RclS Sensor Kinase Modulates Virulence of Pseudomonas capeferrum
Abstract
:1. Introduction
2. Results
2.1. RNA Sequencing and Mapping to the Reference Genome
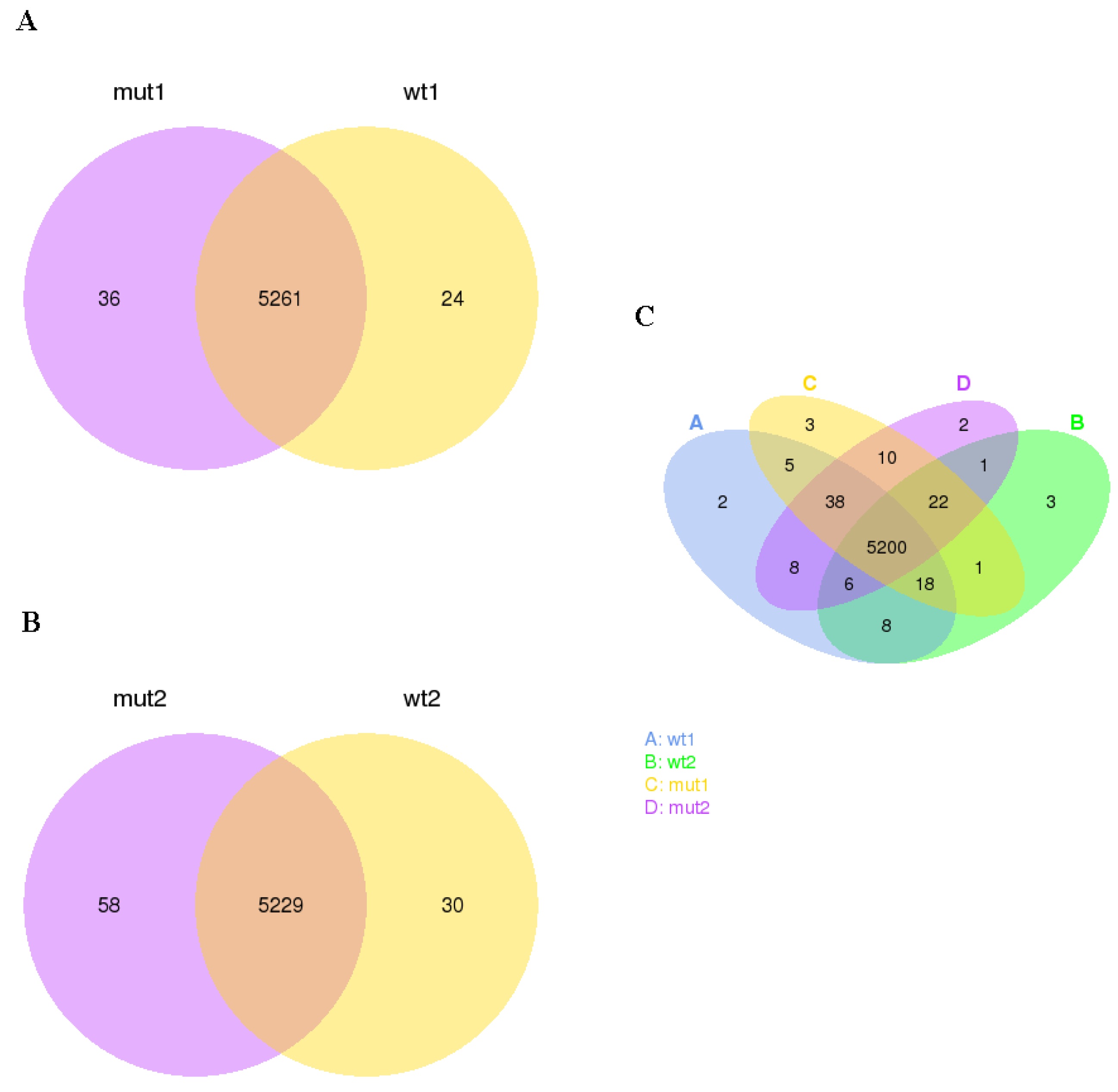
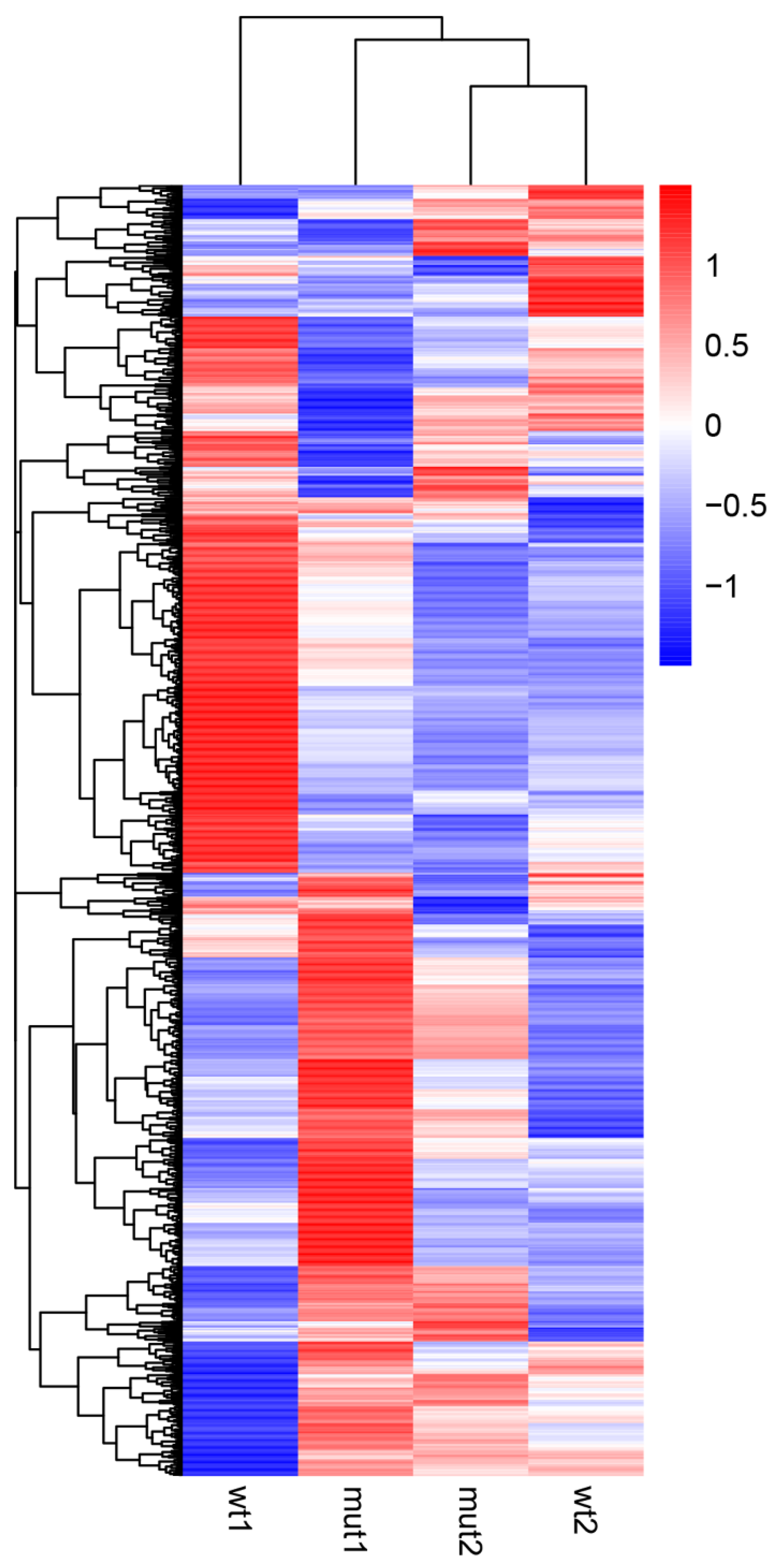
2.2. Gene Co-Expression and Differentially Expressed Genes (DEGs) Analysis
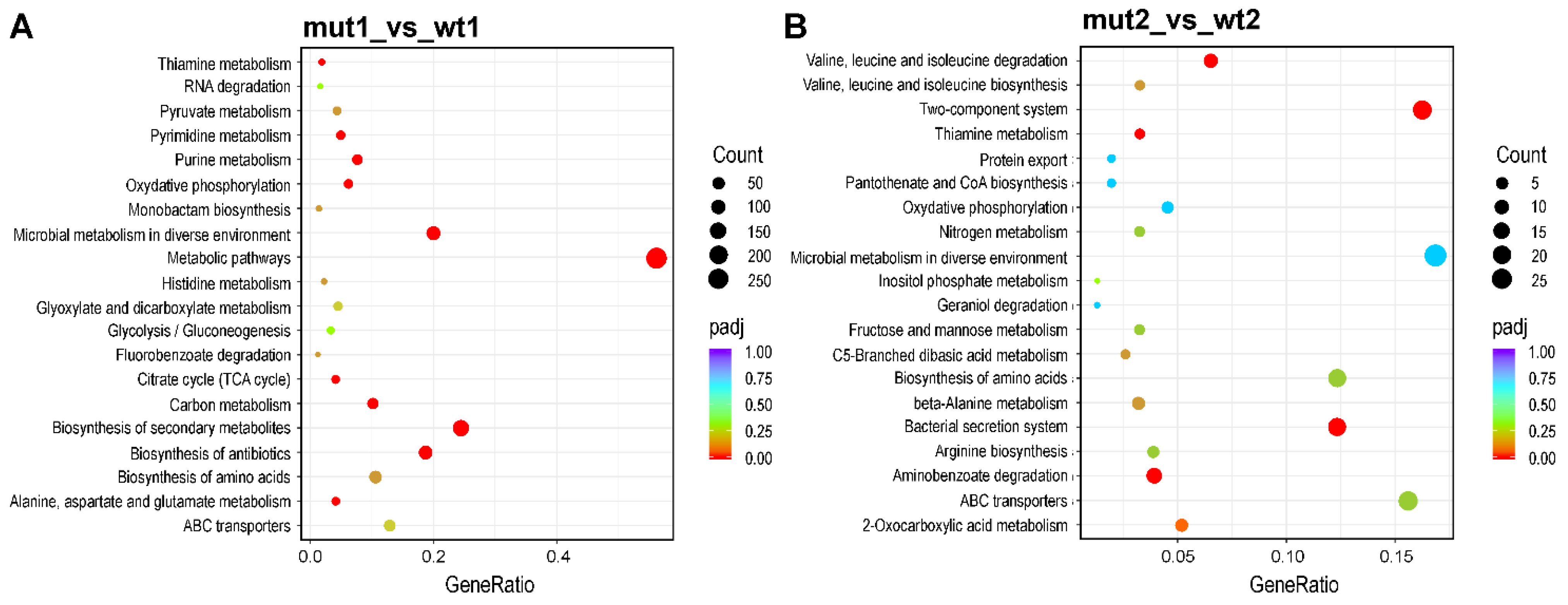
2.3. GO and KEGG Enrichment Analysis of DEGs
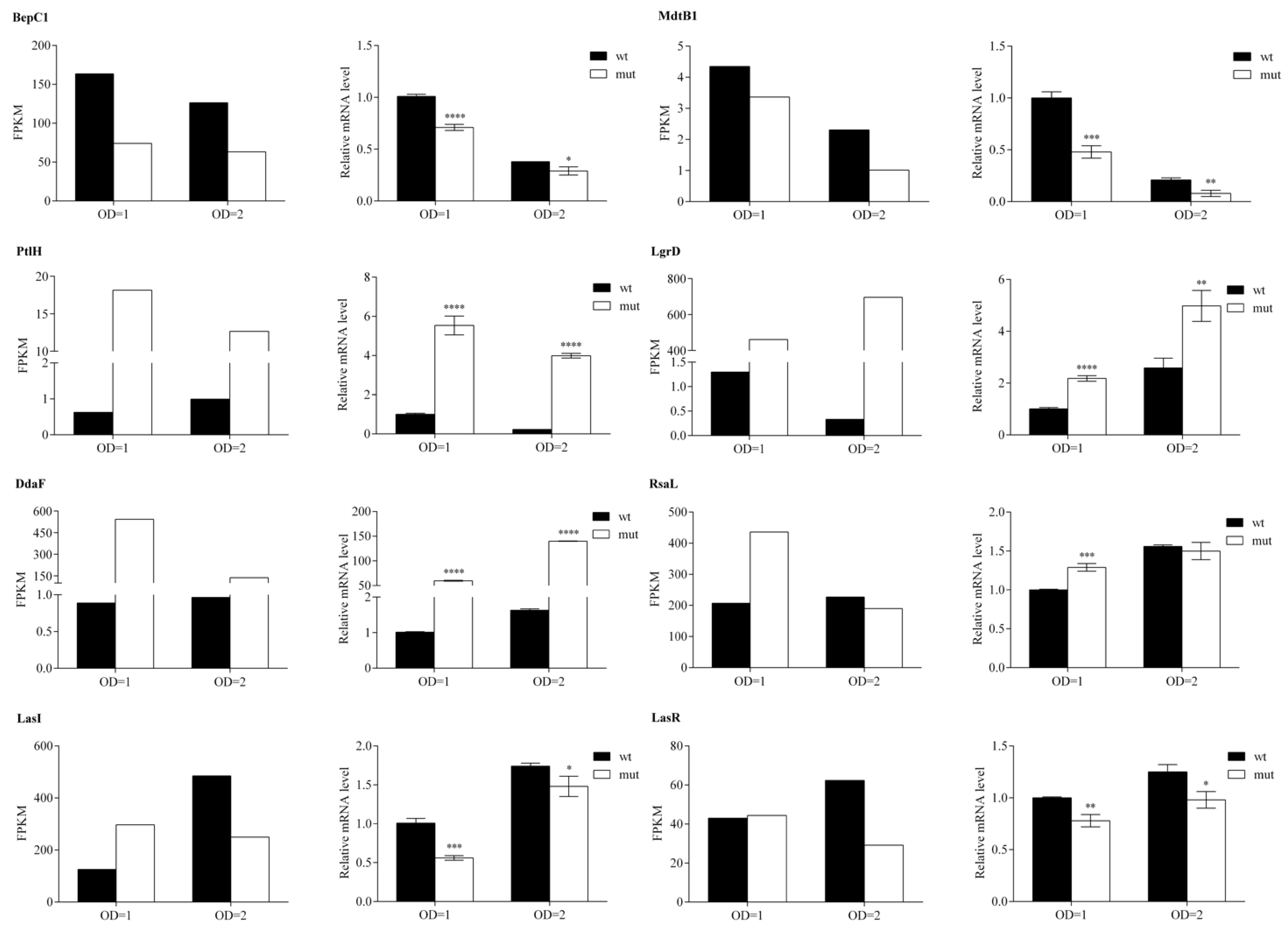
2.4. Validation of RNA Sequencing Using RT-qPCR
2.5. Antimicrobial Susceptibility of WCS358 Wild Type and ΔrclS Mutant
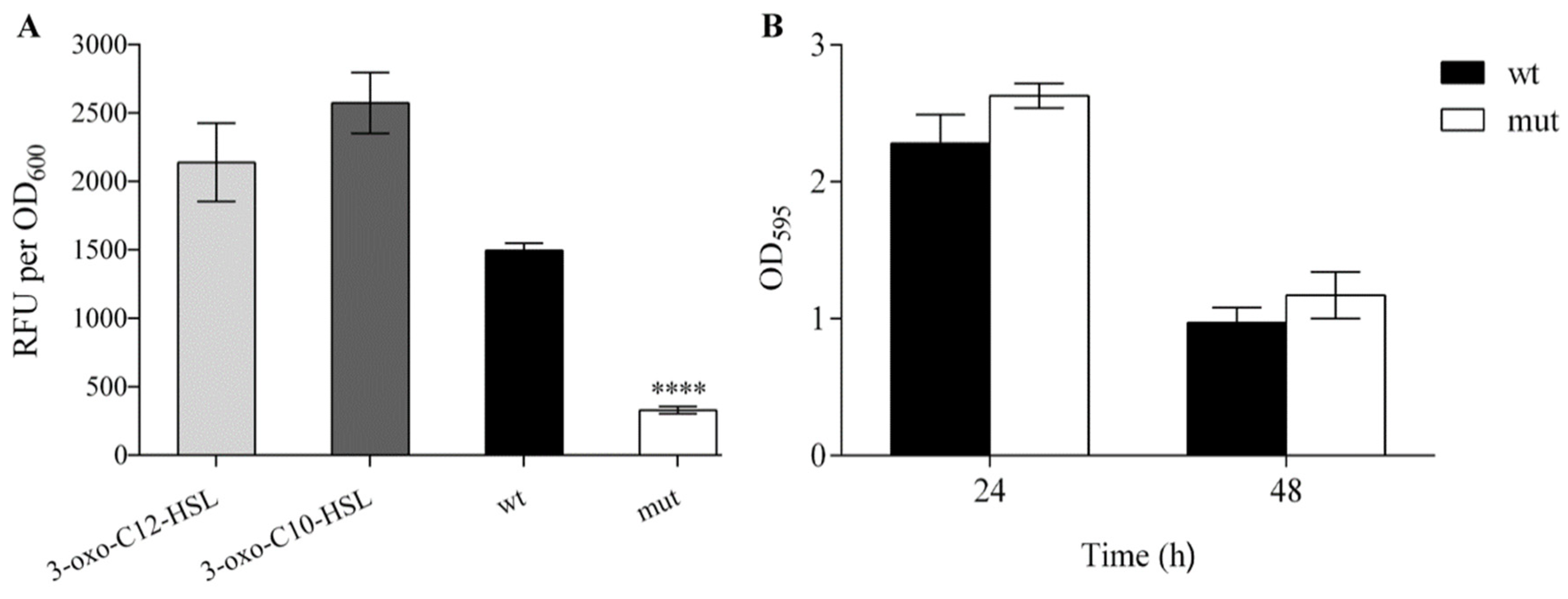
2.6. AHLs, Pseudobactin 358 and Biofilm Production of WCS358 Wild Type and ΔrclS Mutant
2.7. Cytotoxic Effect and Adhesion Ability of WCS358 Wild Type and ΔrclS Mutant
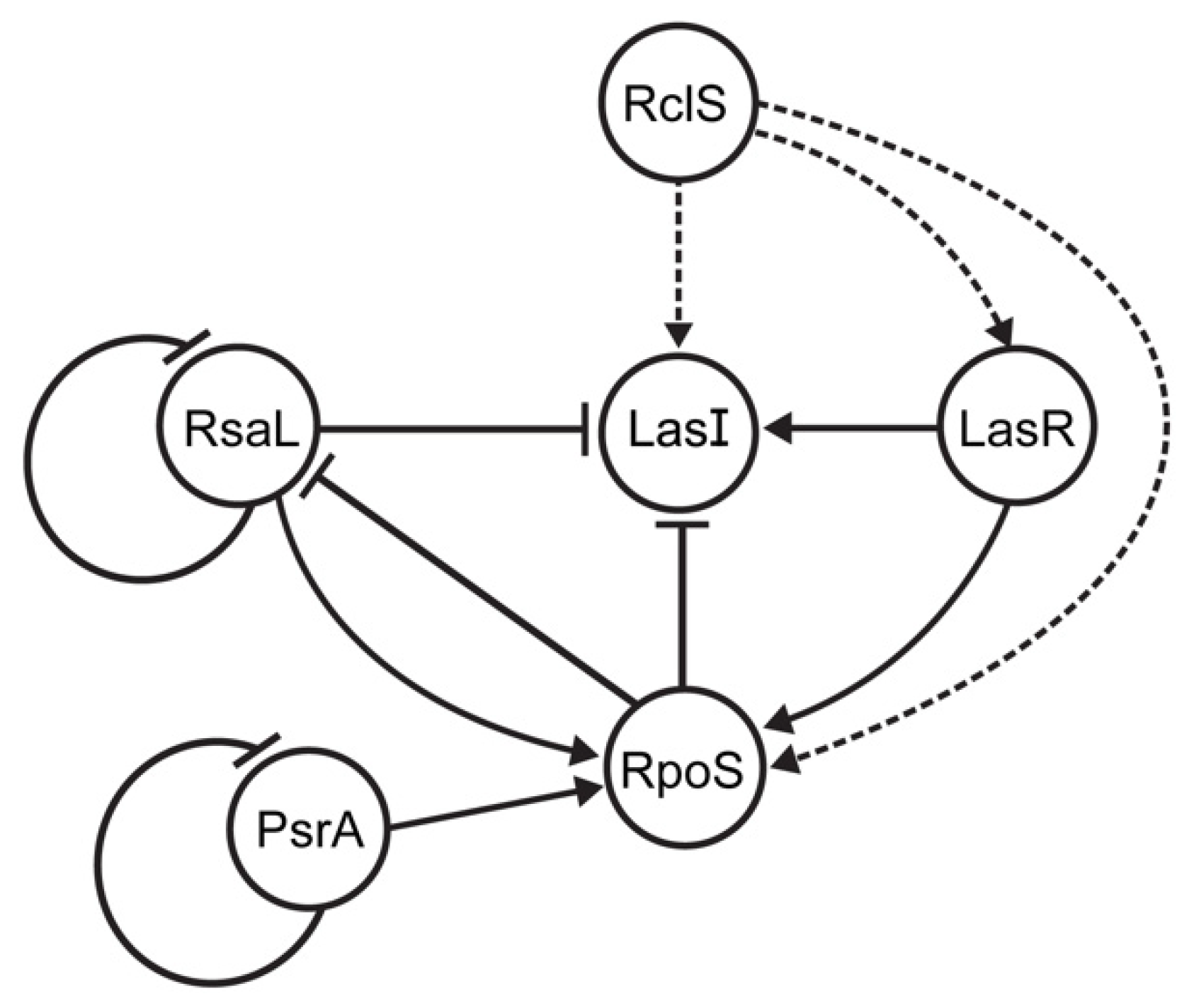
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Chemicals
4.2. RNA Extraction and Sequencing
4.3. Data Analysis
4.4. GO and KEGG Enrichment Analysis
4.5. Real Time Quantitative PCR (RT-qPCR) Analysis
4.6. Antimicrobial Susceptibility Testing
4.7. Preparation of Crude Ethyl Acetate Extract
4.8. Fluorescence Assay
4.9. Biofilm Formation Assay
4.10. Isolation of Siderophore Pseudobactin 358
4.11. Cytotoxicity and Adhesion Assays
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gooderham, W.J.; Hancock, R.E. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 2009, 33, 279–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob-Dubuisson, F.; Mechaly, A.; Betton, J.M.; Antoine, R. Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 2018, 16, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, D.; Zhu, J.; Liu, W. Two-component signal transduction systems: A major strategy for connecting input stimuli to biofilm formation. Front. Microbiol. 2019, 9, 3279. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.J.; Barros, C.; Stibitz, S. Characterization of the bvgR locus of Bordetella pertussis. J. Bacteriol. 1998, 180, 1682–1690. [Google Scholar] [CrossRef] [Green Version]
- Kulasekara, H.D.; Ventre, I.; Kulasekara, B.R.; Lazdunski, A.; Filloux, A.; Lory, S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol. Microbiol. 2005, 55, 368–380. [Google Scholar] [CrossRef]
- Liu, C.; Yang, J.; Liu, L.; Li, B.; Yuan, H.; Liu, W. Sodium lactate negatively regulates Shewanella putrefaciens CN32 biofilm formation via a three-component regulatory system (LrbS-LrbA-LrbR). Appl. Environ. Microbiol. 2017, 83, e00712-17. [Google Scholar] [CrossRef] [Green Version]
- Novović, K.; Malešević, M.; Gardijan, L.; Kojić, M.; Jovčić, B. Novel RclSAR three-component system regulates expression of the intI1 gene in the stationary growth phase. Res. Microbiol. 2022, 173, 103885. [Google Scholar] [CrossRef]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [Green Version]
- Francis, V.I.; Stevenson, E.C.; Porter, S.L. Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2017, 364, fnx104. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Schneper, L.; Kumari, H.; Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013, 41, 1–20. [Google Scholar] [CrossRef]
