Behavior of Ni20Cr Alloy in Molten Nitrate Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Corrosive Medium
2.3. Corrosion Tests
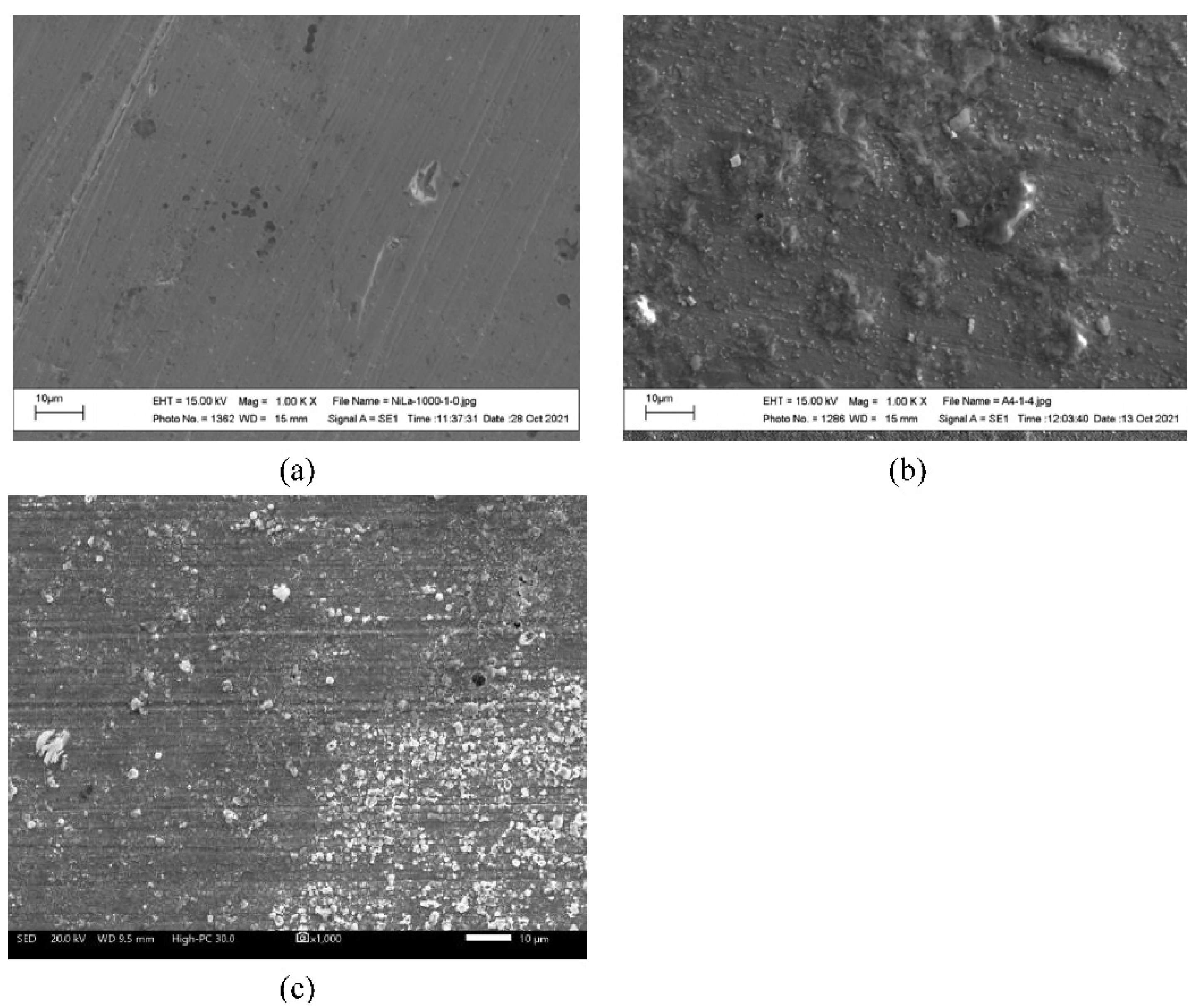
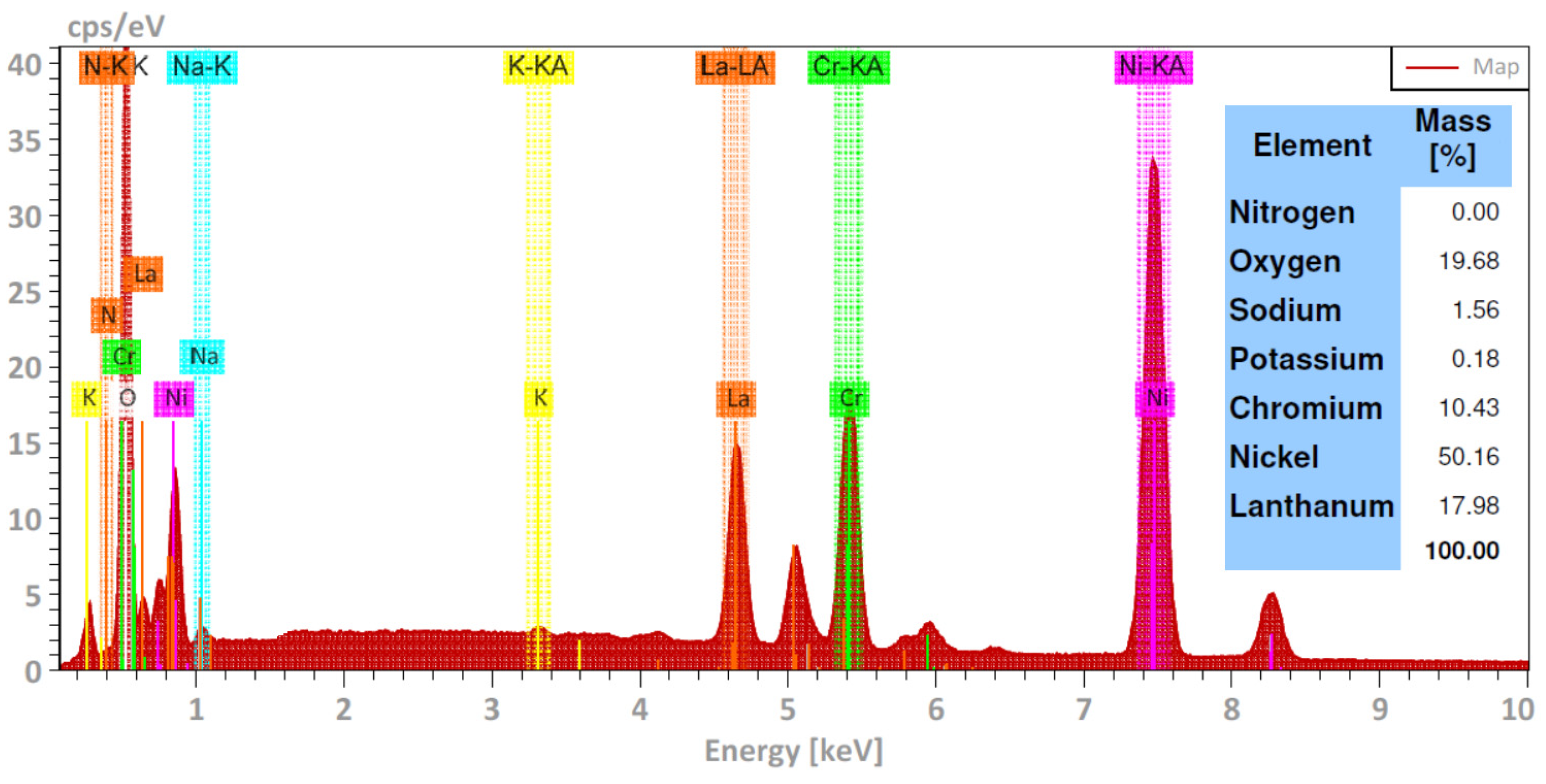
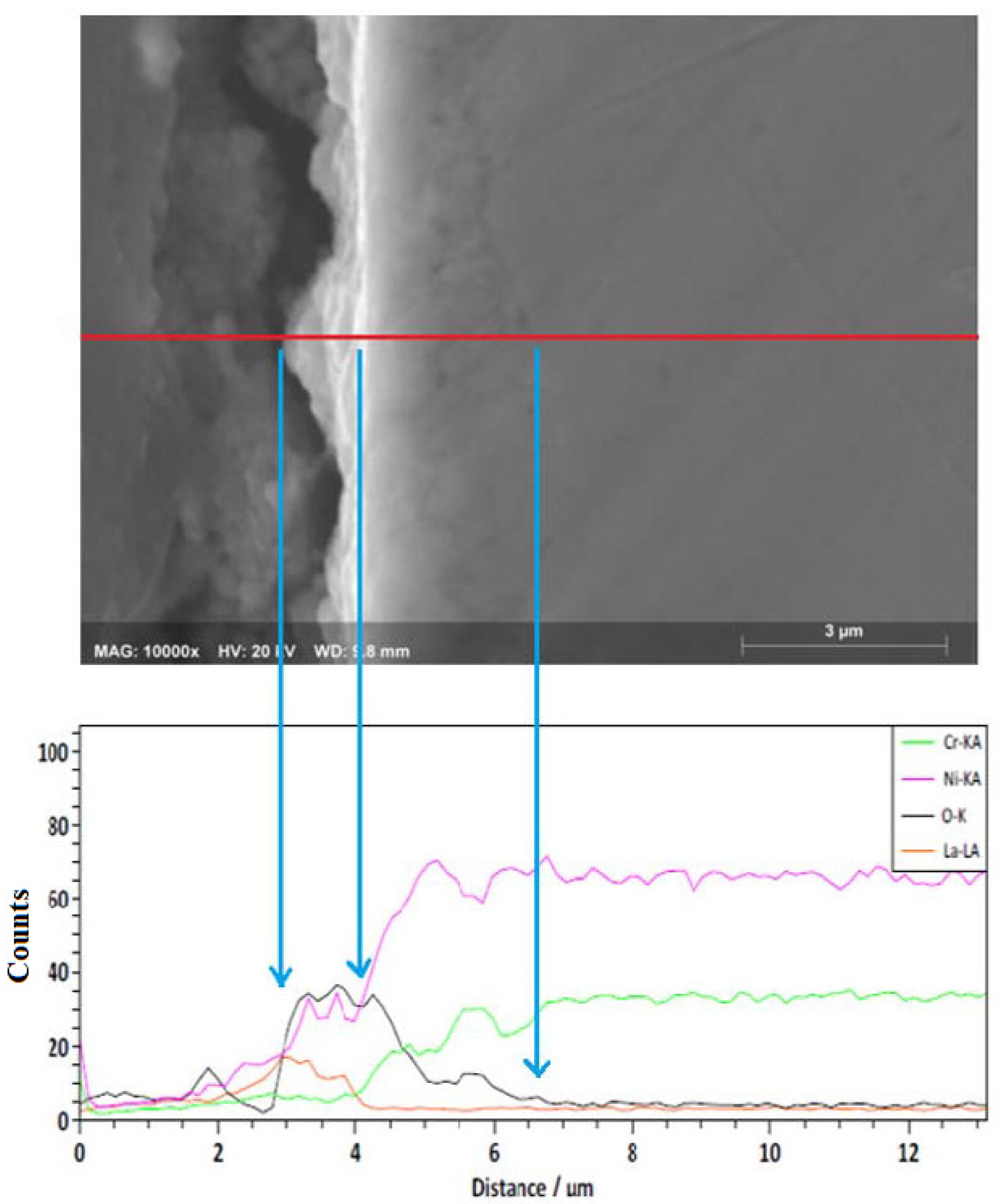
2.4. Complementary Analysis
