Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review
Abstract
:1. Introduction
2. Synthesis and Properties of Biomedical Polyurethanes Used in Anti-Cancer Drug Delivery Systems
3. Polyurethane Anti-Cancer Drug Delivery Systems
4. Anti-Cancer Drug Delivery Systems Obtained from Biodegradable Polyurethanes
5. Anti-Cancer Drug Delivery Systems Obtained from Non-Biodegradable Polyurethanes
6. Anti-Cancer Polyurethane Prodrug
7. Conclusions, Challenges, and Prospects
- -
- some of the PUs (mainly based on aromatic isocyanates), products of their degradation, used solvents, etc., may exhibit toxic, irritating, and allergenic properties.
- -
- PU nano- and microcarriers have an active and large surface and can “negatively” interact with biomolecules.
- -
- The immune system may incorrectly recognize PU-DDSs.
- -
- Nano-PU-DDSs have the size of some proteins and can interfere with the transmission of information between cells.
- -
- A small number of developed PU-DDSs are characterized by a fully controlled release of the anti-cancer drug.
- -
- In many cases, the occurrence of the phenomenon of the drug’s burst release is observed.
- -
- Some methods of obtaining PU-DDSs are multi-stage and complex.
- -
- The cost of raw materials and technologies for obtaining PU-DDSs is, in many cases, high.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APUs | amphiphilic aliphatic polyurethanes |
| BDI | tetramethylene diisocyanate |
| BDO | 1,4-butanediol |
| BioPUs | biomedical PUs |
| CL | ε-caprolactone |
| CYCLOPHO | cyclophosphamide |
| CS | chitosan |
| CYS | Cystaminedihydrochloride |
| CDI | bis(2-isocyanatoethyl) disulfide |
| DABCO | 1,4-diazabicyclo-[2.2.2]-octane |
| 1,4-DAB | 1,4-diaminobutane |
| 1,2-DAE | 1,2-diaminoethane |
| 1,6-DAH | 1,6-diaminohexane |
| 1,8-DAO | 1,8-diaminooctane |
| DBTDL | dibutyltin dilaurate |
| DBTDO | dibutyltin dioctanate |
| DEG | diethylene glycol |
| DDS/DDSs | drug delivery system(s) |
| DMPA | 2,2-bis(hydroxymethyl)propionic acid |
| DOX | doxorubicin |
| EG | ethylene glycol |
| ECG | epigallocatechin gallate |
| FA | folic acid |
| 5-FU | 5-fluorouracil |
| GG | glycolide |
| GEF | gefitinib |
| GSH | glutathione |
| HDI | hexamethylene diisocyanate |
| HMDI | dicyclohexylmethane 4,4′-diisocyanate |
| HEP | bis-1,4-(hydroxyethyl) piperazine |
| HPEG | hydrazone-ended methoxyl-poly(ethylene glycol) |
| HPCL | hydrazone-embedded poly(ε-caprolactone) diol |
| IC/ICs | diisocyanate/diisocyanates |
| IPDI | isophorone diisocyanate |
| LA | lactide |
| LCST | lower critical solution temperature |
| LDI | L-Lysine diisocyanate ethyl ester |
| L-LYS | L-lysine |
| L-LYS-GQA | L-lysine-derivatized gemini quaternary ammonium salts with two primary amine groups |
| L-LYS-ABA-ABA tripeptide | L-lysine-γ-aminobutyric acid-γ-aminobutyric acid tripeptide |
| mPEG | methoxyl-poly(ethylene glycol) |
| MDEA | N-methyl-diethanolamine |
| MDI | 4,4′-methylenebis(phenyl isocyanate) |