- Putrinš, M.; Ilves, H.; Lilje, L.; Kivisaar, M.; Hõrak, R. The impact of ColRS two-component system and Ttg ABC efflux pump on phenol tolerance of Pseudomonas putida becomes evident only in growing bacteria. BMC Microbiol. 2010, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsaar, K.; Mumm, K.; Ilves, H.; Hõrak, R. The ColRS signal transduction system responds to the excess of external zinc, iron, manganese, and cadmium. BMC Microbiol. 2014, 14, 162. [Google Scholar] [CrossRef] [Green Version]
- Hõrak, R.; Ilves, H.; Pruunsild, P.; Kuljus, M.; Kivisaar, M. The ColR-ColS two-component signal transduction system is involved in regulation of Tn4652 transposition in Pseudomonas putida under starvation conditions. Mol. Microbiol. 2004, 54, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, X.; Huang, Q.; Chen, W. The role of CzcRS two-component systems in the heavy metal resistance of Pseudomonas putida X4. Int. J. Mol. Sci. 2015, 16, 17005–17017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Pinar, R.; Ramos, J.L.; Rodríguez-Herva, J.J.; Espinosa-Urgel, M. A two-component regulatory system integrates redox state and population density sensing in Pseudomonas putida. J. Bacteriol. 2008, 190, 7666–7674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertani, I.; Venturi, V. Regulation of the N-acyl homoserine lactone-dependent quorum-sensing system in rhizosphere Pseudomonas putida WCS358 and cross-talk with the stationary-phase RpoS sigma factor and the global regulator GacA. Appl. Environ. Microbiol. 2004, 70, 5493–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, R.L.; van Verk, M.C.; Stringlis, I.A.; Zamioudis, C.; Tommassen, J.; Pieterse, C.M.J.; Bakker, P.A.H.M. Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genom. 2015, 16, 539. [Google Scholar] [CrossRef] [Green Version]