3. Results and Discussion
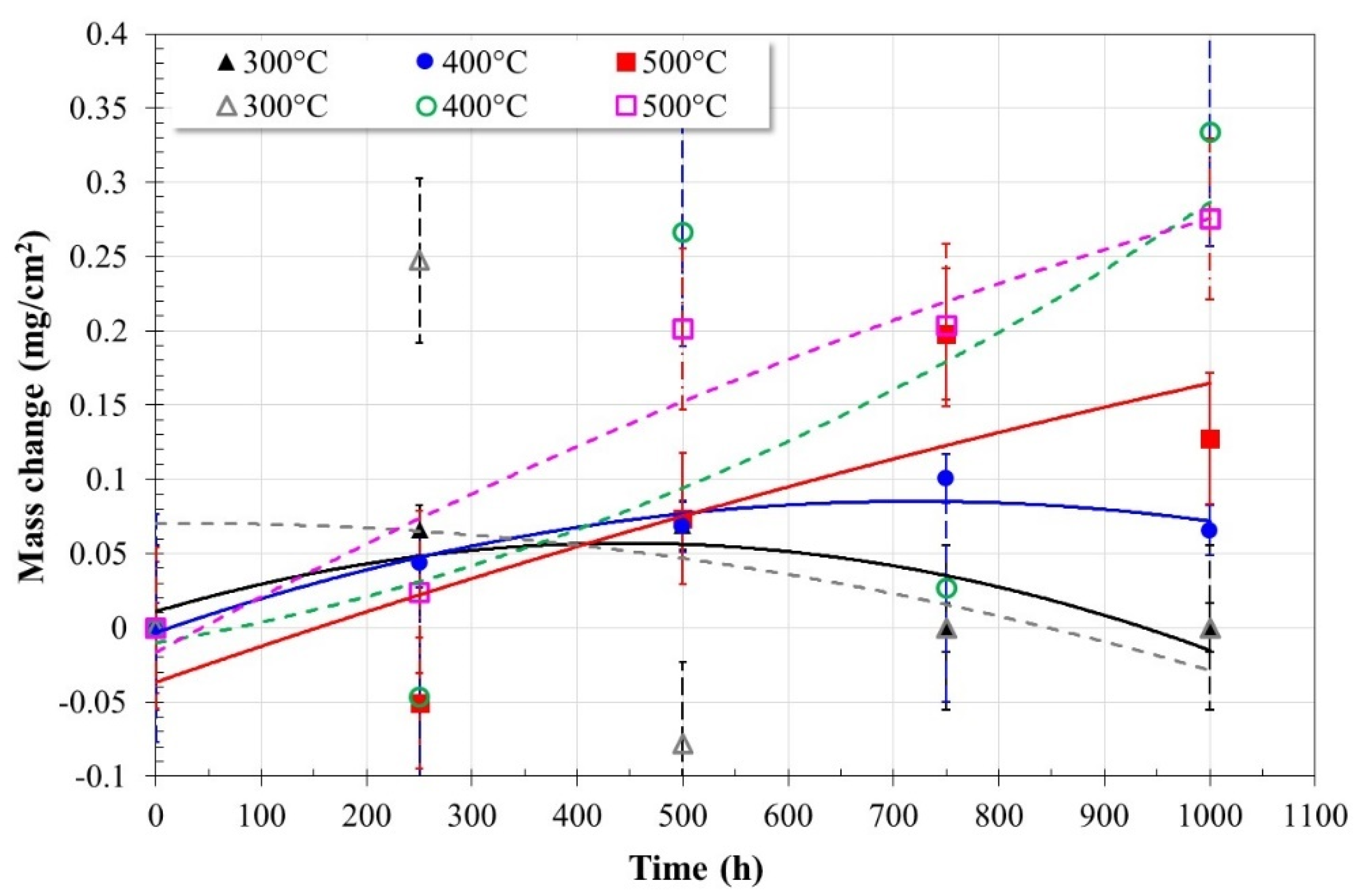
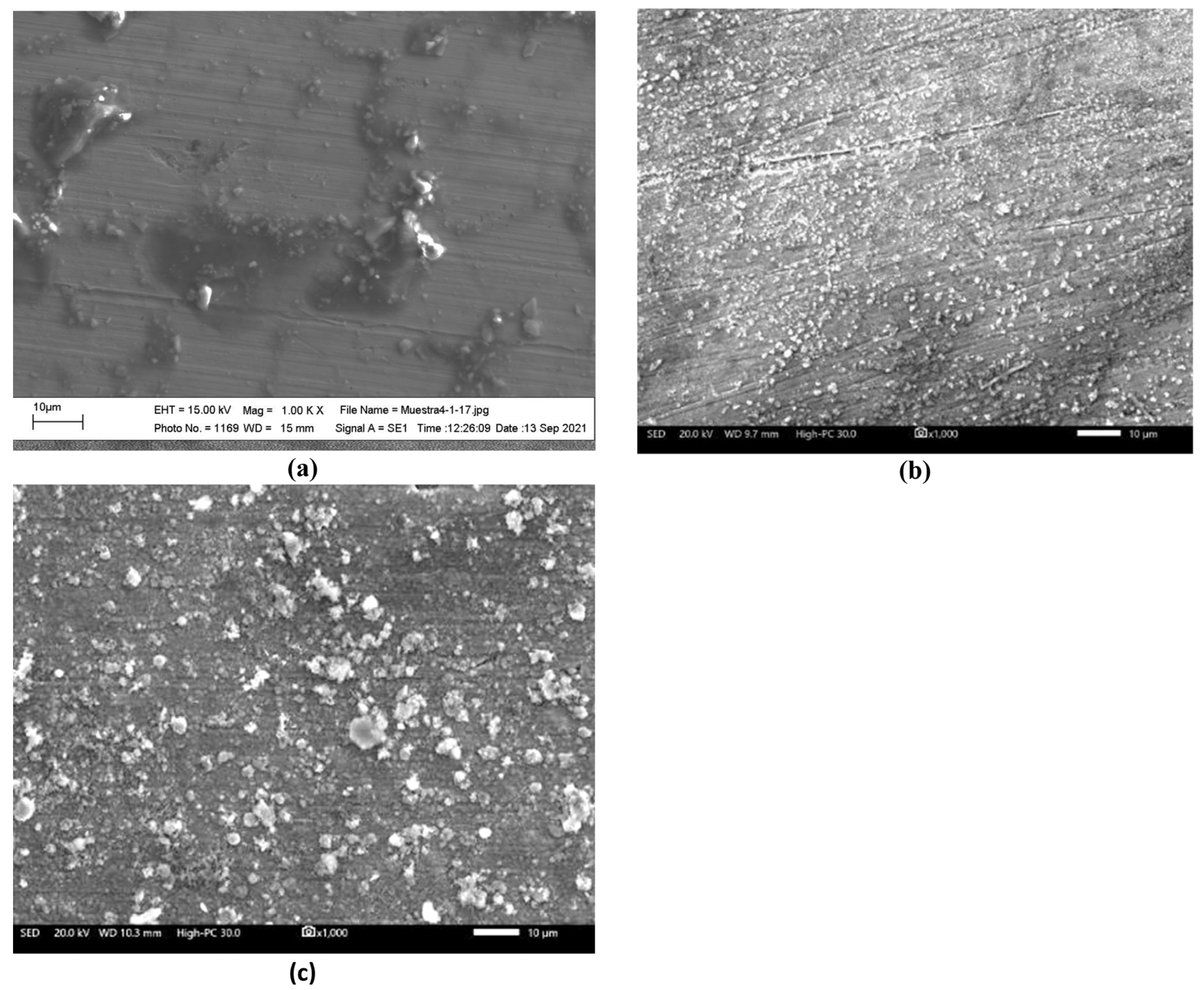
3.1. Corrosion Tests
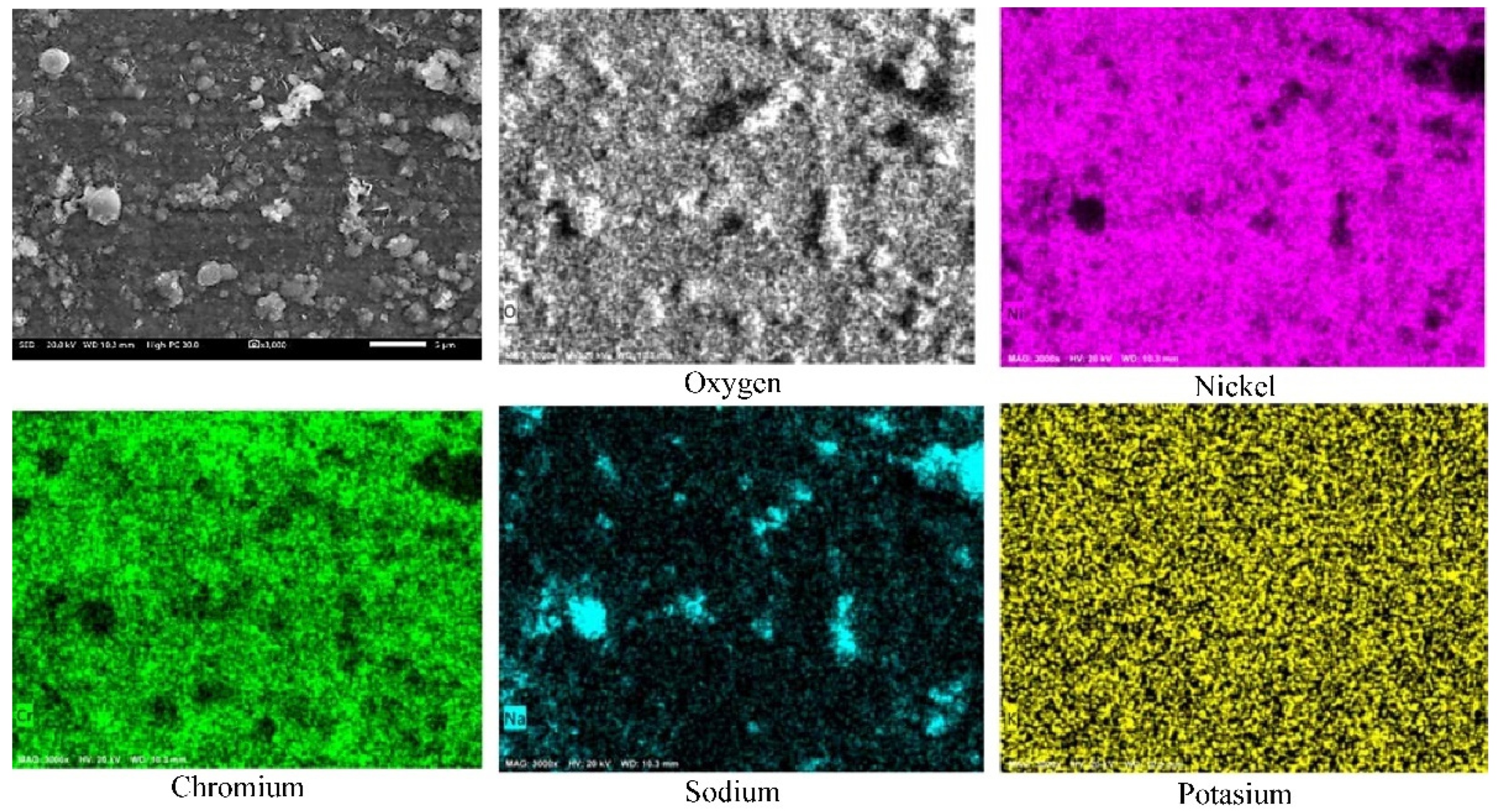