| EG | ethylene glycol |
| METX | Methotrexate |
| bis-MPA | 2,2-bis(hydroxymethyl)-propionic acid |
| NP | nanoparticles |
| O-DTT | trans-4,5-dihydroxy-1,2-dithiane |
| OEDA | dihydroxy(polyethylene adipate) |
| PACL | paclitaxel |
| PBS | phosphate buffered saline |
| PCL | poly(ε-caprolactone) |
| PEG-PU(SS)-PEG | polyurethane with disulfide bonds and PEG fragments |
| PEOtz-OH | poly(2-ethyl-2-oxazoline) |
| PHBHx | poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| PLGA | copolymer of lactide and glycolide |
| PDO | 1,3-propanediol |
| PEG | poly(ethylene glycol) |
| PLA | polylactide |
| PLACL | copolymer of lactide and ε-caprolactone |
| PLA-SS-PLA | PLA with disulfide bonds |
| PNIPAAm-g-chitosan | poly(N-isopropylacrylamide)-grafted-chitosan |
| POEUs | poly(ortho ester urethanes) |
| POx | poly(2-oxazoline)s |
| PPG | poly(propylene glycol) |
| PPG-N3 | azide-grafted PEG |
| PPEG | Propargyl-grafted PEG |
| PPS | poly(1,3-propylene succinate) diols |
| PTMC | poly(trimethylene carbonate) |
| PTMC-SS-PTMC | PTMC with disulfide bonds |
| PTMG | poly(tetramethylene ether) glycol |
| PUs | polyurethanes |
| PU-DDSs | polyurethane drug delivery systems |
| PU-Prodrugs | polyurethane prodrugs |
| PU-SS-COOH | polyurethane obtained from PEG-1000, PCL-2000, HDI, CYS and DMPA |
| PU-SS-COOH-NH2 | product of condensation reaction between the PU-SS-COOH and 1,6-diaminohexane |
| ROP | ring-opening polymerization |
| SnOct2 | tin(II) 2-ethylhexanoate |
| SPION | superparamagnetic iron oxide nanoparticles |
| TDI | toluene 2,4-diisocyanate |
| TMC | trimethylene carbonate |
| TMZ | temozolomide |
| WPU | waterborne polyurethane dispersions |
References
- Shanthi, M. Global Status Report on Noncommunicable Diseases 2014; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Mulas, K.; Stefanowicz, Z.; Oledzka, E.; Sobczak, M. Current state of the polymeric delivery systems of fuoroquinolones—A review. J. Control. Release 2019, 294, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Kim, J.H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.K.; Ankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Cai, L.; Xu, J.; Yang, Z.; Tong, R.; Dong, Z.; Wang, C.; Leong, K.W. Engineered biomaterials for cancer immunotherapy. MedComm 2020, 1, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Osi, B.; Khoder, M.; Al-Kinani, A.A.; Alany, R.G. Pharmaceutical, biomedical and ophthalmic applications of biodegradable polymers (BDPs): Literature and patent review. Pharm. Dev. Technol. 2022, 3, 341–356. [Google Scholar] [CrossRef]
- Hajebi, S.; Nasr, S.M.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. 2021, 70, 926–940. [Google Scholar] [CrossRef]
- Sobczak, M. Biodegradable polyurethane elastomers for biomedical applications—Synthesis methods and properties. Polym. Plast. Technol. Eng. 2015, 54, 155–172. [Google Scholar] [CrossRef]
