Recombinant Human Arresten and Canstatin Inhibit Angiogenic Behaviors of HUVECs via Inhibiting the PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Construction, Expression, and Identification of Recombinant Human Arresten and Canstatin
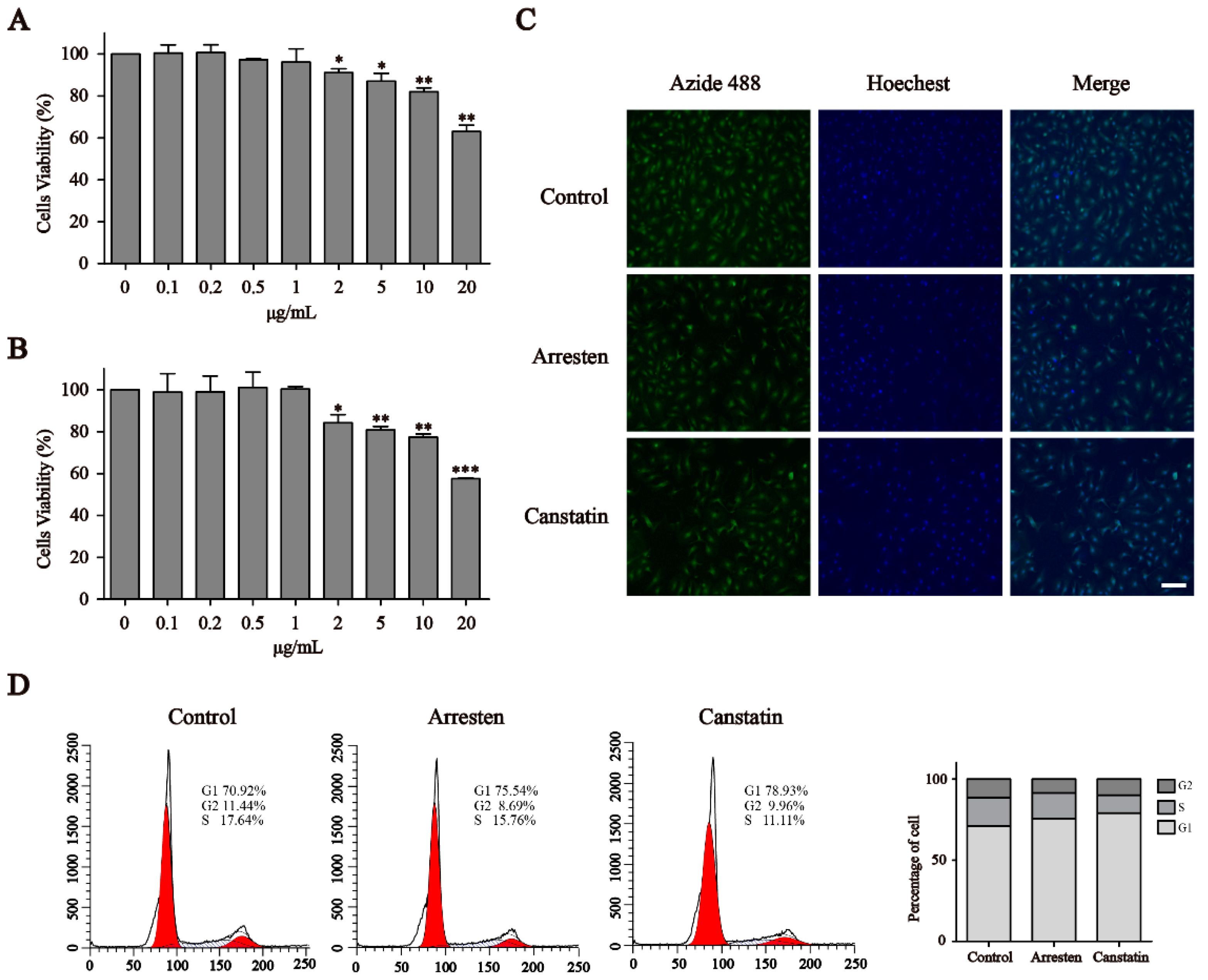
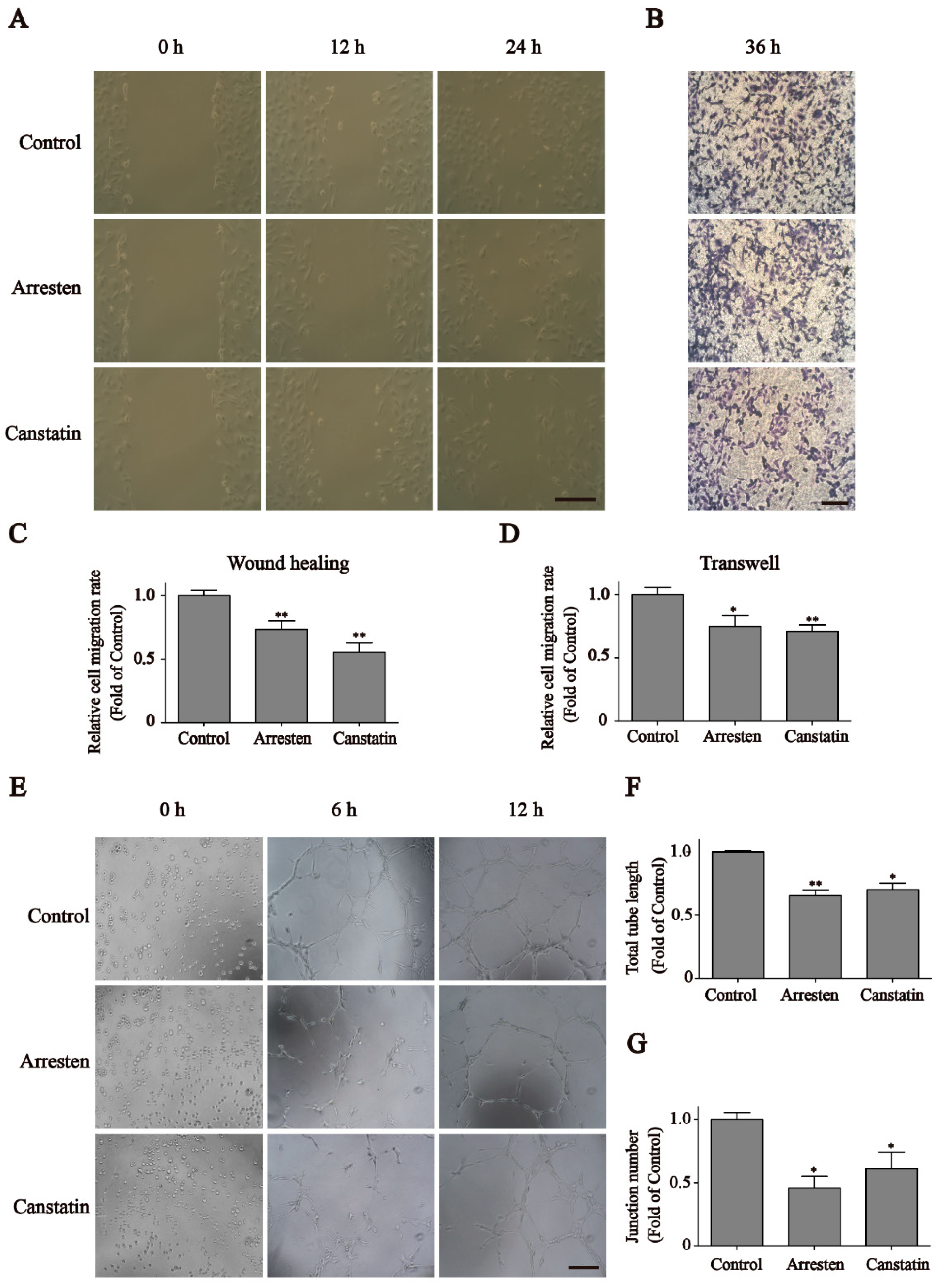
2.2. Effects of Recombinant Human Arresten and Canstatin on Proliferation and Migration of HUVECs
2.3. Effects of Recombinant Human Arresten and Canstatin on Tube Formation of HUVECs
2.4. Effects of Recombinant Arresten and Canstatin on PI3K/Akt Signaling Pathway in HUVECs
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Construction of Expression Plasmids
4.3. Recombinant Expression and Purification of Arresten and Canstatin
4.4. Cell Culture
4.5. CCK-8 Assay
4.6. EdU Assay
4.7. Flow Cytometry Analysis
4.8. Wound Healing Assay
4.9. Transwell Assay
4.10. Tube Formation Assay
4.11. Western Blot Assay
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Colorado, P.C.; Torre, A.; Kamphaus, G.; Maeshima, Y.; Hopfer, H.; Takahashi, K.; Volk, R.; Zamborsky, E.D.; Herman, S.; Sarkar, P.K.; et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000, 60, 2520–2526. [Google Scholar]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57, 1–11. [Google Scholar]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar]
- Streit, M.; Riccardi, L.; Velasco, P.; Brown, L.F.; Hawighorst, T.; Bornstein, P.; Detmar, M. Thrombospondin-2: A potent endogenous inhibitor of tumor growth and angiogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 14888–14893. [Google Scholar]
- Kawaguchi, T.; Yamashita, Y.; Kanamori, M.; Endersby, R.; Bankiewicz, K.S.; Baker, S.J.; Bergers, G.; Pieper, R.O. The PTEN/Akt pathway dictates the direct alphaVbeta3-dependent growth-inhibitory action of an active fragment of tumstatin in glioma cells in vitro and in vivo. Cancer Res. 2006, 66, 11331–11340. [Google Scholar]
- Petitclerc, E.; Boutaud, A.; Prestayko, A.; Xu, J.; Sado, Y.; Ninomiya, Y.; Sarras, M.P., Jr.; Hudson, B.G.; Brooks, P.C. New functions for non-collagenous domains of human collagen type IV: Novel integrin ligands inhibiting angiogenesis and tumor growthin vivo. J. Biol. Chem. 2000, 275, 8051–8061. [Google Scholar]