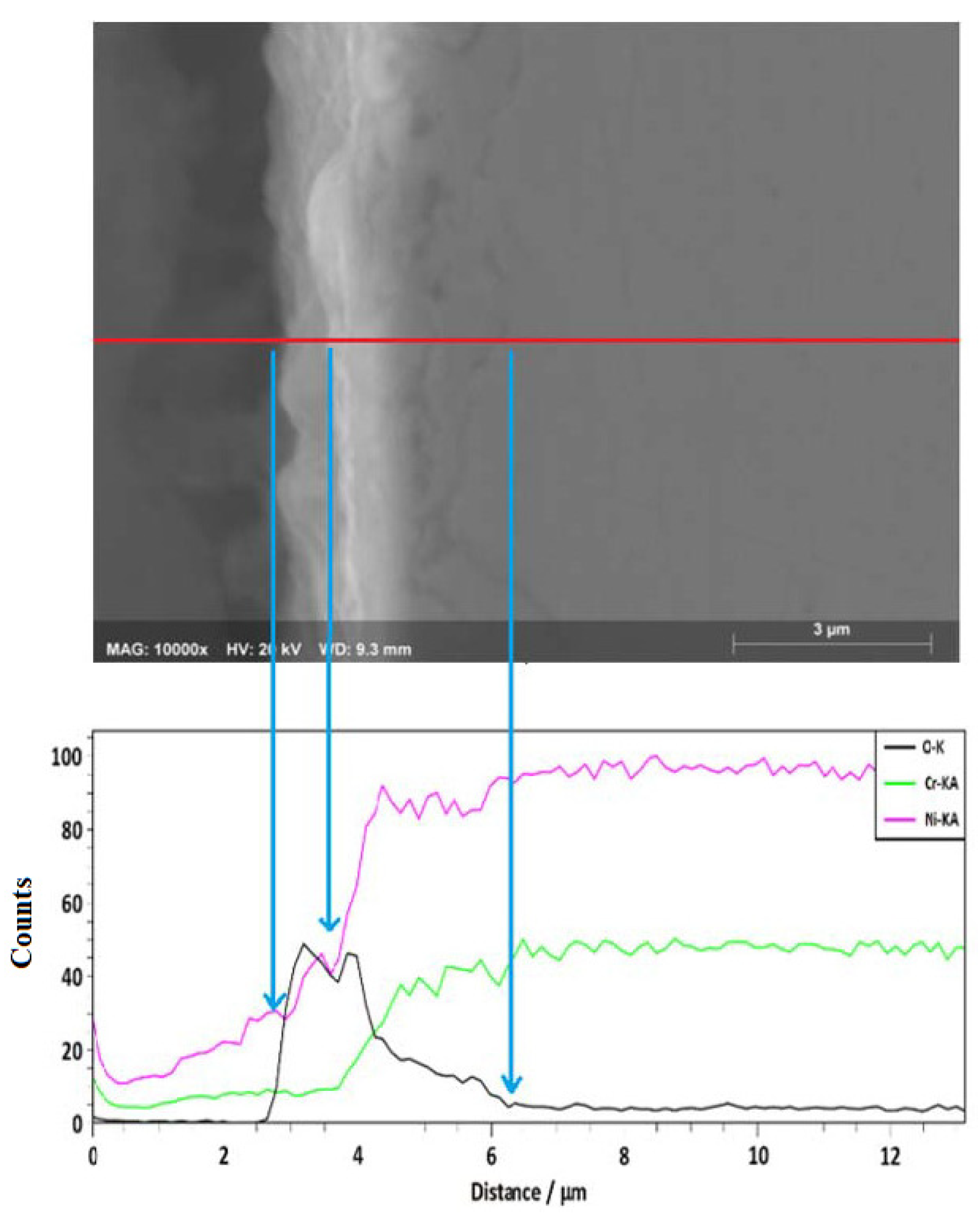
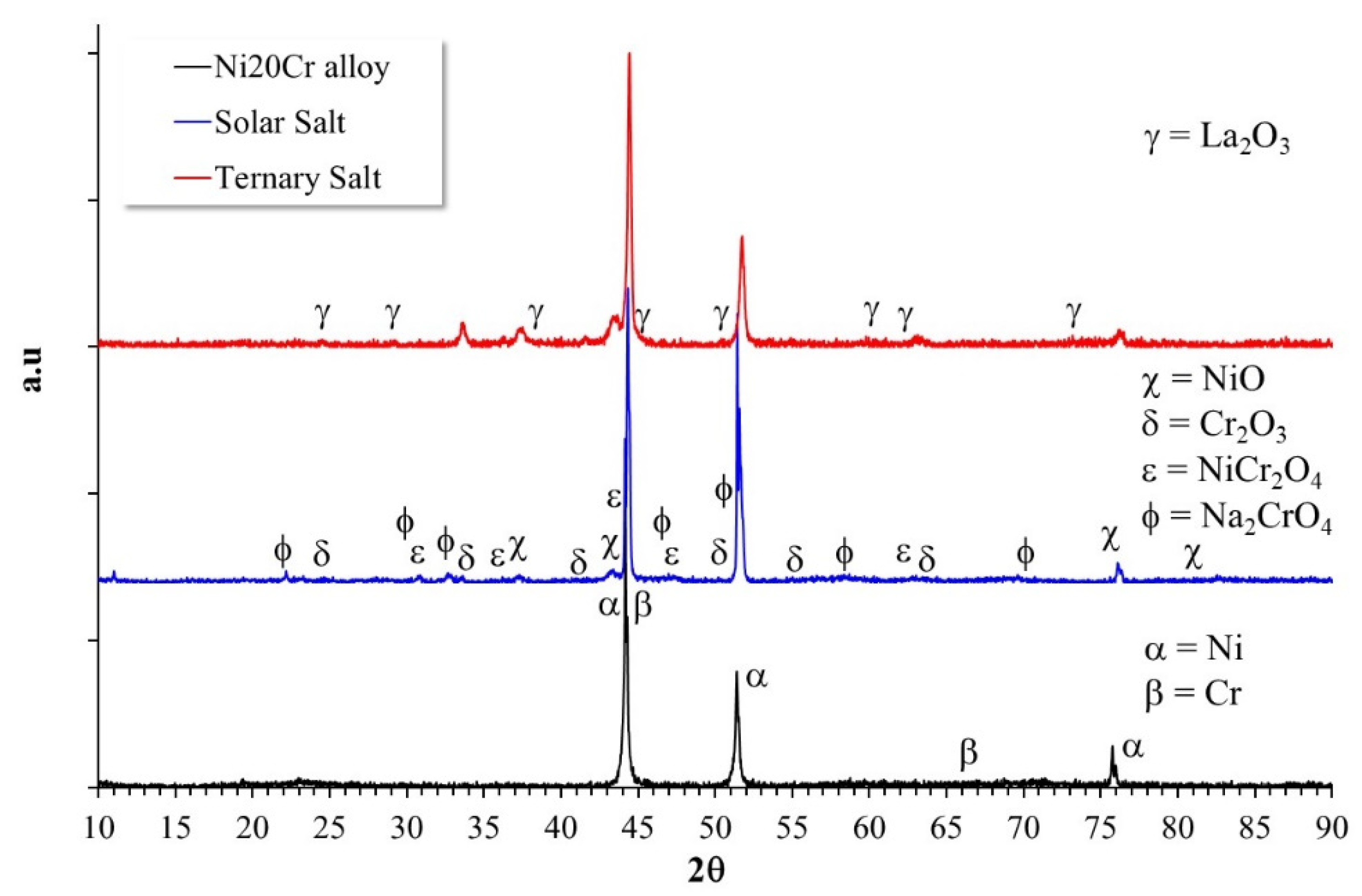
3.2. Reaction Mechanisms
4. Conclusions
- (1)
- The addition of La(NO3)3 into Solar Salt, at the concentration evaluated here, decreased its thermal stability and increased its corrosivity.