- Sobczak, M.; Dębek, C.; Goś, P. Preparation polyurethanes as potential biomaterials for short-term applications. e-Polymers 2010, 148. [Google Scholar]
- Ozimek, J.; Pielichowski, K. Recent Advances in Polyurethane/POSS Hybrids for Biomedical Applications. Molecules 2022, 27, 40. [Google Scholar] [CrossRef]
- Wendels, S.; Averous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Feldman, D. Polyurethane and Polyurethane Nanocomposites: Recent Contributions to Medicine. Biointerface Res. Appl. Chem. 2021, 11, 8179–8189. [Google Scholar]
- Li, Y.; Thouas, G.A.; Chen, Q.-Z. Biodegradable soft elastomers: Synthesis/properties of materials and fabrication of scaffolds. RSC Adv. 2012, 2, 8229–8242. [Google Scholar] [CrossRef]
- Taraghi, I.; Paszkiewicz, S.; Grebowicz, J.; Fereidoon, A.; Roslaniec, Z. Nanocomposites of Polymeric Biomaterials Containing Carbonate Groups: An Overview. Macromol. Mater. Eng. 2017, 302, 1700042. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Shukla, H.; Kalia, K.; Torchilin, V.P.; Tekade, R.K. Multifunctional polymeric micellar nanomedicine in the diagnosis and treatment of cancer. Mater. Sci. Eng. C 2021, 126, 112186. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Butt, M.T.Z.; Khan, S.M. Synthesis of Mono Ethylene Glycol (MEG)-Based Polyurethane and Effect of Chain Extender on Its Associated Properties. Polymers 2021, 13, 3436. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Hai, T.A.P.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable Polyurethanes from Sustainable Biological Precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar]
- Farzan, A.; Borandeh, S.; Zanjanizadeh Ezazi, N.; Lipponen, S.; Santos, H.A.; Seppälä, J. 3D scaffolding of fast photocurable polyurethane for soft tissue engineering by stereolithography: Influence of materials and geometry on growth of fibroblast cells. Eur. Polym. J. 2020, 139, 109988. [Google Scholar] [CrossRef]
- Aduba, D.C., Jr.; Zhang, K.; Kanitkar, A.; Sirrine, J.M.; Verbridge, S.S.; Long, T.E. Electrospinning of plant oil-based, non-isocyanate polyurethanes for biomedical applications. J. Appl. Polym. Sci. 2018, 135, 46464. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, V.; Craig, A. Retrospective: On Clinical Studies with 5-Fluorouracil. Cancer Res. 2016, 76, 767–768. [Google Scholar] [CrossRef] [Green Version]
- Muhsin, M.; Graham, J.; Kirkpatrick, P. Gefitinib. Nat. Rev. Cancer 2003, 3, 556–557. [Google Scholar] [CrossRef]
- Schweitzer, B.I.; Dicker, A.D.; Bertino, J.R. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990, 4, 2441–2452. [Google Scholar] [CrossRef]
- Newlands, E.; Stevens, M.; Wedge, S.; Wheelhouse, R.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A compressive review about Taxol®: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Sardon, H.; Tan, J.P.K.; Chan, J.M.W.; Mantione, D.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y. Thermoresponsive random poly(ether urethanes) with tailorable LCSTs for anticancer drug delivery. Macromol. Rapid Commun. 2015, 36, 1761–1767. [Google Scholar] [CrossRef]