- Bhagirath, A.Y.; Li, Y.; Patidar, R.; Yerex, K.; Ma, X.; Kumar, A.; Duan, K. Two component regulatory systems and antibiotic resistance in Gram-negative pathogens. Int. J. Mol. Sci. 2019, 20, 1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivisaar, M. Narrative of a versatile and adept species Pseudomonas putida. J. Med. Microbiol. 2020, 69, 324–338. [Google Scholar] [CrossRef]
- Molina, L.; Udaondo, Z.; Duque, E.; Fernández, M.; Molina-Santiago, C.; Roca, A.; Porcel, M.; de la Torre, J.; Segura, A.; Plesiat, P.; et al. Antibiotic resistance determinants in a Pseudomonas putida strain isolated from a hospital. PLoS ONE 2014, 9, e81604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, S.; Oberhettinger, P.; Schuele, L.; Dinkelacker, A.; Vogel, W.; Dörfel, D.; Bezdan, D.; Ossowski, S.; Marschal, M.; Liese, J.; et al. Genomic characterisation of clinical and environmental Pseudomonas putida group strains and determination of their role in the transfer of antimicrobial resistance genes to Pseudomonas aeruginosa. BMC Genom. 2017, 18, 859. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xi, Y.; Yuan, P.; Sun, Z.; Yang, D. Risk factors and antimicrobial resistance profiles of Pseudomonas putida infection in Central China, 2010–2017. Medicine 2019, 98, e17812. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Porcel, M.; de la Torre, J.; Molina-Henares, M.A.; Daddaoua, A.; Llamas, M.A.; Roca, A.; Carriel, V.; Garzón, I.; Ramos, J.L.; et al. Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 2015, 6, 871. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Zamorano, L.; Mena, A.; Albertí, S.; Pérez, J.L.; Oliver, A. Metallo-β-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J. Antimicrob. Chemother. 2010, 65, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.D.; Emerson, N.; Gahan, C.G.; Hill, C. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 1999, 181, 6840–6843. [Google Scholar] [CrossRef] [Green Version]
- Frederick, J.R.; Rogers, E.A.; Marconi, R.T. Analysis of a growth-phase-regulated two-component regulatory system in the periodontal pathogen Treponema denticola. J. Bacteriol. 2008, 190, 6162–6169. [Google Scholar] [CrossRef] [Green Version]
- Dhiman, A.; Bhatnagar, S.; Kulshreshtha, P.; Bhatnagar, R. Functional characterization of WalRK: A two-component signal transduction system from Bacillus anthracis. FEBS Open Bio 2014, 4, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Jiang, F.; Huang, X.; Li, G.; Cai, W. Transcriptomic and metabolomic profiling reveals that KguR broadly impacts the physiology of uropathogenic Escherichia coli under in vivo relevant conditions. Front. Microbiol. 2021, 12, 793391. [Google Scholar] [CrossRef]
- Hadjifrangiskou, M.; Kostakioti, M.; Chen, S.L.; Henderson, J.P.; Greene, S.E.; Hultgren, S.J. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol. Microbiol. 2011, 80, 1516–1529. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, A.M.; Singh, B.; Röhm, K.H. The AauR-AauS two-component system regulates uptake and metabolism of acidic amino acids in Pseudomonas putida. Appl. Environ. Microbiol. 