- (2)
- The observed changes showed a decrease of 13.51 °C in the melting point, and a decrease of 100 °C in the beginning of the decomposition point of Solar Salt.
- (3)
- These changes caused an increase in the concentration of oxidizing species, fluidity of the ternary salt, as well as its basicity.
- (4)
- The presence of the (La3+) formed a protective layer on the surface of the alloy.
- (5)
- The Ni20Cr alloy, immersed in the Solar Salt, developed protective corrosion products such as Cr and Ni oxides (Cr2O3, NiO) and Ni chromate (NiCr2O4), and when immersed in the ternary salt, the additional presence of a La-based protective layer (La2O3) was detected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Encinas-Sánchez, V.; de Miguel, M.T.; Lasanta, M.I.; García-Martín, G.; Pérez, F.J. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol. Energy Mater. Sol. Cells 2019, 191, 157–163. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Wu, Y.; Lu, Y. Comparative review of different influence factors on molten salt corrosion characteristics for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 235, 111485. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Wang, K.; Molina, E.E.; Li, P.; Gervasio, D.; Kannan, A.M. Vapor pressure and corrosivity of ternary metal-chloride molten-salt based heat transfer fluids for use in concentrating solar power systems. Appl. Energy 2015, 159, 206–221. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Zhao, Y.; Esmaily, M.; Allanore, A.; Vidal, J.; Birbilis, N. High-temperature corrosion of a nickel-based alloy in a molten chloride environment—The effect of thermal and chemical purifications. Sol. Energy Mater. Sol. Cells 2022, 236, 111542. [Google Scholar] [CrossRef]
- Fernández, A.G.; Cabeza, L.F. Corrosion monitoring and mitigation techniques on advanced thermal energy storage materials for CSP plants. Sol. Energy Mater. Sol. Cells 2019, 192, 179–187. [Google Scholar] [CrossRef]
- Sutter, F.; Oskay, C.; Galetz, M.C.; Diamantino, T.; Pedrosa, F.; Figueira, I.; Glumm, S.; Bonk, A.; Agüero, A.; Rodríguez, S.; et al. Dynamic corrosion testing of metals in solar salt for concentrated solar power. Sol. Energy Mater. Sol. Cells 2021, 232, 111331. [Google Scholar] [CrossRef]
- Fernandez, G.; Rey, A.; Lasanta, I.; Mato, S.; Brady, M.; Perez, F.J. Corrosion of alumina-forming austenitic steel in molten nitrate salts by gravimetric analysis and impedance spectroscopy. Mater. Corros. 2014, 65, 267–275. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Chen, D.-J.; Wang, C.-J. High-temperature corrosion of Cr–Mo steel in molten LiNO3-NaNO3-KNO3 eutectic salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2015, 132, 563–569. [Google Scholar] [CrossRef]
- Ignatiev, V.; Surenkov, A.; Gnidoy, I. Intergranular tellurium cracking of nickel-based alloys in molten salt mixture. J. Nucl. Mater. 2013, 440, 243–249. [Google Scholar] [CrossRef]
- Ahn, S.H.; Oh, K.N.; Kim, M.J.; Youn, J.Y.; Jo, K.H.; Kim, K.M.; Kwon, H.S. Electrochemical analysis on the growth of oxide formed on stainless steels in molten carbonate at 650 °C. Hydrog. Energy 2014, 39, 12291–12299. [Google Scholar] [CrossRef]
- Slusser, J.W.; Titcomb, J.B.; Heffelfinger, M.T. Corrosion in molten nitrate-nitrite salts. J. Met. 1985, 37, 24–27. [Google Scholar] [CrossRef]
- Meißner, T.M.; Oskay, C.; Bonk, A.; Gregoire, B.; Donchev, A.; Solimani, A.; Galetz, M.C. Improving the corrosion resistance of ferritic-martensitic steels at 600 °C in molten solar salt via diffusion coatings. Sol. Energy Mater. Sol. Cells 2021, 227, 111105. [Google Scholar] [CrossRef]