- Niu, Y.; Stadler, F.J.; Song, J.; Chen, S.; Chen, S. Facile fabrication of polyurethane microcapsules carriers for tracing cellular internalization and intracellular pH-triggered drug release. Colloids Surf. B 2017, 153, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, C.; Qiao, Z.; Yao, Y.; Luo, J. Reduction responsive and surface charge switchable polyurethane micelles with acid cleavable crosslinks for intracellular drug delivery. RSC Adv. 2018, 8, 17888–17897. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Hu, J.; Bu, L.; Zhang, H.; Du, B.; Zhu, C.; Li, Y. Facile preparation of reduction-responsive micelles based on biodegradable amphiphilic polyurethane with disulfide bonds in the backbone. Polymers 2019, 11, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Luo, Z.; Loh, X.J.; Wu, Y.-L.; Li, Z. PHA-based thermogel as a controlled zero-order chemotherapeutic delivery system for the effective treatment of melanoma. ACS Appl. Bio Mater. 2019, 2, 3591–3600. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Q.; Cai, Y.; Zhu, X.; Zhu, C.; Li, Y.; Li, B. Poly(ethylene glycol)-sheddable reduction-sensitive polyurethane micelles for triggered intracellular drug delivery for osteosarcoma treatment. J. Orthop. Translat. 2020, 21, 57–65. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Bu, L.; Zhang, H.; Zhu, C.; Li, Y. Stimuli-responsive micelles with detachable poly(2-ethyl-2-oxazoline) shell based on amphiphilic polyurethane for improved intracellular delivery of doxorubicin. Polymers 2020, 12, 2642. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, Y.; Xiang, Y.; Shu, M.; Chen, H.; Yanga, B.; Liao, B.X. Polyurethane/doxorubicin nanoparticles based on electrostatic interactions as pH-sensitive drug delivery carriers. Polym. Int. 2018, 67, 1186–1193. [Google Scholar] [CrossRef]
- He, W.; Zheng, X.; Zhao, Q.; Duan, L.; Lv, Q.; Gao, G.H.; Yu, S. pH-triggered charge-reversal polyurethane micelles for controlled release of doxorubicin. Macromol. Biosci. 2016, 16, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wang, J.; Qi, Y.; Wen, J.; Wei, S.; Liu, D.; Yu, S. One pot preparation of polyurethane-based GSH-responsive core-shell nanogels for controlled drug delivery. J. Appl. Polym. Sci. 2020, 137, 48473. [Google Scholar] [CrossRef]
- Zhanga, H.; Liua, X.; Xua, K.; Dua, B.; Zhub, C.; Li, Y. Biodegradable polyurethane PMeOx-PU(SS)-PMeOx micelles with redox and pH-sensitivity for efficient delivery of doxorubicin. Eur. Polym. J. 2020, 140, 110054. [Google Scholar] [CrossRef]
- Liu, J.; Dai, Z.; Zhou, Y.; Tao, W.; Chen, H.; Zhao, Z.; Lia, X. Acid-sensitive charge-reversal co-assembled polyurethane nanomicelles as drug delivery carriers. Colloids Surf. B 2022, 209, 112203. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Zhu, Y.; Wang, G. Synthesis, self-assembly, biodegradation and drug delivery of polyurethane copolymers from bio-based poly(1,3-propylene succinate). React. Funct. Polym. 2019, 141, 9–20. [Google Scholar] [CrossRef]