2006, 72, 6569–6577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, H.; Rico, S.; Díaz, M.; Santamaría, R.I. Two-component systems in Streptomyces: Key regulators of antibiotic complex pathways. Microb. Cell Factories 2013, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alduina, R.; Tocchetti, A.; Costa, S.; Ferraro, C.; Cancemi, P.; Sosio, M.; Donadio, S. A two-component regulatory system with opposite effects on glycopeptide antibiotic biosynthesis and resistance. Sci. Rep. 2020, 10, 6200. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Li, X.; Ke, X.; Chu, J. A two-component system gene SACE_0101 regulates copper homeostasis in Saccharopolyspora erythraea. Bioresour. Bioprocess. 2020, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Bricio-Moreno, L.; Sheridan, V.H.; Goodhead, I.; Armstrong, S.; Wong, J.K.; Waters, E.M.; Sarsby, J.; Panagiotou, S.; Dunn, J.; Chakraborty, A.; et al. Evolutionary trade-offs associated with loss of PmrB function in host-adapted Pseudomonas aeruginosa. Nat. Commun. 2018, 9, 2635. [Google Scholar] [CrossRef]
- Marunga, J.; Goo, E.; Kang, Y.; Hwang, I. Mutations in the two-component GluS-GluR regulatory system confer resistance to β-lactam antibiotics in Burkholderia glumae. Front. Microbiol. 2021, 12, 721444. [Google Scholar] [CrossRef]
- Rampioni, G.; Bertani, I.; Pillai, C.R.; Venturi, V.; Zennaro, E.; Leoni, L. Functional characterization of the quorum sensing regulator RsaL in the plant-beneficial strain Pseudomonas putida WCS358. Appl. Environmen. Microbiol. 2012, 78, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Huang, J.H.; Feng, Q.S.; Liu, Z.Q.; Lin, Q.Q.; Xu, Z.; Zhang, L.H. A two-component system FleS/FleR regulates multiple virulence-related traits in Pseudomonas aeruginosa. bioRxiv 2021. [Google Scholar] [CrossRef]