- Calvarin, G.; Molins, R.; Huntz, A.M. Oxidation Mechanism of Ni–20Cr Foils and Its Relation to the Oxide-Scale Microstructure. Oxid. Met. 2000, 53, 25–48. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Sotelo-Mazon, O.; Salinas-Bravo, V.M.; Arrieta-Gonzalez, C.D.; Ramos-Hernandez, J.J.; Cuevas-Arteaga, C. Electrochemical Performance of Ni20Cr Coatings Applied by Combustion Powder Spray in ZnCl2-KCl Molten Salts. Int. J. Electrochem. Sci. 2012, 7, 1134–1148. [Google Scholar]
- Porcayo-Calderon, J.; Sotelo-Mazon, O.; Casales-Diaz, M.; Ascencio-Gutierrez, J.A.; Salinas-Bravo, V.M.; Martinez-Gomez, L. Electrochemical Study of Ni20Cr Coatings Applied by HVOF Process in ZnCl2-KCl at High Temperatures. J. Anal. Methods Chem. 2014, 2014, 503618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Leon, P.D.; Sotelo-Mazon, O.; Salinas-Solano, G.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Valdez, S.; Martinez-Gomez, L. Hot corrosion behavior of Ni20Cr alloy in NaVO3 molten salt. J. Mater. Eng. Perform. 2019, 28, 5047–5062. [Google Scholar] [CrossRef]
- Kruizenga, A.; Gill, D. Corrosion of iron stainless steels in molten nitrate salt. Energy Procedia 2014, 49, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Petrovic, S.; Bundaleski, N.; Radovic, M.; Ristic, Z.; Gligoric, G.; Perusko, D.; Zec, S. Structure and Surface Composition of NiCr Sputtered Thin Films. Sci. Sinter. 2006, 38, 155–160. [Google Scholar] [CrossRef]
- Gou, T.; Xu, G.; Cheng, Y.; Jiang, Y.; Tan, S. Effect of Ni20Cr alloy on infrared emissivity of inorganic silicate heat-resistant composite coating. Surf. Coat. Technol. 2016, 288, 46–51. [Google Scholar] [CrossRef]
- Mohamed, L.Z.; El Kady, O.A.; Lotfy, M.M.; Ahmed, H.A.; Elrefaie, F.A. Characteristics of Ni-Cr Binary Alloys Produced by Conventional Powder Metallurgy. Key Eng. Mater. 2020, 835, 214–222. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rapp, R.A. The solubilities of NiO, Co3O4, and ternary oxides in fused Na2SO4 at 1200 K. J. Electrochem. Soc. 1980, 127, 2194–2202. [Google Scholar] [CrossRef]
- Zhang, Y.S. Solubilities of Cr2O3 in fused Na2SO4 at 1200 K. J. Electrochem. Soc. 1986, 133, 655–657. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Rapp, R.A. Solubilities of CeO2, HfO2 and Y2O3 in Fused Na2SO4-30 mol% NaVO3 and CeO2 in Pure Na2SO4 at 900 °C. Corrosion 1987, 43, 348–352. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Rapp, R.A. Thermochemistry and Solubilities of Oxides in Sodium Sulfate-Vanadate Solutions. Corrosion 1989, 45, 933–937. [Google Scholar] [CrossRef]
- Rapp, R.A. Hot corrosion of materials: A fluxing mechanism? Corros. Sci. 2002, 44, 209–221. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Rodriguez-Diaz, R.A.; Martinez-Gomez, L. Effect of the NaVO3-V2O5 ratio on the high temperature corrosion of chromium. Int. J. Electrochem. Sci. 2015, 10, 4928–4945. [Google Scholar]
- Porcayo-Calderon, J.; Ramos-Hernandez, J.J.; Mayén, J.; Porcayo-Palafox, E.; Pedraza-Basulto, G.K.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. High Temperature Corrosion of Nickel in NaVO3-V2O5 Melts. Adv. Mater. Sci. Eng. 2017, 2017, 8929873. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.; Steinberg, T.; Will, G. Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power. Renew. Sustain. Energy Rev. 2019, 114, 109328. [Google Scholar] [CrossRef]
- Bonk, A.; Rückle, D.; Kaesche, S.; Braun, M.; Bauer, T. Impact of Solar Salt aging on corrosion martensitic and austenitic steel for concentrating solar power plants. Sol. Energy Mater. Sol. Cells 2019, 203, 110160. [Google Scholar] [CrossRef]