- Saeedi, S.; Omrani, I.; Bafkary, R.; Sadeh, E.; Shendi, H.K.; Nabid, M.R. Facile preparation of biodegradable dual stimuli-responsive micelles from waterborne polyurethane for efficient intracellular drug delivery. New J. Chem. 2019, 43, 18534–18545. [Google Scholar] [CrossRef]
- Feng, Z.; Zhenga, Y.; Zhao, L.; Zhang, Z.; Suna, Y.; Qiao, K.; Xie, Y.; Wang, Y.; He, W. An ultrasound-controllable release system based on waterborne polyurethane/chitosan membrane for implantable enhanced anticancer therapy. Mater. Sci. Eng. C 2019, 104, 109944. [Google Scholar] [CrossRef]
- Wei, J.; Shuai, X.; Wang, R.; He, X.; Li, Y.; Ding, M.; Li, J.; Tan, H.; Fu, Q. Clickable and imageable multiblock polymer micelles with magnetically guided and PEG-switched targeting and release property for precise tumor theranosis. Biomaterials 2017, 145, 138–153. [Google Scholar] [CrossRef]
- Fu, S.; Yang, G.; Wang, J.; Wang, X.; Cheng, X.; Zha, Q.; Tang, R. pH-sensitive poly(ortho ester urethanes) copolymers with controlled degradation kinetic: Synthesis, characterization, and in vitro evaluation as drug carriers. Eur. Polym. J. 2017, 95, 275–288. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, R.; Meng, F.; Deng, C.; Zhong, Z. Micelles based on acid degradable poly(acetal urethane): Preparation, pH-Sensitivity, and triggered intracellular drug release. Biomacromolecules 2015, 16, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ding, J.; He, C.; Cao, Y.; Xu, W.; Chen, X. Disulfide cross-linked polyurethane micelles as a reduction-triggered drug delivery system for cancer therapy. Adv. Healthc. Mater. 2014, 3, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, C.; Lv, Q.; Sun, H.; Chen, X. pH and reduction dual responsive cross-linked polyurethane micelles as an intracellular drug delivery system. RSC Adv. 2014, 4, 63070–63078. [Google Scholar] [CrossRef]
- Pan, Z.; Yu, L.; Song, N.; Zhou, L.; Li, J.; Ding, M.; Tan, H.; Fu, Q. Synthesis and characterization of biodegradable polyurethanes with folate side chains conjugated to hard segments. Polym. Chem. 2014, 5, 2901–2910. [Google Scholar] [CrossRef]
- Alisani, R.; Rakhshani, N.; Abolhallaj, M.; Motevalli, F.; Abadi, P.G.; Akrami, M.; Shahrousvand, M.; Jazi, F.S.; Irani, M. Adsorption, and controlled release of doxorubicin from cellulose acetate/polyurethane/multi-walled carbon nanotubes composite nanofibers. Nanotechnology 2022, 33, 155102. [Google Scholar] [CrossRef]
- He, Q.; Yan, R.; Hou, W.; Wang, H.; Tian, Y. A pH-responsive zwitterionic polyurethane prodrug as drug delivery system for enhanced cancer therapy. Molecules 2021, 26, 5274. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, P. Dendritic polyurethane-based prodrug as unimolecular micelles for precise ultrasound-activated localized drug delivery. Mater. Today Chem. 2022, 24, 100819. [Google Scholar] [CrossRef]
