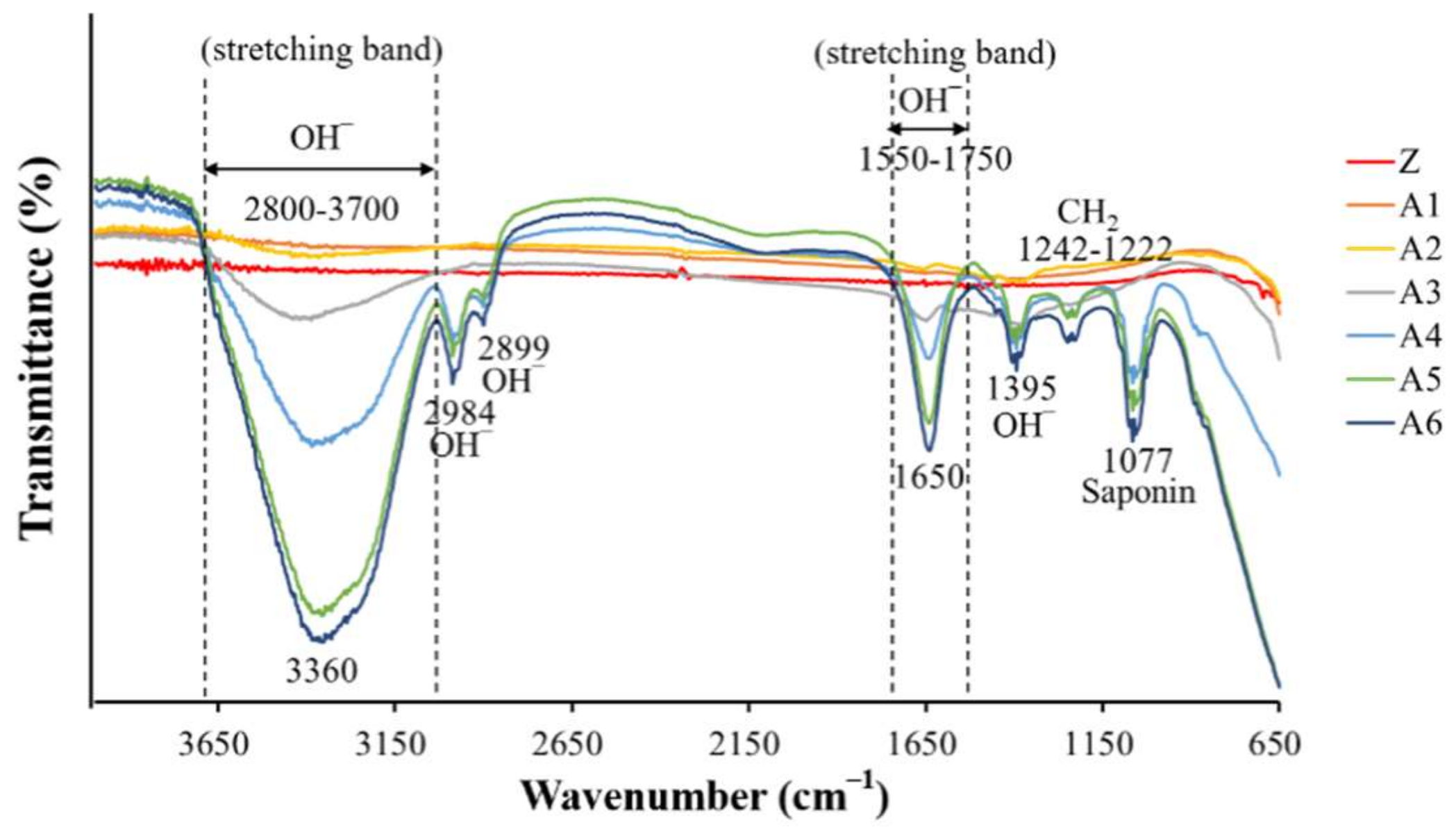
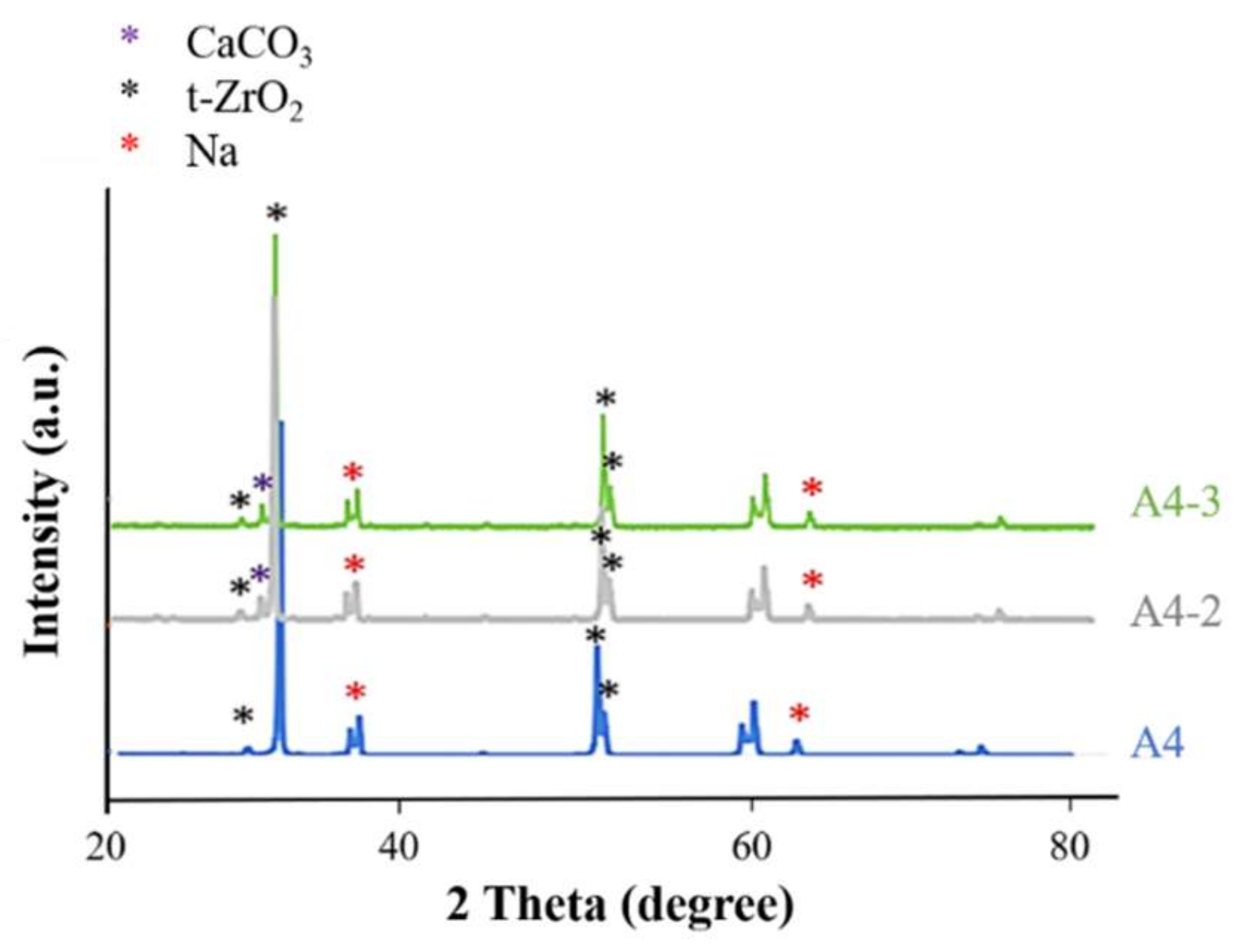
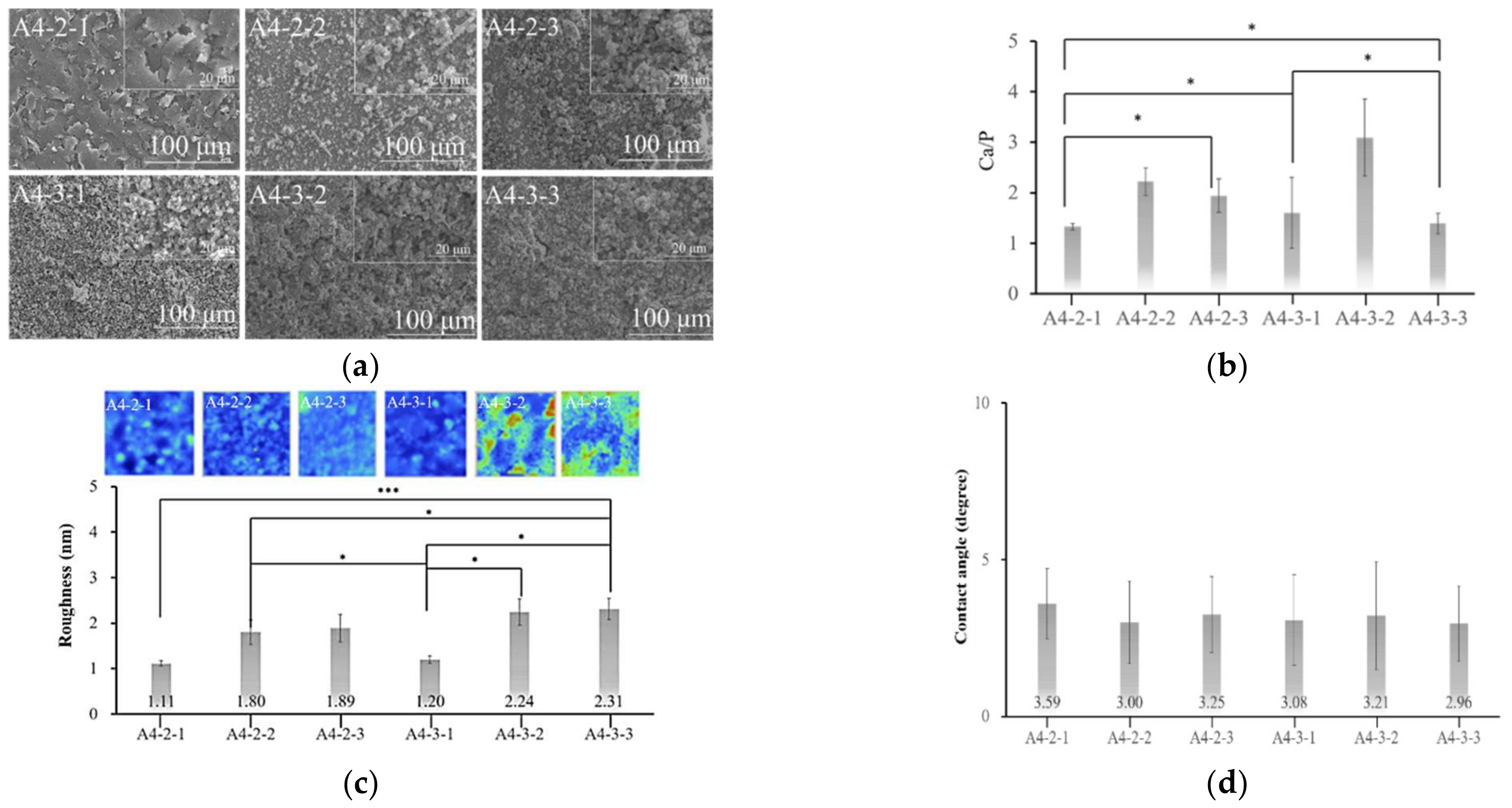
2.4. Analysis of Calcium Phosphate on the Surface of Specimens
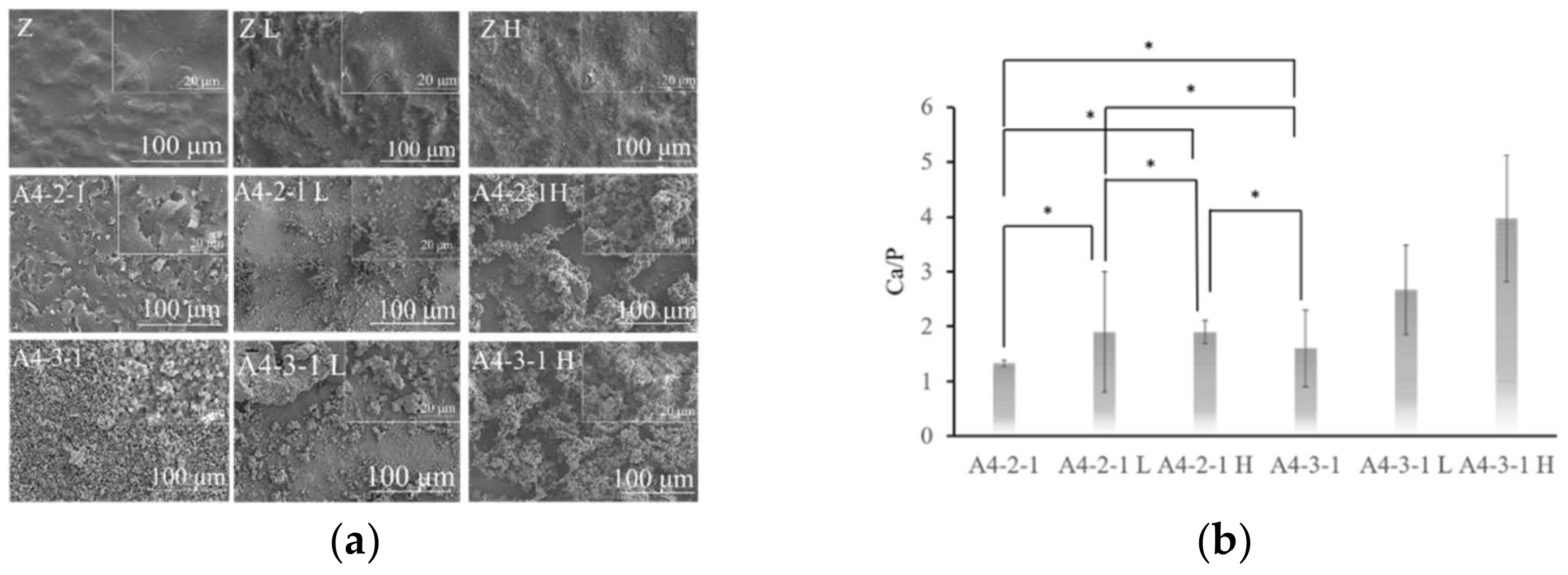
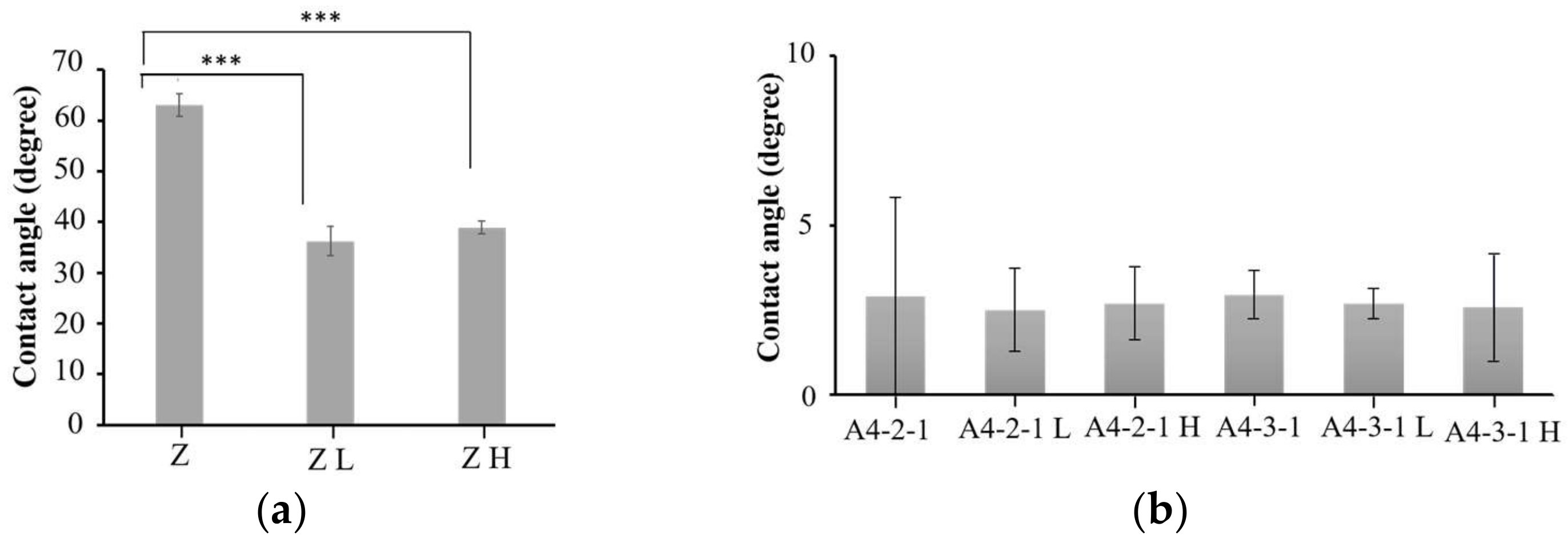
2.5. Surface Properties of Various Materials after Sintering
3. Discussion
3.1. Implant Materials
3.2. Alkaline Treatment
3.3. Calcium Phosphate Coatings
3.4. Sintering Treatment
4. Materials and Methods
4.1. Specimen Preparation
4.2. Surface Treatment
4.2.1. Alkaline Treatment
Alkaline Treatment in Sodium Hydroxide (NaOH)
4.2.2. Coating Calcium Phosphate
Stage 1: Soaking in Calcium Hydroxide Solution (Ca(OH)2)
Stage 2: Soaking in Sodium Phosphate Solution (Na3PO4)
4.2.3. Sintering Treatment
4.3. Material Characterization
4.3.1. Characterization of Surface Morphology
4.3.2. Energy-Dispersive Spectrometry
4.3.3. X-ray Photoelectron Spectroscopy
4.3.4. Fourier-Transform Infrared Spectroscopy
4.3.5. White Light Interferometry
4.3.6. Contact Angle Analyzer
