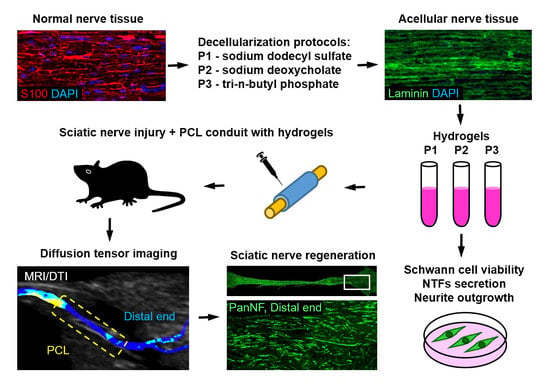
Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization
Abstract
:1. Introduction
2. Results
2.1. Characterisation of Decellularized Nerve Tissue
2.2. Characterisation of Hydrogels Prepared from Decellularized Nerve Tissue
2.3. Effects of Hydrogels on Axon Regeneration after Peripheral Nerve Injury
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Decellularization of Nerve Tissue
4.3. DNA Quantification
4.4. Quantification of Collagen and Glycosaminoglycans
4.5. Preparation of Hydrogels and Gelation Kinetics
4.6. Total Protein Isolation and Quantification
4.7. Analysis of Cytokines and Chemokines in the Hydrogel
4.8. Schwann Cell Culture
4.9. Cell Proliferation Assay on Hydrogels
4.10. Enzyme Linked Immunosorbent Assay (ELISA)
4.11. Neurite Outgrowth Assay in Hydrogels
4.12. Preparation of the Tubular Conduits with Hydrogels
4.13. Sciatic Nerve Injury Model
4.14. Diffusion Tensor Imaging (DTI) and Data Analysis
4.15. Tissue Processing
4.16. Histological Staining and Immunohistochemistry
4.17. Image Analysis and Quantification
4.18. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palispis, W.A.; Gupta, R. Surgical repair in humans after traumatic nerve injury provides limited functional neural regeneration in adults. Exp. Neurol. 2017, 290, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, L.B.; Wiberg, M. Nerve injuries of the upper extremity and hand. EFORT Open Rev. 2017, 2, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, J.A. Peripheral nerve injuries: A retrospective survey of 456 cases. Muscle Nerve 2006, 34, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral nerve injury, scarring, and recovery. Connect. Tissue Res. 2019, 60, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ruijs, A.C.; Jaquet, J.B.; Kalmijn, S.; Giele, H.; Hovius, S.E. Median and ulnar nerve injuries: A meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast. Reconstr. Surg. 2005, 116, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Scholz, T.; Krichevsky, A.; Sumarto, A.; Jaffurs, D.; Wirth, G.A.; Paydar, K.; Evans, G.R. Peripheral nerve injuries: An international survey of current treatments and future perspectives. J. Reconstr. Microsurg. 2009, 25, 339–344. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Z.; Shi, H.; Yao, Y.; Du, W.; Lu, P.; Liang, K.; Hong, L.; Gao, C. Micropatterns and peptide gradient on the inner surface of a guidance conduit synergistically promotes nerve regeneration in vivo. Bioact. Mater. 2022, 9, 134–146. [Google Scholar] [CrossRef]
- Lohmeyer, J.A.; Shen, Z.L.; Walter, G.F.; Berger, A. Bridging extended nerve defects with an artifcial nerve graft containing Schwann cells pre-seeded on polyglactin filaments. Int. J. Artif. Organs 2007, 30, 64–74. [Google Scholar] [CrossRef]
- Dietzmeyer, N.; Huang, Z.; Schuning, T.; Rochkind, S.; Almog, M.; Nevo, Z.; Lieke, T.; Kankowski, S.; Haastert-Talini, K. In Vivo and In Vitro Evaluation of a Novel Hyaluronic Acid-Laminin Hydrogel as Luminal Filler and Carrier System for Genetically Engineered Schwann Cells in Critical Gap Length Tubular Peripheral Nerve Graft in Rats. Cell Transpl. 2020, 29, 963689720910095. [Google Scholar] [CrossRef]
- Verdu, E.; Labrador, R.O.; Rodriguez, F.J.; Ceballos, D.; Fores, J.; Navarro, X. Alignment of collagen and laminin-containing gels improve nerve regeneration within silicone tubes. Restor. Neurol. Neurosci. 2002, 20, 169–179. [Google Scholar] [PubMed]
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 459–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Kemp, G.J.; Yu, L.G.; Wagstaff, S.C.; Frostick, S.P. Neurotrophin-4 delivered by fibrin glue promotes peripheral nerve regeneration. Muscle Nerve 2001, 24, 345–351. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, W.; Zhang, K.; Qiu, S.; Xu, J.; Wang, C.; Chai, Y. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 2019, 83, 291–301. [Google Scholar] [CrossRef]