- Yu, C.; Tan, X.; Xu, Z.; Zhu, G.; Teng, W.; Zhao, Q.; Liang, Z.; Wu, Z.; Xiong, D. Smart drug carrier based on polyurethane material for enhanced and controlled DOX release triggered by redox stimulus. React. Funct. Polym. 2020, 148, 104507. [Google Scholar] [CrossRef]
- Marin, A.; Marin, M.A.; Ene, I.; Poenaru, M. Polyurethane structures used as a drug carrier for epigallocatechin gallate. Mater. Plast. 2021, 58, 210–217. [Google Scholar] [CrossRef]
- Bahadur, A.; Shoaib, M.; Iqba, S.; Saeed, A.; Saif ur Rahman, M.; Channar, P.A. Regulating the anticancer drug release rate by controlling the composition of waterborne polyurethane. React. Funct. Polym. 2018, 131, 134–141. [Google Scholar] [CrossRef]
- Farboudi, A.; Nouri, A.; Shirinzad, S.; Sojoudi, P.; Davaran, S.; Akrami, M.; Irani, M. Synthesis of magnetic gold coated poly(ε-caprolactonediol) based polyurethane/poly(N-isopropylacrylamide)-grafted chitosan core-shell nanofibers for controlled release of paclitaxel and 5-FU. Int. J. Biol. Macromol. 2020, 150, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Federico, S.; Pitarresi, G.; Fiorica, C.; Giammona, G. Synthesis and characterization of redox-sensitive polyurethanes based on L-glutathione oxidized and poly(ether ester) triblock copolymers. React. Funct. Polym. 2021, 166, 104986. [Google Scholar] [CrossRef]
- Ding, M.; Song, N.; He, X.; Li, J.; Zhou, L.; Tan, H.; Fu, Q.; Gu, Q. Toward the next-generation nanomedicines: Design of multifunctional multiblock polyurethanes for effective cancer treatment. ACS Nano 2013, 7, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Su, Y.; Zhao, L.; Meng, F.; Wang, Q.; Yao, Y.; Luo, J. Biodegradable polyurethane micelles with pH and reduction responsive properties for intracellular drug delivery. Mater. Sci. Eng. C 2017, 75, 1221–1230. [Google Scholar] [CrossRef]
- Piras, A.M.; Sandreschi, S.; Malliappan, S.P.; Dash, M.; Bartoli, C.; Dinucci, D.; Guarna, F.; Ammannati, E.; Masa, M.; Múcková, M.; et al. Surface decorated poly(ester-ether-urethane)s nanoparticles: A versatile approach towards clinical translation. Int. J. Pharm. 2014, 475, 523–535. [Google Scholar] [CrossRef]
- Bazzazzadeha, A.; Dizajia, B.F.; Kianinejadb, N.; Nouric, A.; Irani, M. Fabrication of poly(acrylic acid) grafted-chitosan/polyurethane/magnetic MIL-53 metal organic framework composite core-shell nanofibers for codelivery of temozolomide and paclitaxel against glioblastoma cancer cells. Int. J. Pharm. 2020, 587, 119674. [Google Scholar] [CrossRef]
- Irani, M.; Sadeghi, G.M.M.; Haririan, I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int. J. Biol. Macromol. 2017, 97, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Saxena, S.; Jayakannan, M. Development of L-lysine based biodegradable polyurethanes and their dual-responsive amphiphilic nanocarriers for drug delivery to cancer cells. ACS Appl. Polym. Mater. 2019, 1, 1866–1880. [Google Scholar] [CrossRef]