4.3.7. X-ray Diffraction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Chen, X.; Gutierrez-Gonzalez, C.F.; Fernández, A.; Lopez-Esteban, S.; Aparicio, C. Peptide-functionalized zirconia and new zirconia/titanium biocermets for dental applications. J. Dent. 2015, 43, 1162–1174. [Google Scholar] [CrossRef]
- Dehestani, M.; Ilver, L.; Adolfsson, E. Enhancing the bioactivity of zirconia and zirconia composites by surface modification. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 832–840. [Google Scholar] [CrossRef]
- Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR Induced by Alkali Treatment. Int. J. Mol. Sci. 2017, 18, 780. [Google Scholar] [CrossRef] [Green Version]
- Endres, K.; Marx, R.; Tinschert, J.; Wirtz, D.C.; Stoll, C.; Riediger, D.; Smeets, R. A new adhesive technique for internal fixation in midfacial surgery. Biomed. Eng. Online 2008, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S.; Nakamura, S.; Nakamura, M.; Shinomiya, K.; Yamashita, K. Enhanced Bone Regeneration by Electrical Polarization of Hydroxyapatite. Artif. Organs 2006, 30, 863–869. [Google Scholar] [CrossRef]
- Yin, D.; Komasa, S.; Yoshimine, S.; Sekino, T.; Okazaki, J. Effect of mussel adhesive protein coating on osteogenesis in vitro and osteointegration in vivo to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2019, 14, 3831–3843. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-D.T.; Jang, Y.-S.; Lee, M.-H.; Bae, T.-S. Effect of strontium doping on the biocompatibility of calcium phosphate-coated titanium substrates. J. Appl. Biomater. Funct. Mater. 2019, 17, 1803–1812. [Google Scholar] [CrossRef] [Green Version]
- Biesuz, M.; Grasso, S.; Sglavo, V.M. What’s new in ceramics sintering? A short report on the latest trends and future prospects. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100868. [Google Scholar] [CrossRef]
- Ortali, C.; Julien, I.; Vandenhende, M.; Drouet, C.; Champion, E. Consolidation of bone-like apatite bioceramics by spark plasma sintering of amorphous carbonated calcium phosphate at very low temperature. J. Eur. Ceram. Soc. 2018, 38, 2098–2109. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.M.; Troczynski, T.; Mccullagh, A.P.; Wyatt, C.C.L.; Carvalho, R.M. The influence of altering sintering protocols on the optical and mechanical properties of zirconia: A review. J. Esthet. Restor. Dent. 2019, 31, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Özcan, M.; Hallmann, L.; Ender, A.; Mehl, A.; Hämmerlet, C.H. The effect of zirconia sintering temperature on flexural strength, grain size, and contrast ratio. Clin. Oral Investig. 2013, 17, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierre, C.; Bertrand, G.; Rey, C.; Benhamou, O.; Combes, C. Calcium phosphate coatings elaborated by the soaking process on titanium dental implants: Surface preparation, processing and physical–chemical characterization. Dent. Mater. 2019, 35, e25–e35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.-T. Bone formation of a porous Gelatin-Pectin-biphasic calcium phosphate composite in presence of BMP-2 and VEGF. Int. J. Biol. Macromol. 2015, 76, 10–24. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2001, 23, 1065–1072. [Google Scholar] [CrossRef]
- Macan, J.; Sikirić, M.D.; Deluca, M.; Bermejo, R.; Baudin, C.; Plodinec, M.; Salamon, K.; Čeh, M.; Gajović, A. Mechanical properties of zirconia ceramics biomimetically coated with calcium deficient hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2020, 111, 104006. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Kim, H.-E.; Kim, M.-J.; Kim, U.-C.; Kim, J.-H.; Koh, Y.-H. Production and characterization of calcium phosphate (CaP) whisker-reinforced poly(ε-caprolactone) composites as bone regenerative. Mater. Sci. Eng. C 2010, 30, 1280–1284. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef]
- Jakovac, M.; Klaser, T.; Radatović, B.; Bafti, A.; Skoko, Ž.; Pavić, L.; Žic, M. Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material. Materials 2021, 14, 2834. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Yamaguchi, S.; Matsushita, T.; Kokubo, T. Nanostructured positively charged bioactive TiO2 layer formed on Ti metal by NaOH, acid and heat treatments. J. Mater. Sci. Mater. Electron. 