- Kamphaus, G.D.; Colorado, P.C.; Panka, D.J.; Hopfer, H.; Ramchandran, R.; Torre, A.; Maeshima, Y.; Mier, J.W.; Sukhatme, V.P.; Kalluri, R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J. Biol. Chem. 2000, 275, 1209–1215. [Google Scholar]
- Maragoudakis, M.E.; Missirlis, E.; Karakiulakis, G.D.; Sarmonica, M.; Bastakis, M.; Tsopanoglou, N. Basement membrane biosynthesis as a target for developing inhibitors of angiogenesis with anti-tumor properties. Kidney Int. 1993, 43, 147–150. [Google Scholar]
- Mundel, T.M.; Kalluri, R. Type IV collagen-derived angiogenesis inhibitors. Microvasc. Res. 2007, 74, 85–89. [Google Scholar]
- Zeng, Z.-C.; He, A.-B.; Ma, L.-X.; Liao, F. Expression and identification of recombinant arresten in Pichia pastoris. Chin. J. Biotechnol. 2003, 19, 572–576. [Google Scholar]
- Hwang-Bo, J.; Yoo, K.H.; Park, J.-H.; Jeong, H.-S.; Chung, I.S. Recombinant canstatin inhibits angiopoietin-1-induced angiogenesis and lymphangiogenesis. Int. J. Cancer 2012, 131, 298–309. [Google Scholar]
- Li, W.; Song, Z.; Zheng, Q.; Xiong, J.; Shang, D.; Guan, S.; Shu, X. Construction of eukaryotic expression vector of human arresten gene and its secreted expression in HEK 293 cells. Front. Med. China 2009, 3, 297–302. [Google Scholar]
- Nyberg, P.; Xie, L.; Sugimoto, H.; Colorado, P.; Sund, M.; Holthaus, K.; Sudhakar, A.; Salo, T.; Kalluri, R. Characterization of the anti-angiogenic properties of arresten, an α1β1 integrin-dependent collagen-derived tumor suppressor. Exp. Cell Res. 2008, 314, 3292–3305. [Google Scholar]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar]
- Rao, N.; Lee, Y.F.; Ge, R. Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol. Sin. 2015, 36, 1177–1190. [Google Scholar]
- Chen, J.; Sivan, U.; Tan, S.L.; Lippo, L.; De Angelis, J.; Labella, R.; Singh, A.; Chatzis, A.; Cheuk, S.; Medhghalchi, M.; et al. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci. Adv. 2021, 7, eabd7819. [Google Scholar]