- Chen, W.; di Carlo, C.; Devery, D.; McGrath, D.J.; McHugh, P.E.; Kleinsteinberg, K.; Jockenhoevel, S.; Hennink, W.E.; Kok, R.J. Fabrication and characterization of gefitinib-releasing polyurethane foam as a coating for drug-eluting stent in the treatment of bronchotracheal cancer. Int. J. Pharm. 2018, 548, 803–811. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, R.; Zhou, J.; Fan, H.; Shi, B. On-demand drug delivery from temperature-responsive polyurethane membrane. React. Funct. Polym. 2011, 71, 525–535. [Google Scholar] [CrossRef]
- Iskakov, R.; Batyrbekov, E.O.; Leonova, M.B.; Zhubanov, B.A. Preparation and release profiles of cyclophosphamide from segmented polyurethanes. J. Appl. Polym. Sci. 2000, 75, 35–43. [Google Scholar] [CrossRef]
- Liao, Z.-S.; Huang, S.-Y.; Huang, J.-J.; Chen, J.-K.; Lee, A.-W.; Lai, J.-Y.; Lee, D.-J.; Cheng, C.-C. Self-assembled pH-responsive polymeric micelles for highly efficient, noncytotoxic delivery of doxorubicin chemotherapy to inhibit macrophage activation: In vitro investigation. Biomacromolecules 2018, 19, 2772–2781. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Hajdaniak, M.; Gos, P.; Oledzka, E.; Kolodziejski, W.L. Use of aliphatic poly(amide urethane)s for the controlled release of 5-fluorouracil. Eur. J. Med. Chem. 2011, 46, 914–918. [Google Scholar] [CrossRef] [PubMed]

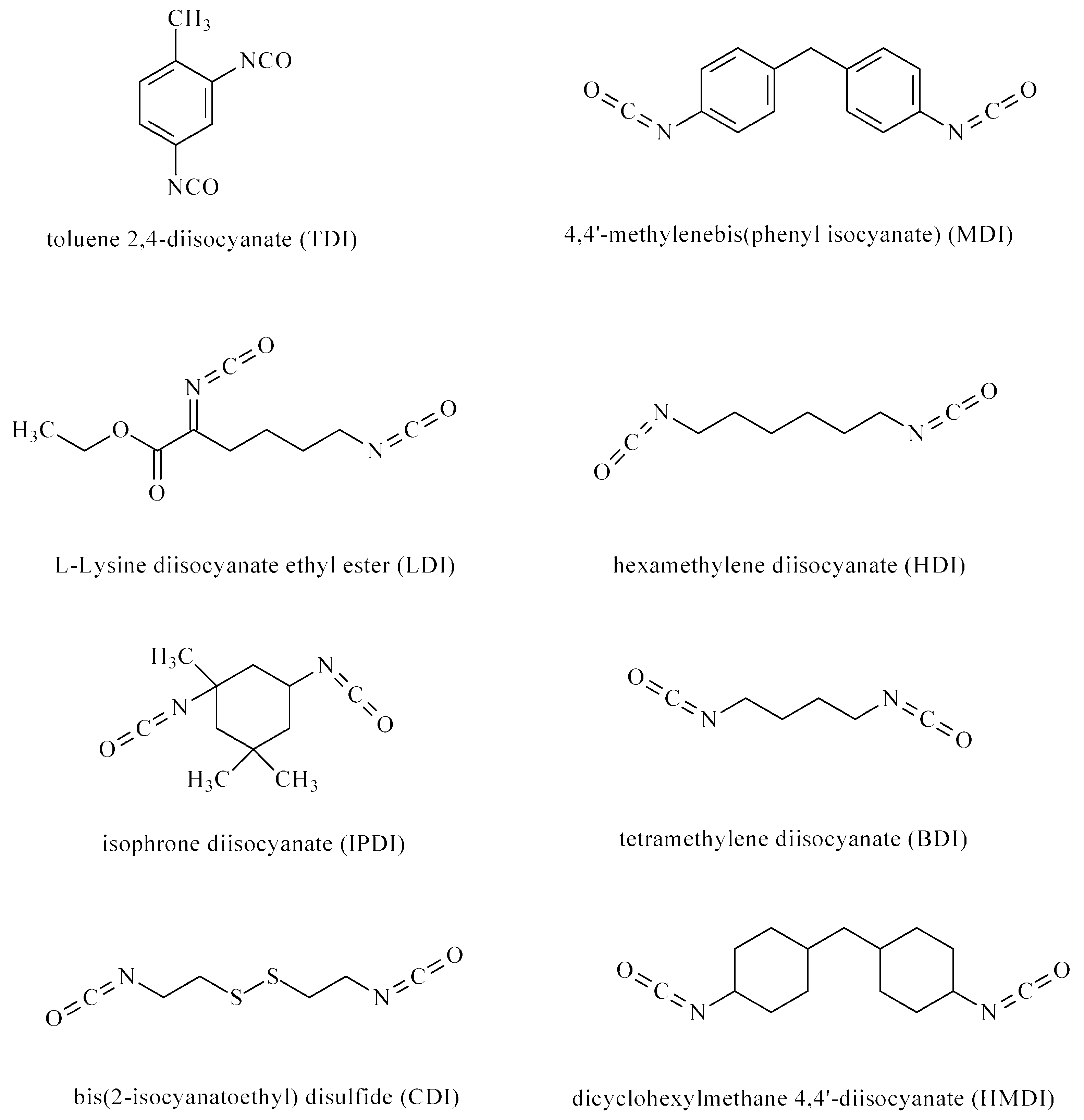
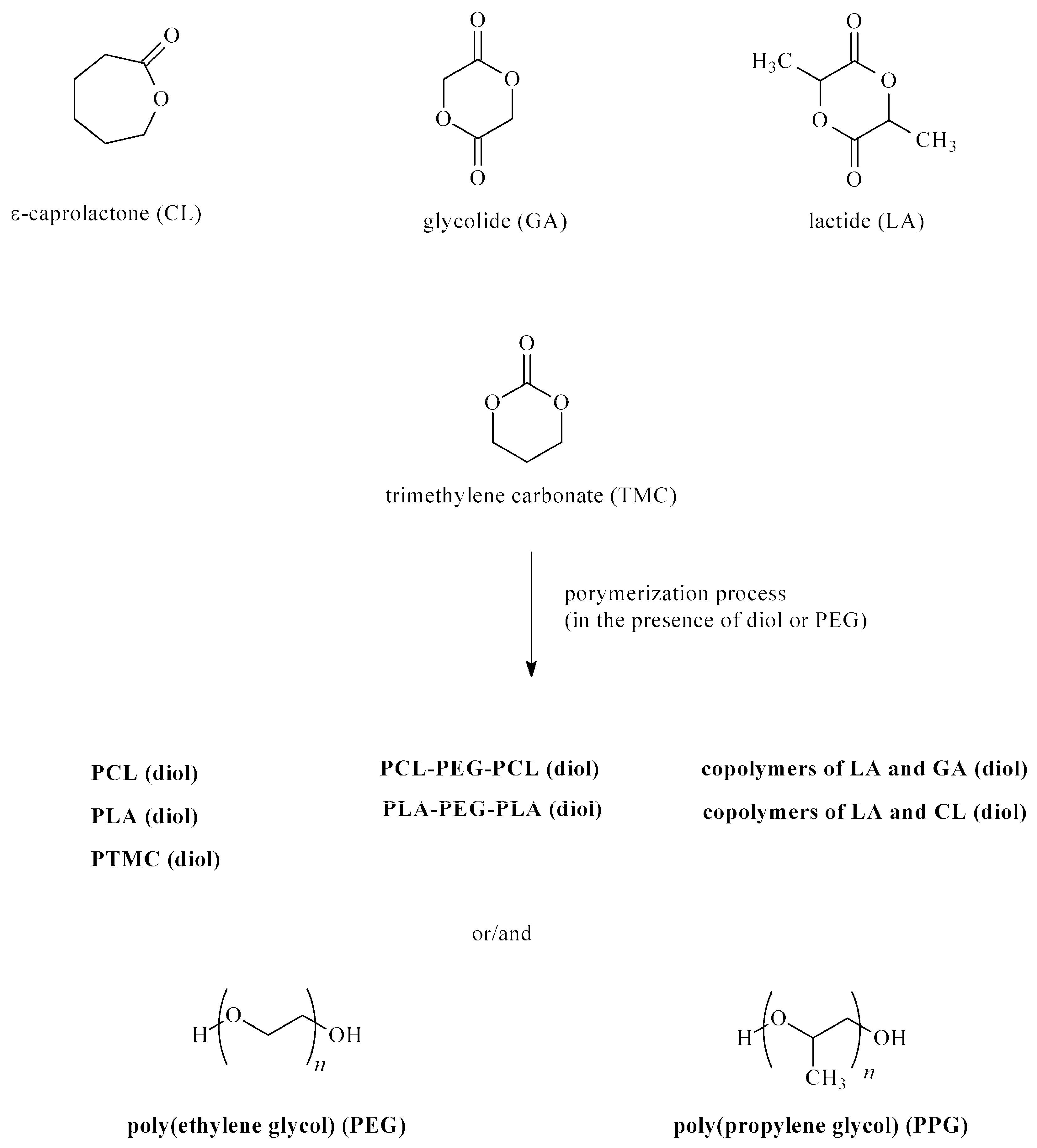
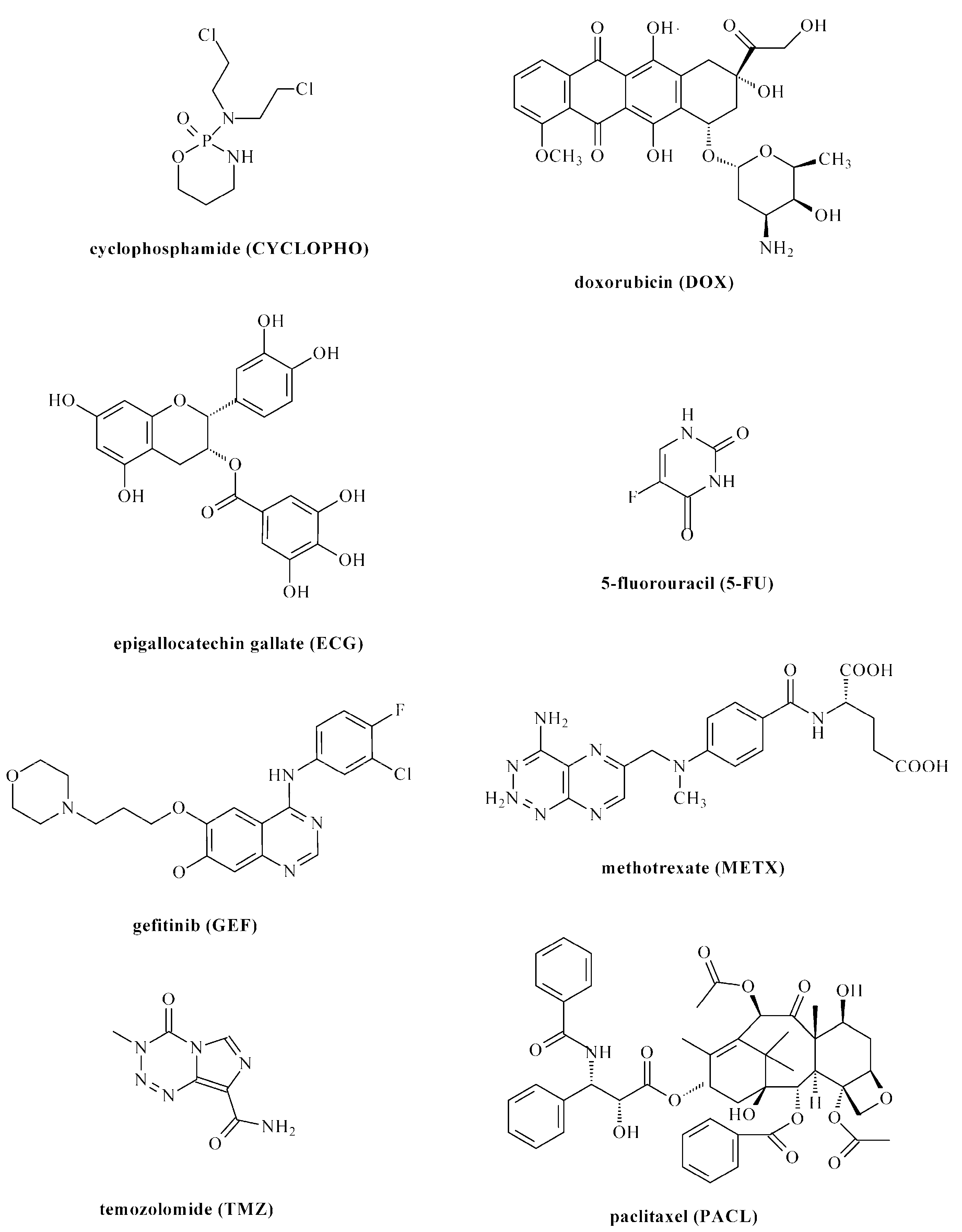


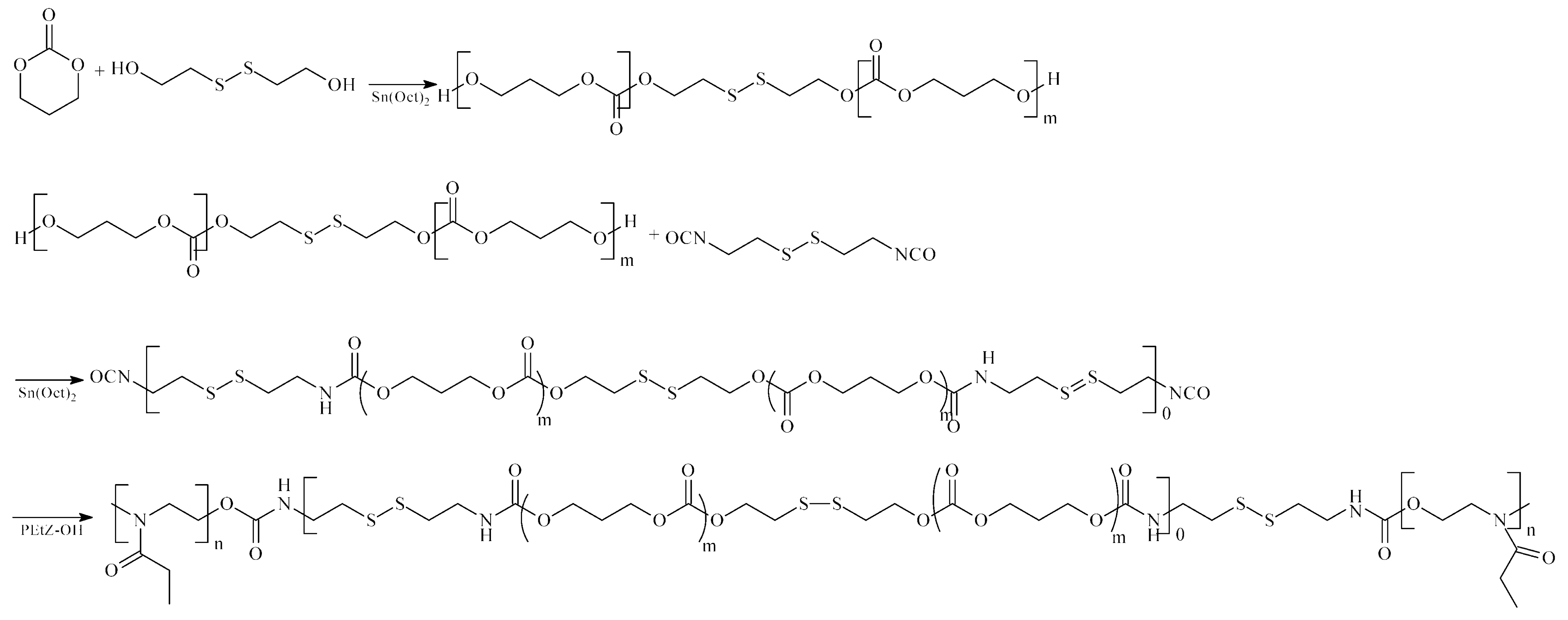
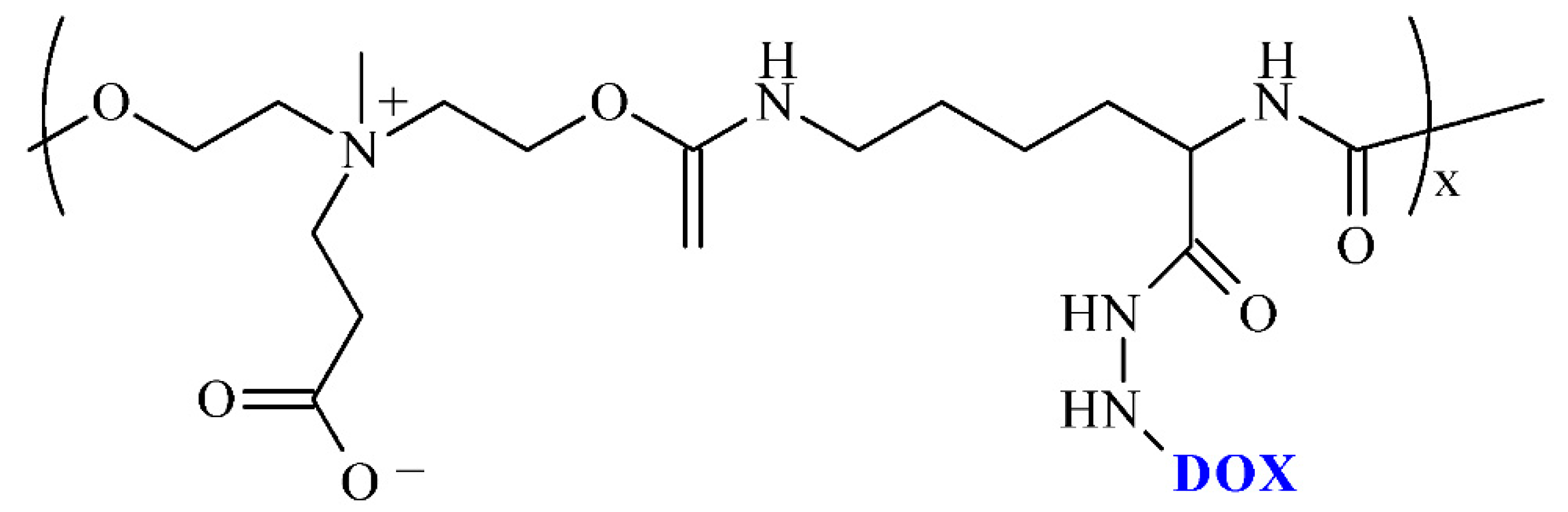
| Drug/Drugs | Type of PUs or Composites | Type of DDSs | Main Conclusions | Ref. |
|---|---|---|---|---|
| DOX | PEG-1500/bis-MPA/IPDI | nano- and microparticles/injectable carriers |
| [35] |
| DOX | HDI/PCL/PEG | microcapsules |
| [36] |
| DOX | PU-SS-COOH: PEG-1000/PCL-2000/HDI/CYS/DMPA; PU-SS-COOH-NH2: PEG-1000/PCL-2000/HDI/CYS/DMPA/1,6-diaminohexane | micelles |
| [37] |
| DOX | LDI/PEG-PU(SS)-PEG/ | micelles |
| [38] |
| DOX | PHBHx/PEG-2000/PPG-2050/HDI | thermogel |
| [39] |
| DOX | LDI/mPEG-OH-5000/PCL; PCL obtained form ε-CL to 2,20-dithiodiethanol | micelles |
| [40] |
| DOX | PTMC-SS-PTMC/CDI/PEOtz-OH | micelles |
| [41] |
| DOX | HDI/2,2-bis(hydroxymethyl) propionic acid/PEG; amphiphilic PUs with carboxyl pendent groups | nanoparticles |
| [42] |
| DOX | IPDI/methoxyl-poly(ethylene glycol) (mPEG)/carboxylic acid/piperazine | micelles |
| [43] |
| DOX | mPEG-5000/HDI/trimethylolpropane/bis(2-hydroxyethyl) disulfide | core-shell nanogels |
| [44] |
| DOX | poly(2-oxazoline)s/PLA-SS-PLA/LDI | micelles |
| [45] |
| DOX | PEG-2000/HDI and PCL-2000/PEG-2000/HDI | nanomicelles |
| [46] |
| DOX | mPEG-1000 (or PEG-2000)/poly(1,3-propylene succinate) diols (PPS)/IPDI | micelles |
| [47] |
| DOX | PLA-SS-PLA/LDI/PEG | micelles |
| [48] |
| DOX | WPU/CS | membranes |
| [49] |
| DOX | mPEG-1900/PCL/LDI; PUs with benzoic-imine linkage | micelles |
| [50] |