- Yang, C.; Wei, X.; Wang, W.; Lin, Z.; Ding, J.; Wang, Y.; Peng, Q.; Yang, J. NOx emissions and the component changes of ternary molten nitrate salts in thermal energy storage process. Appl. Energy 2016, 184, 346–352. [Google Scholar] [CrossRef]
- Wei, X.; Quin, B.; Yang, C.; Wang, W.; Ding, J.; Wang, Y.; Peng, Q. Nox emission of ternary nitrate molten salts in high-temperature heat storage and transfer process. Appl. Energy 2019, 26, 147–154. [Google Scholar] [CrossRef]
- Kerridge, D.H.; Tariq, S.A. Molten sodium nitrite-potassium nitrite eutectic: The reactions of some compounds of chromium. Inorg. Chim. Acta 1969, 3, 667–670. [Google Scholar] [CrossRef]
- Kim, W.-B.; Choi, W.-S.; Lim, K.-S.; Cho, S.-H.; Lee, J.-H. High-Temperature Corrosion Behavior of Al-Coated Ni-Base Alloys in Lithium Molten Salt for Electroreduction. Coatings 2021, 11, 328. [Google Scholar] [CrossRef]
- Pettersson, C.; Pettersson, J.; Asteman, H. KCl-induced high temperature corrosion of the austenitic Fe-Cr-Ni alloys 304L and Sanicro 28 at 600 °C. Corros. Sci. 2016, 48, 1368–1378. [Google Scholar] [CrossRef]
- Stern, K.H. High temperature properties and decomposition of inorganic salts Part 3. Nitrates and nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, P.; Fereres, S. Effect of heating rates and composition on the termal decomposition of nitrate based molten salts. Energy Procedia 2015, 69, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Villada, C.; Bonk, A.; Bauer, T.; Bolívar, F. High-temperature stability of nitrate/nitrite molten salt mixtures under different atmospheres. Appl. Energy 2018, 226, 107–115. [Google Scholar] [CrossRef]


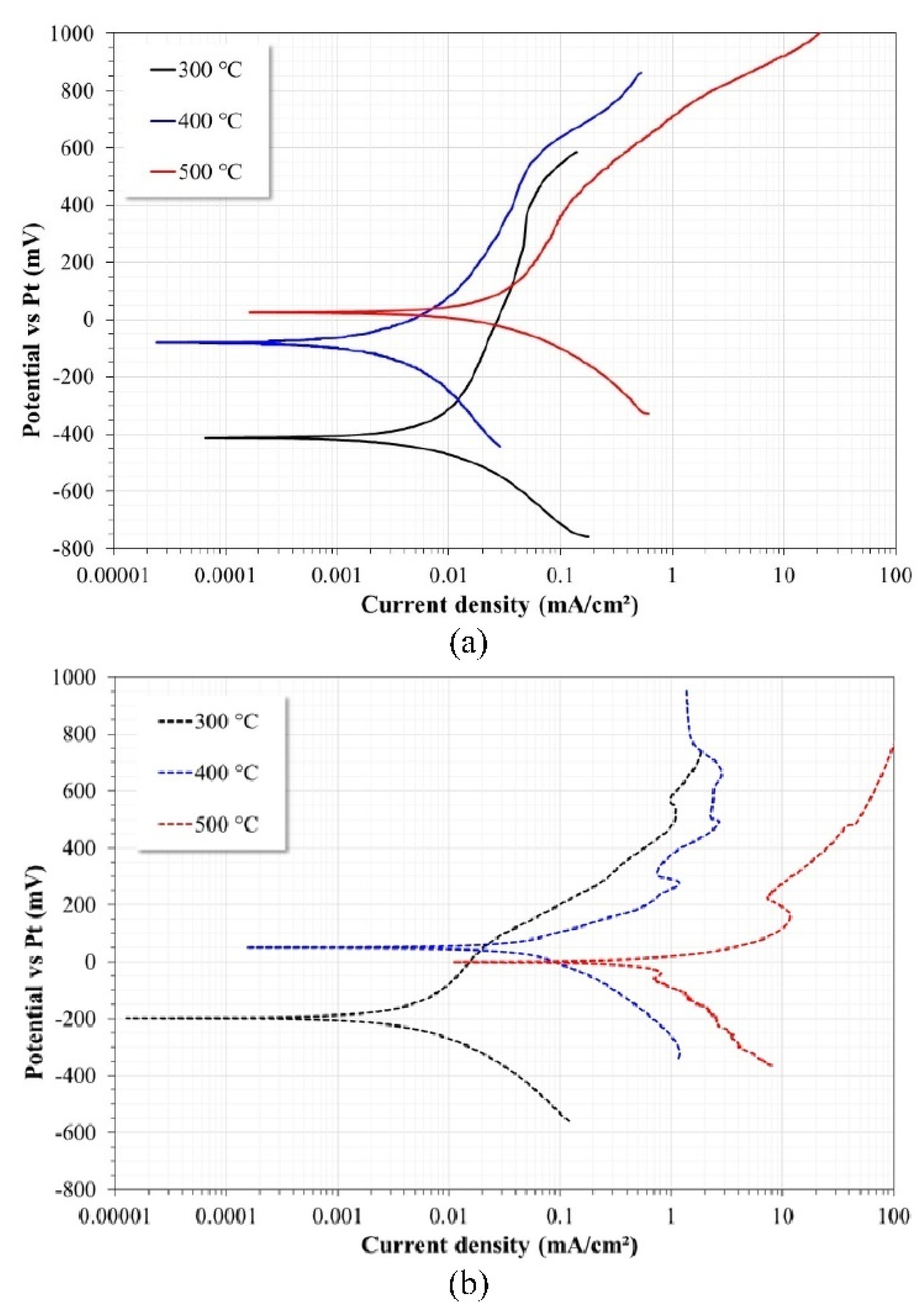

| Temperature | Ecorr | Icorr | βa | βc | CR | Rp |
|---|---|---|---|---|---|---|
| (mV) | (μA/cm2) | (mV/Decade) | (mV/Decade) | (mm/Year) | (Ohm-cm2) | |
| 300 °C | −414 ± 7 | 9.50 ± 1 | 897 ± 15 | −272 ± 15 | 0.093 ± 0.005 | 7300 ± 100 |
| 400 °C | −80 ± 6 | 3.60 ± 2 | 405 ± 10 | −392 ± 8 | 0.035 ± 0.006 | 17,000 ± 150 |
| 500 °C | 23 ± 3 | 30.3 ± 3 | 626 ± 7 | −236 ± 10 | 0.298 ± 0.010 | 1870 ± 50 |
| Temperature | Ecorr | Icorr | βa | βc | CR | Rp |
|---|---|---|---|---|---|---|
| (mV) | (μA/cm2) | (mV/Decade) | (mV/Decade) | (mm/Year) | (Ohm-cm2) | |
| 300 °C | −195 ± 6 | 4.80 ± 0.5 | 380 ± 15 | −211 ± 10 | 0.048 ± 0.003 | 9500 ± 200 |
| 400 °C | 49 ± 3 | 75.60 ± 3 | 151 ± 7 | −249 ± 15 | 0.745 ± 0.05 | 500 ± 50 |
| 500 °C | −3 ± 2 | 672.60 ± 10 | 92 ± 6 | −359 ± 20 | 6.626 ± 0.50 | 30 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Guzman, N.B.; Lopez-Dominguez, D.; Arrieta-Gonzalez, C.D.; Mayen, J.; Porcayo-Palafox, E.; Chacon-Nava, J.G.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Rodriguez-Diaz, R.A. Behavior of Ni20Cr Alloy in Molten Nitrate Salts. Int. J. Mol. Sci. 2022, 23, 7895. https://doi.org/10.3390/ijms23147895
Gomez-Guzman NB, Lopez-Dominguez D, Arrieta-Gonzalez CD, Mayen J, Porcayo-Palafox E, Chacon-Nava JG, Gonzalez-Rodriguez JG, Porcayo-Calderon J, Rodriguez-Diaz RA. Behavior of Ni20Cr Alloy in Molten Nitrate Salts. International Journal of Molecular Sciences. 2022; 23(14):7895. https://doi.org/10.3390/ijms23147895
Chicago/Turabian StyleGomez-Guzman, Nestor Belisario, Daniel Lopez-Dominguez, Cinthya Dinorah Arrieta-Gonzalez, Jan Mayen, Eduardo Porcayo-Palafox, Jose Guadalupe Chacon-Nava, Jose Gonzalo Gonzalez-Rodriguez, Jesus Porcayo-Calderon, and Roberto Ademar Rodriguez-Diaz. 2022. "Behavior of Ni20Cr Alloy in Molten Nitrate Salts" International Journal of Molecular Sciences 23, no. 14: 7895. https://doi.org/10.3390/ijms23147895