- Singh, A.; Veeriah, V.; Xi, P.; Labella, R.; Chen, J.; Romeo, S.G.; Ramasamy, S.; Kusumbe, A.P. Angiocrine signals regulate quiescence and therapy resistance in bone metastasis. JCI insight 2019, 4, e125679. [Google Scholar]
- Chen, J.; Lippo, L.; Labella, R.; Tan, S.L.; Marsden, B.D.; Dustin, M.L.; Ramasamy, S.K.; Kusumbe, A. P Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system. EMBO J. 2021, 40, e105242. [Google Scholar]
- Grunewald, M.; Kumar, S.; Sharife, H.; Volinsky, E.; Gileles-Hillel, A.; Licht, T.; Permyakova, A.; Hinden, L.; Azar, S.; Friedmann, Y.; et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 2021, 373, eabc8479. [Google Scholar]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar]
- Gacche, R.N.; Meshram, R.J. Angiogenic factors as potential drug target: Efficacy and limitations of anti-angiogenic therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1846, 161–179. [Google Scholar]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance mechanisms to anti-angiogenic therapies in cancer. Front. Oncol. 2020, 10, 221. [Google Scholar]
- Limaverde-Sousa, G.; Sternberg, C.; Ferreira, C.G. Antiangiogenesis beyond VEGF inhibition: A journey from antiangiogenic single-target to broad-spectrum agents. Cancer Treat. Rev. 2014, 40, 548–557. [Google Scholar]
- Yin, H.; Liu, Z.; Zhang, A.; Zhang, T.; Luo, J.; Shen, J.; Chen, L.; Zhou, B.; Fu, X.; Fu, C.; et al. Intracellular expression and purification of the Canstatin-N protein in Pichia pastoris. Gene 2012, 504, 122–126. [Google Scholar]
- Dhanabal, M.; Volk, R.; Ramchandran, R.; Simons, M.; Sukhatme, V.P. Cloning, Expression, andin VitroActivity of Human Endostatin. Biochem. Biophys. Res. Commun. 1999, 258, 345–352. [Google Scholar]
- Fan, C.; Feng, L.; Fan, J. Recent advances on the expression systems for recombinant protein production. Biotechnology 2012, 22, 76–80. [Google Scholar]
- Du, C.; Yi, X.; Zhang, Y. Expression and purification of soluble recombinant human endostatin in Escherichia coli. Biotechnol. Bioprocess Eng. 2010, 15, 229–235. [Google Scholar]
- Wei, D.-M.; Gao, Y.; Cao, X.-R.; Zhu, N.-C.; Liang, J.-F.; Xie, W.-P.; Zhen, M.-Y.; Zhu, M.-S. Soluble multimer of recombinant endostatin expressed in E. coli has anti-angiogenesis activity. Biochem. Biophys. Res. Commun. 2006, 345, 1398–1404. [Google Scholar]