- Fothergill, J.L.; Neill, D.R.; Loman, N.; Winstanley, C.; Kadioglu, A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 2014, 5, 4780. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.L.; Lee, V.T. Cyclic-di-GMP regulation of virulence in bacterial pathogens. Wiley Interdiscip. Rev. RNA 2018, 9, e1454. [Google Scholar] [CrossRef]
- Mikkelsen, H.; Sivaneson, M.; Filloux, A. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 2011, 13, 1666–1681. [Google Scholar] [CrossRef]
- Steidle, A.; Sigl, K.; Schuhegger, R.; Ihring, A.; Schmid, M.; Gantner, S.; Stoffels, M.; Riedel, K.; Givskov, M.; Hartmann, A.; et al. Visualization of N-acyl homoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl. Environ. Microbiol. 2001, 67, 5761–5770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, M.I.; Jacobs, A.C.; Sayood, K.; Dunman, P.M.; Skaar, E.P. Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations. Antimicrob. Agents Chemother. 2010, 54, 1029–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kojic, M.; Degrassi, G.; Venturi, V. Cloning and characterisation of the rpoS gene from plant growth-promoting Pseudomonas putida WCS358: RpoS is not involved in siderophore and homoserine lactone production. Biochim. Biophys. Acta 1999, 1489, 413–3420. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Meyer, J.M.; Abdallah, M.A. The fluorescent pigment of Pseudomonas fluorescens: Biosynthesis, purification and physicochemical properties. Microbiology 1978, 107, 319–328. [Google Scholar] [CrossRef] [Green Version]