| DOX | polycondensation products of ortho ester-based diols and HDI (or HMDI) | microparticles |
| [51] |
| DOX | polycondensation product of terephthalilidene-bis(trimethylolethane) and LDI (and next termination process with allyl alcohol) | nanomicelles |
| [52] |
| DOX | trans-4,5-dihydroxy-1,2-dithiane (O-DTT)/HDI/mPEG | nanomicelles |
| [53] |
| DOX | PEG-2000/bis-1,4-(hydroxyethyl) piperazine (HEP)/O-DTT/HDI | nanomicelles |
| [54] |
| DOX | LDI/PDO/PEG/PCL/folic acid (FA) | nano- and micelles |
| [55] |
| DOX | PCL/poly (tetramethylene ether) glycol/HDI | cellulose acetate/PU/carbon nanotubes/composite nanofibers |
| [56] |
| DOX | LDI/hydrazine/dihydroxy carboxybetaine | conjugates/nano- and micromicelles |
| [57] |
| DOX | Dipentaerythritol/HDI/mPEG-2000/glycerol | conjugates/nanomicelles/dendritic PU |
| [58] |
| DOX and PACL | PLA-SS-PLA/IPDI/PEG | micelles |
| [59] |
| ECG | MEG/BDO/PEG-200/HDI/IPDI | microparticles |
| [60] |
| 5-FU | HDI/PEG-650 or -1250 or -1500 or -2000/1,2−DAE or 1,6-DAH or 1,4-DAB or 1,8-DAO/L-LYS | WPU |
| [61] |
| 5-FU and PACL | (PCL/HDI)/PNIPAAm grafted-chitosan core-shell nanofibers | core-shell nanofibers |
| [62] |
| METX | PCL-b-PEG-b-PCL/BDI/ L-glutathione oxidized | films |
| [63] |
| PACL | L-LYS-GQA/L-LYS-ABA-ABA tripeptide/HPCL/HPEG/LDI/PDO | nanomicelles |
| [64] |
| PACL | PEG-1000/PCL-2000/LDI/BDO/CYS or PEG-1000/PCL-2000/LDI/MDEA/BDO or PEG-1000/PCL-2000/LDI/CYS/MDEA | micelles |
| [65] |
| PACL | PCL-co-PEG/HMDI | nanoparticles |
| [66] |
| PACL and TMZ | PU purchased from Lubrizol Co | magnetic particles incorporated into nanofibers |
| [67] |
| TMZ | PCL/HDI/BDO |
|
| [68] |
| DOX | polycondensation products of multi-functional L-lysine monomers/1,12-dodecanediol | nanomicelles |
| [69] |
| GEF | TDI/unknown polyol/unknown cross-linker (Vysera Biomedical Ltd.); GEF-loaded PLGA-based microspheres | PU foams either as micronized drug or as GEF-PLGA microspheres |
| [70] |
| PACL | MDI/PCL-4000/BDO | membrane |
| [71] |
| CYCLOPHO | TDI/PEG-600 (or -1500 or -3500)/DEG | implant |
| [72] |
| DOX | MDI/PPG-N3/PPEG-2000 or PPEG-4000 | micelles |
| [73] |
| 5-FU | PCL (or PLA, CL/LA copolymers)/HDI | conjugates |
| [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, M.; Kędra, K. Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 8181. https://doi.org/10.3390/ijms23158181
Sobczak M, Kędra K. Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review. International Journal of Molecular Sciences. 2022; 23(15):8181. https://doi.org/10.3390/ijms23158181
Chicago/Turabian StyleSobczak, Marcin, and Karolina Kędra. 2022. "Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review" International Journal of Molecular Sciences 23, no. 15: 8181. https://doi.org/10.3390/ijms23158181
APA StyleSobczak, M., & Kędra, K. (2022). Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review. International Journal of Molecular Sciences, 23(15), 8181. https://doi.org/10.3390/ijms23158181