- Lu, W.-J.; Liang, H.-B.; Li, Y.-F.; Tu, X.-Q.; He, J.-R.; Ding, K.-Q.; Yang, G.-Y.; Xin, X.-Y.; Zeng, L.-L. MicroRNA-210-3p targets RGMA to enhance the angiogenic functions of endothelial progenitor cells under hypoxic conditions. Front. Cell. Neurosci. 2019, 13, 223. [Google Scholar]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; Mousa, S.A.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: A potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003, 63, 2020–2023. [Google Scholar]
- Kanazawa, H.; Imoto, K.; Okada, M.; Yamawaki, H. Canstatin inhibits hypoxia-induced apoptosis through activation of integrin/focal adhesion kinase/Akt signaling pathway in H9c2 cardiomyoblasts. PLoS ONE 2017, 12, e0173051. [Google Scholar]
- Turaga, R.C.; Yin, L.; Yang, J.J.; Lee, H.; Ivanov, I.; Yan, C.; Yang, H.; Grossniklaus, H.E.; Wang, S.; Ma, C.; et al. Rational design of a protein that binds integrin αvβ3 outside the ligand binding site. Nat. Commun. 2016, 7, 1–11. [Google Scholar]
- Guan, J.-L.; Shalloway, D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature 1992, 358, 690–692. [Google Scholar]
- Ben-Mahdi, M.H.; Dang, P.M.-C.; Gougerot-Pocidalo, M.-A.; O’Dowd, Y.; El-Benna, J.; Pasquier, C. Xanthine oxidase-derived ROS display a biphasic effect on endothelial cells adhesion and FAK phosphorylation. Oxidative Med. Cell. Longev. 2016, 2016, 9346242. [Google Scholar]
- Duan, M.X.; Zhou, H.; Wu, Q.Q.; Liu, C.; Xiao, Y.; Deng, W.; Tang, Q.Z. Andrographolide protects against HG-induced inflammation, apoptosis, migration, and impairment of angiogenesis via PI3K/AKT-eNOS signalling in HUVECs. Mediat. Inflamm. 2019, 2019, 6168340. [Google Scholar]
- McPhail, J.A.; Burke, J.E. Drugging the phosphoinositide 3-kinase (PI3K) and phosphatidylinositol 4-kinase (PI4K) family of enzymes for treatment of cancer, immune disorders, and viral/parasitic infections. Druggable Lipid Signal. Pathw. 2020, 1274, 203–222. [Google Scholar]