- Lovati, A.B.; D’Arrigo, D.; Odella, S.; Tos, P.; Geuna, S.; Raimondo, S. Nerve Repair Using Decellularized Nerve Grafts in Rat Models. A Review of the Literature. Front. Cell. Neurosci. 2018, 12, 427. [Google Scholar] [CrossRef]
- Cai, M.; Huang, T.; Hou, B.; Guo, Y. Role of Demyelination Efficiency within Acellular Nerve Scaffolds during Nerve Regeneration across Peripheral Defects. Biomed Res. Int. 2017, 2017, 4606387. [Google Scholar] [CrossRef]
- Kaizawa, Y.; Kakinoki, R.; Ikeguchi, R.; Ohta, S.; Noguchi, T.; Takeuchi, H.; Oda, H.; Yurie, H.; Matsuda, S. A Nerve Conduit Containing a Vascular Bundle and Implanted With Bone Marrow Stromal Cells and Decellularized Allogenic Nerve Matrix. Cell Transplant. 2017, 26, 215–228. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef]
- Sondell, M.; Lundborg, G.; Kanje, M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998, 795, 44–54. [Google Scholar] [CrossRef]
- Whitlock, E.L.; Tuffaha, S.H.; Luciano, J.P.; Yan, Y.; Hunter, D.A.; Magill, C.K.; Moore, A.M.; Tong, A.Y.; Mackinnon, S.E.; Borschel, G.H. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009, 39, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Li, G.; Wu, T.T.; Lin, Z.J.; Zou, J.L.; Huang, L.J.; Xu, H.Y.; Wang, J.H.; Ma, Y.H.; Zeng, Y.S. Decellularization optimizes the inhibitory microenvironment of the optic nerve to support neurite growth. Biomaterials 2020, 258, 120289. [Google Scholar] [CrossRef] [PubMed]
- Rinker, B.; Zoldos, J.; Weber, R.V.; Ko, J.; Thayer, W.; Greenberg, J.; Leversedge, F.J.; Safa, B.; Buncke, G. Use of Processed Nerve Allografts to Repair Nerve Injuries Greater Than 25 mm in the Hand. Ann. Plast. Surg. 2017, 78, S292–S295. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.N.; Weber, R.V.; Chao, J.D.; Rinker, B.D.; Zoldos, J.; Robichaux, M.R.; Ruggeri, S.B.; Anderson, K.A.; Bonatz, E.E.; Wisotsky, S.M.; et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 2012, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Manzano, G.; Gupta, R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013, 29, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Rbia, N.; Shin, A.Y. The Role of Nerve Graft Substitutes in Motor and Mixed Motor/Sensory Peripheral Nerve Injuries. J. Hand Surg. Am. 2017, 42, 367–377. [Google Scholar] [CrossRef]
- Farber, S.J.; Hoben, G.M.; Hunter, D.A.; Yan, Y.; Johnson, P.J.; Mackinnon, S.E.; Wood, M.D. Vascularization is delayed in long nerve constructs compared with nerve grafts. Muscle Nerve 2016, 54, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef] [Green Version]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Buckenmeyer, M.J.; Meder, T.J.; Prest, T.A.; Brown, B.N. Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods 2020, 171, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.L.; Menache, D. Prevention of infectious disease transmission by blood and blood products. Prog. Hematol. 1987, 15, 221–241. [Google Scholar] [PubMed]
- El Soury, M.; Garcia-Garcia, O.D.; Moretti, M.; Perroteau, I.; Raimondo, S.; Lovati, A.B.; Carriel, V. Comparison of Decellularization Protocols to Generate Peripheral Nerve Grafts: A Study on Rat Sciatic Nerves. Int. J. Mol. Sci. 2021, 22, 2389. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003, 4, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Halasz, K.; Kassner, A.; Morgelin, M.; Heinegard, D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 2007, 282, 31166–31173. [Google Scholar] [CrossRef] [Green Version]
- Dugan, T.A.; Yang, V.W.; McQuillan, D.J.; Hook, M. Decorin modulates fibrin assembly and structure. J. Biol. Chem. 2006, 281, 38208–38216. [Google Scholar] [CrossRef] [Green Version]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.C.; Khoo, Z.S.; Lo, N.W.; Tan, W.J.; Chemmangattuvalappil, N.G. Design and performance optimisation of detergent product containing binary mixture of anionic-nonionic surfactants. Heliyon 2020, 6, e03861. [Google Scholar] [CrossRef]