| Samples | wt1 | mut1 | wt2 | mut2 |
|---|---|---|---|---|
| Raw reads | 7,982,150 | 11,145,045 | 7,978,294 | 11,921,075 |
| Clean reads | 7,940,628 | 11,117,800 | 7,965,543 | 11,895,759 |
| Q20 (%) | 98.42 | 98.37 | 98.42 | 98.46 |
| Q30 (%) | 95.24 | 95.11 | 95.14 | 95.22 |
| GC content (%) | 60.96 | 57.76 | 55.87 | 55.73 |
| Total mapped (%) | 97.84 | 98.79 | 98.87 | 99.02 |
| Uniquely mapped (%) | 96.27 | 98.00 | 98.17 | 98.41 |
| Antibiotic | MIC (μg/mL) | |
|---|---|---|
| Wt | Mut | |
| Ampicillin | >800 | >800 |
| Ceftazidime | 5 | 10 |
| Chloramphenicol | 80 | 80 |
| Erythromycin | 800 | 800 |
| Gentamicin | 5 | 2.5 |
| Novobiocin | 800 | 400 |
| Levofloxacine | 1.25 | 0.625 |
| Tetracycline | 10 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novović, K.; Malešević, M.; Dinić, M.; Gardijan, L.; Kojić, M.; Jovčić, B. RclS Sensor Kinase Modulates Virulence of Pseudomonas capeferrum. Int. J. Mol. Sci. 2022, 23, 8232. https://doi.org/10.3390/ijms23158232
Novović K, Malešević M, Dinić M, Gardijan L, Kojić M, Jovčić B. RclS Sensor Kinase Modulates Virulence of Pseudomonas capeferrum. International Journal of Molecular Sciences. 2022; 23(15):8232. https://doi.org/10.3390/ijms23158232
Chicago/Turabian StyleNovović, Katarina, Milka Malešević, Miroslav Dinić, Lazar Gardijan, Milan Kojić, and Branko Jovčić. 2022. "RclS Sensor Kinase Modulates Virulence of Pseudomonas capeferrum" International Journal of Molecular Sciences 23, no. 15: 8232. https://doi.org/10.3390/ijms23158232