- Zhao, J.; Zhang, X.; Gao, T.; Wang, S.; Hou, Y.; Yuan, P.; Yang, Y.; Yang, T.; Xing, J.; Li, J.; et al. SIK2 enhances synthesis of fatty acid and cholesterol in ovarian cancer cells and tumor growth through PI3K/Akt signaling pathway. Cell Death Dis. 2020, 11, 25. [Google Scholar]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar]
- Zhu, Y.-B.; Zhang, Y.-P.; Zhang, J.; Zhang, Y.-B. Evaluation of vitamin C supplementation on kidney function and vascular reactivity following renal ischemic injury in mice. Kidney Blood Press. Res. 2016, 41, 460–470. [Google Scholar]
- Yan, X.; Hui, Y.; Hua, Y.; Huang, L.; Wang, L.; Peng, F.; Tang, C.; Liu, D.; Song, J.; Wang, F. EG-VEGF silencing inhibits cell proliferation and promotes cell apoptosis in pancreatic carcinoma via PI3K/AKT/mTOR signaling pathway. Biomed. Pharmacother. 2019, 109, 762–769. [Google Scholar]
- Kang, H.-Y.; Shim, D.-H.; Kang, S.-S.; Chang, S.-I.; Kim, H.-Y. Protein kinase B inhibits endostatin-induced apoptosis in HUVECs. BMB Rep. 2006, 39, 97–104. [Google Scholar]



| Primers | Sequences (5′-3′) |
|---|---|
| arr-f | CATGCCATGGATGTCAGTTGATCACGGCTTC |
| arr-r | GGGAAGCTTGGTACGACGCATGCAAACC |
| can-f | CATGCCATGGATGGTGAGCATCGGCTACCT |
| can-r | GGGAAGCTTCAGGTTTTTCATGCAAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Guo, Z.; Zhang, J.; Yang, Y.; Liu, C.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. Recombinant Human Arresten and Canstatin Inhibit Angiogenic Behaviors of HUVECs via Inhibiting the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8995. https://doi.org/10.3390/ijms23168995
Zhu L, Guo Z, Zhang J, Yang Y, Liu C, Zhang L, Gu Z, Li Y, Ding Z, Shi G. Recombinant Human Arresten and Canstatin Inhibit Angiogenic Behaviors of HUVECs via Inhibiting the PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(16):8995. https://doi.org/10.3390/ijms23168995
Chicago/Turabian StyleZhu, Lingyu, Zitao Guo, Ji Zhang, Yuliang Yang, Chunyu Liu, Liang Zhang, Zhenghua Gu, Youran Li, Zhongyang Ding, and Guiyang Shi. 2022. "Recombinant Human Arresten and Canstatin Inhibit Angiogenic Behaviors of HUVECs via Inhibiting the PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 23, no. 16: 8995. https://doi.org/10.3390/ijms23168995