- Jadidi, N.; Adib, B.; Malihi, F.B. Synergism and Performance Optimization in Liquid Detergents Containing Binary Mixtures of Anionic–Nonionic, and Anionic–Cationic Surfactants. J. Surfactants Deterg. 2013, 16, 115–121. [Google Scholar] [CrossRef]
- Elebring, E.; Kuna, V.K.; Kvarnstrom, N.; Sumitran-Holgersson, S. Cold-perfusion decellularization of whole-organ porcine pancreas supports human fetal pancreatic cell attachment and expression of endocrine and exocrine markers. J. Tissue Eng. 2017, 8, 2041731417738145. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Lin, T.; Qiu, S.; Zhou, J.; Liu, S.; Chen, S.; Wang, T.; Liu, X.; Zhu, Q.; Bai, Y.; et al. Decellularized nerve matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral nerve regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111791. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Rao, Z.; He, F.; Wang, T.; Xu, Y.; Du, Z.; Yao, Z.; Lin, T.; Yan, L.; Quan, D.; et al. Decellularized nerve matrix hydrogel and glial-derived neurotrophic factor modifications assisted nerve repair with decellularized nerve matrix scaffolds. J. Tissue Eng. Regen. Med. 2020, 14, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, J.; Rao, Z.; Deng, R.; Xu, Y.; Qiu, S.; Long, H.; Zhu, Q.; Liu, X.; Bai, Y.; et al. Facilitate Angiogenesis and Neurogenesis by Growth Factors Integrated Decellularized Matrix Hydrogel. Tissue Eng. Part A 2021, 27, 771–787. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Z.; Chen, S.; Zhang, F.; Rao, Z.; Zhao, C.; Quan, D.; Bai, Y.; Shen, J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics 2021, 11, 2917–2931. [Google Scholar] [CrossRef]
- Lin, T.; Liu, S.; Chen, S.; Qiu, S.; Rao, Z.; Liu, J.; Zhu, S.; Yan, L.; Mao, H.; Zhu, Q.; et al. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018, 73, 326–338. [Google Scholar] [CrossRef]
- Prest, T.A.; Yeager, E.; LoPresti, S.T.; Zygelyte, E.; Martin, M.J.; Dong, L.; Gibson, A.; Olutoye, O.O.; Brown, B.N.; Cheetham, J. Nerve-specific, xenogeneic extracellular matrix hydrogel promotes recovery following peripheral nerve injury. J. Biomed. Mater. Res. A 2018, 106, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Bulstra, L.F.; Hundepool, C.A.; Friedrich, P.F.; Bishop, A.T.; Hovius, S.E.R.; Shin, A.Y. Functional Outcome after Reconstruction of a Long Nerve Gap in Rabbits Using Optimized Decellularized Nerve Allografts. Plast. Reconstr. Surg. 2020, 145, 1442–1450. [Google Scholar] [CrossRef]
- Yu, T.; Wen, L.; He, J.; Xu, Y.; Li, T.; Wang, W.; Ma, Y.; Ahmad, M.A.; Tian, X.; Fan, J.; et al. Fabrication and evaluation of an optimized acellular nerve allograft with multiple axial channels. Acta Biomater. 2020, 115, 235–249. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, H.; Zhang, Z.; Lu, Y.; Huang, X.; Yang, L.; Xu, J.; Yang, W.; Fan, X.; Du, B.; et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials 2010, 31, 5312–5324. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrary, M.W.; Vaughn, N.E.; Hlavac, N.; Song, Y.H.; Wachs, R.A.; Schmidt, C.E. Novel Sodium Deoxycholate-Based Chemical Decellularization Method for Peripheral Nerve. Tissue Eng. Part C Methods 2020, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, R.C.; Gonzalez-Rothi, E.J.; Porvasnik, S.L.; Wellman, S.M.; Park, J.H.; Fuller, D.D.; Schmidt, C.E. Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord. Biomed. Mater. 2018, 13, 034110. [Google Scholar] [CrossRef] [PubMed]
- Meder, T.; Prest, T.; Skillen, C.; Marchal, L.; Yupanqui, V.T.; Soletti, L.; Gardner, P.; Cheetham, J.; Brown, B.N. Nerve-specific extracellular matrix hydrogel promotes functional regeneration following nerve gap injury. NPJ Regen. Med. 2021, 6, 69. [Google Scholar] [CrossRef]
- Groves, M.L.; McKeon, R.; Werner, E.; Nagarsheth, M.; Meador, W.; English, A.W. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp. Neurol. 2005, 195, 278–292. [Google Scholar] [CrossRef]