2011, 22, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-S.; Salamanca, E.; Lin, J.C.-Y.; Pan, Y.-H.; Wu, Y.-F.; Teng, N.-C.; Watanabe, I.; Sun, Y.-S.; Chang, W.-J. Preparation of Calcium Phosphate Compounds on Zirconia Surfaces for Dental Implant Applications. Int. J. Mol. Sci. 2022, 23, 6675. https://doi.org/10.3390/ijms23126675
Cheng M-S, Salamanca E, Lin JC-Y, Pan Y-H, Wu Y-F, Teng N-C, Watanabe I, Sun Y-S, Chang W-J. Preparation of Calcium Phosphate Compounds on Zirconia Surfaces for Dental Implant Applications. International Journal of Molecular Sciences. 2022; 23(12):6675. https://doi.org/10.3390/ijms23126675
Chicago/Turabian StyleCheng, Mei-Shuan, Eisner Salamanca, Jerry Chin-Yi Lin, Yu-Hwa Pan, Yi-Fan Wu, Nai-Chia Teng, Ikki Watanabe, Ying-Sui Sun, and Wei-Jen Chang. 2022. "Preparation of Calcium Phosphate Compounds on Zirconia Surfaces for Dental Implant Applications" International Journal of Molecular Sciences 23, no. 12: 6675. https://doi.org/10.3390/ijms23126675