- Hudson, T.W.; Liu, S.Y.; Schmidt, C.E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004, 10, 1346–1358. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- De Santis, M.M.; Alsafadi, H.N.; Tas, S.; Bolukbas, D.A.; Prithiviraj, S.; Da Silva, I.A.N.; Mittendorfer, M.; Ota, C.; Stegmayr, J.; Daoud, F.; et al. Extracellular-Matrix-Reinforced Bioinks for 3D Bioprinting Human Tissue. Adv. Mater. 2021, 33, e2005476. [Google Scholar] [CrossRef]
- Gaetani, R.; Aude, S.; DeMaddalena, L.L.; Strassle, H.; Dzieciatkowska, M.; Wortham, M.; Bender, R.H.F.; Nguyen-Ngoc, K.V.; Schmid-Schoenbein, G.W.; George, S.C.; et al. Evaluation of Different Decellularization Protocols on the Generation of Pancreas-Derived Hydrogels. Tissue Eng. Part C Methods 2018, 24, 697–708. [Google Scholar] [CrossRef]
- Uhl, F.E.; Zhang, F.; Pouliot, R.A.; Uriarte, J.J.; Rolandsson Enes, S.; Han, X.; Ouyang, Y.; Xia, K.; Westergren-Thorsson, G.; Malmstrom, A.; et al. Functional role of glycosaminoglycans in decellularized lung extracellular matrix. Acta Biomater. 2020, 102, 231–246. [Google Scholar] [CrossRef]
- Fernandez-Perez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9, 14933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.-L.; Liu, S.; Sun, J.-H.; Yang, W.-H.; Xu, Y.-W.; Rao, Z.-L.; Jiang, B.; Zhu, Q.-T.; Liu, X.-L.; Wu, J.-L.; et al. Peripheral Nerve-Derived Matrix Hydrogel Promotes Remyelination and Inhibits Synapse Formation. Adv. Funct. Mater. 2018, 28, 1705739. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Andersson, G.; Oradd, G.; Sultan, F.; Novikov, L.N. In vivo Diffusion Tensor Imaging, Diffusion Kurtosis Imaging, and Tractography of a Sciatic Nerve Injury Model in Rat at 9.4T. Sci. Rep. 2018, 8, 12911. [Google Scholar] [CrossRef] [PubMed]
- Novikova, L.N.; Pettersson, J.; Brohlin, M.; Wiberg, M.; Novikov, L.N. Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials 2008, 29, 1198–1206. [Google Scholar] [CrossRef]
- Kolar, M.K.; Kingham, P.J.; Novikova, L.N.; Wiberg, M.; Novikov, L.N. The therapeutic effects of human adipose-derived stem cells in a rat cervical spinal cord injury model. Stem Cells Dev. 2014, 23, 1659–1674. [Google Scholar] [CrossRef]
- Tse, K.H.; Sun, M.; Mantovani, C.; Terenghi, G.; Downes, S.; Kingham, P.J. In vitro evaluation of polyester-based scaffolds seeded with adipose derived stem cells for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2010, 95, 701–708. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef]
- McGrath, A.M.; Brohlin, M.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Novikova, L.N. Fibrin conduit supplemented with human mesenchymal stem cells and immunosuppressive treatment enhances regeneration after peripheral nerve injury. Neurosci. Lett. 2012, 516, 171–176. [Google Scholar] [CrossRef]
- Yeh, F.C.; Wedeen, V.J.; Tseng, W.Y. Generalized q-sampling imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [PubMed]
- Griffiths, T.T.; Flather, R.; Teh, I.; Haroon, H.A.; Shelley, D.; Plein, S.; Bourke, G.; Wade, R.G. Diffusion tensor imaging in cubital tunnel syndrome. Sci. Rep. 2021, 11, 14982. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuna, V.K.; Lundgren, A.; Anerillas, L.O.; Kelk, P.; Brohlin, M.; Wiberg, M.; Kingham, P.J.; Novikova, L.N.; Andersson, G.; Novikov, L.N. Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization. Int. J. Mol. Sci. 2022, 23, 8746. https://doi.org/10.3390/ijms23158746
Kuna VK, Lundgren A, Anerillas LO, Kelk P, Brohlin M, Wiberg M, Kingham PJ, Novikova LN, Andersson G, Novikov LN. Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization. International Journal of Molecular Sciences. 2022; 23(15):8746. https://doi.org/10.3390/ijms23158746
Chicago/Turabian StyleKuna, Vijay Kumar, Andre Lundgren, Luis Oliveros Anerillas, Peyman Kelk, Maria Brohlin, Mikael Wiberg, Paul J. Kingham, Ludmila N. Novikova, Gustav Andersson, and Lev N. Novikov. 2022. "Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization" International Journal of Molecular Sciences 23, no. 15: 8746. https://doi.org/10.3390/ijms23158746