Increased Epidermal Nerve Growth Factor without Small-Fiber Neuropathy in Dermatomyositis
Abstract
:1. Introduction
2. Results
2.1. Patients Characteristics
2.2. A Significant Reduction in IENFD in Patients with CLE, but No Change in IENFD in Patients with DM
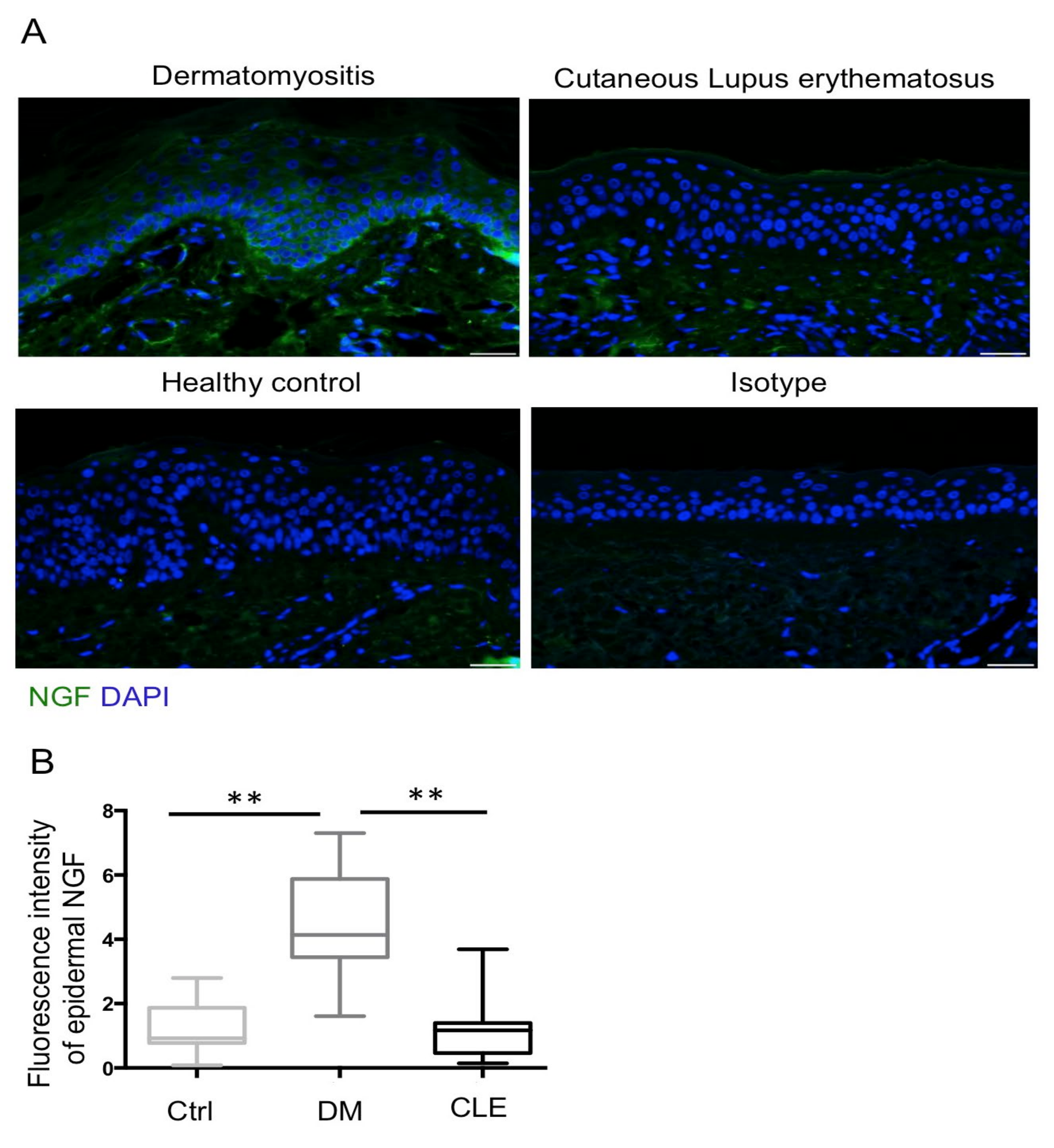
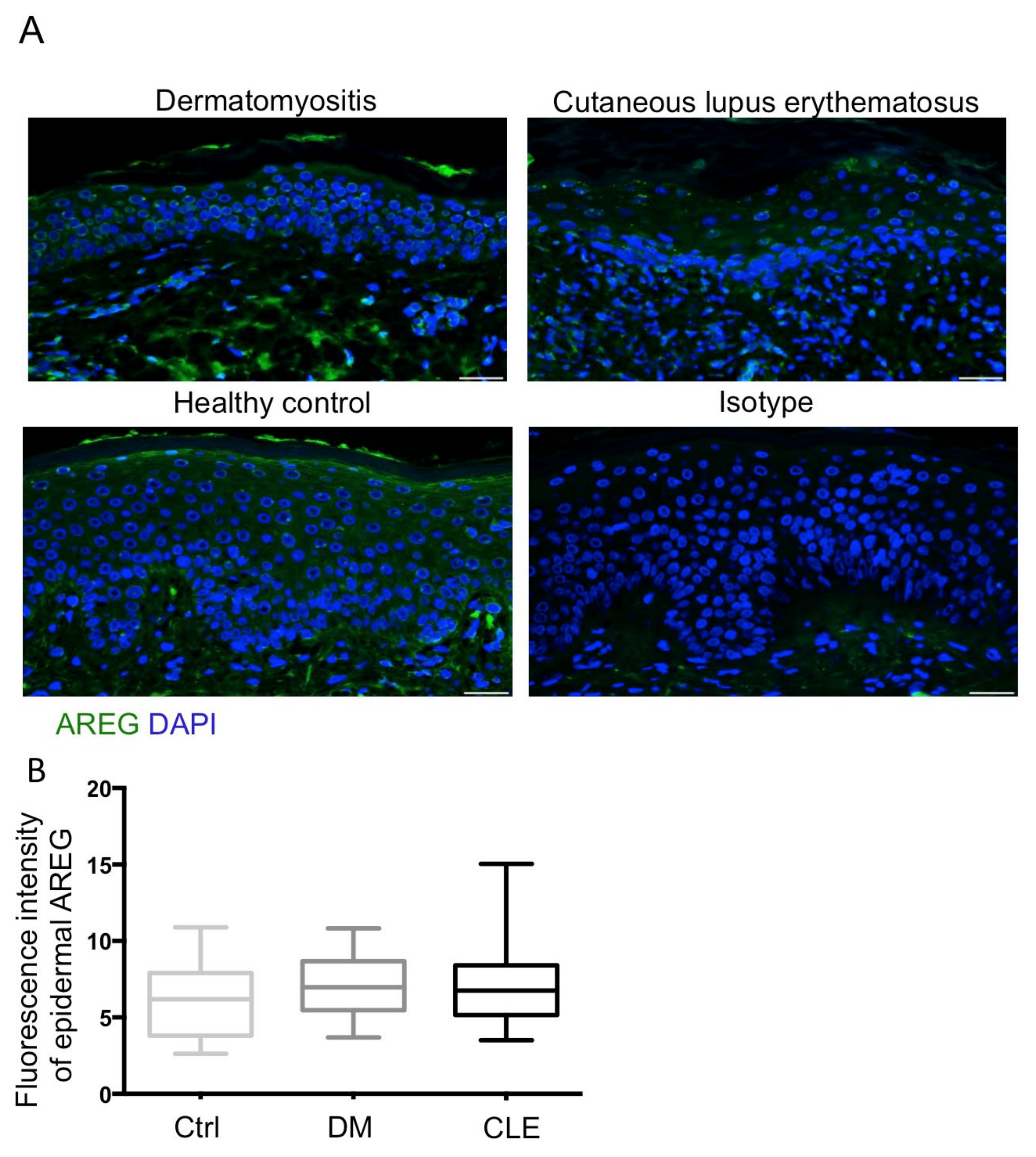
2.3. Upregulation of Epidermal NGF with No Change of AREG in Patients with DM
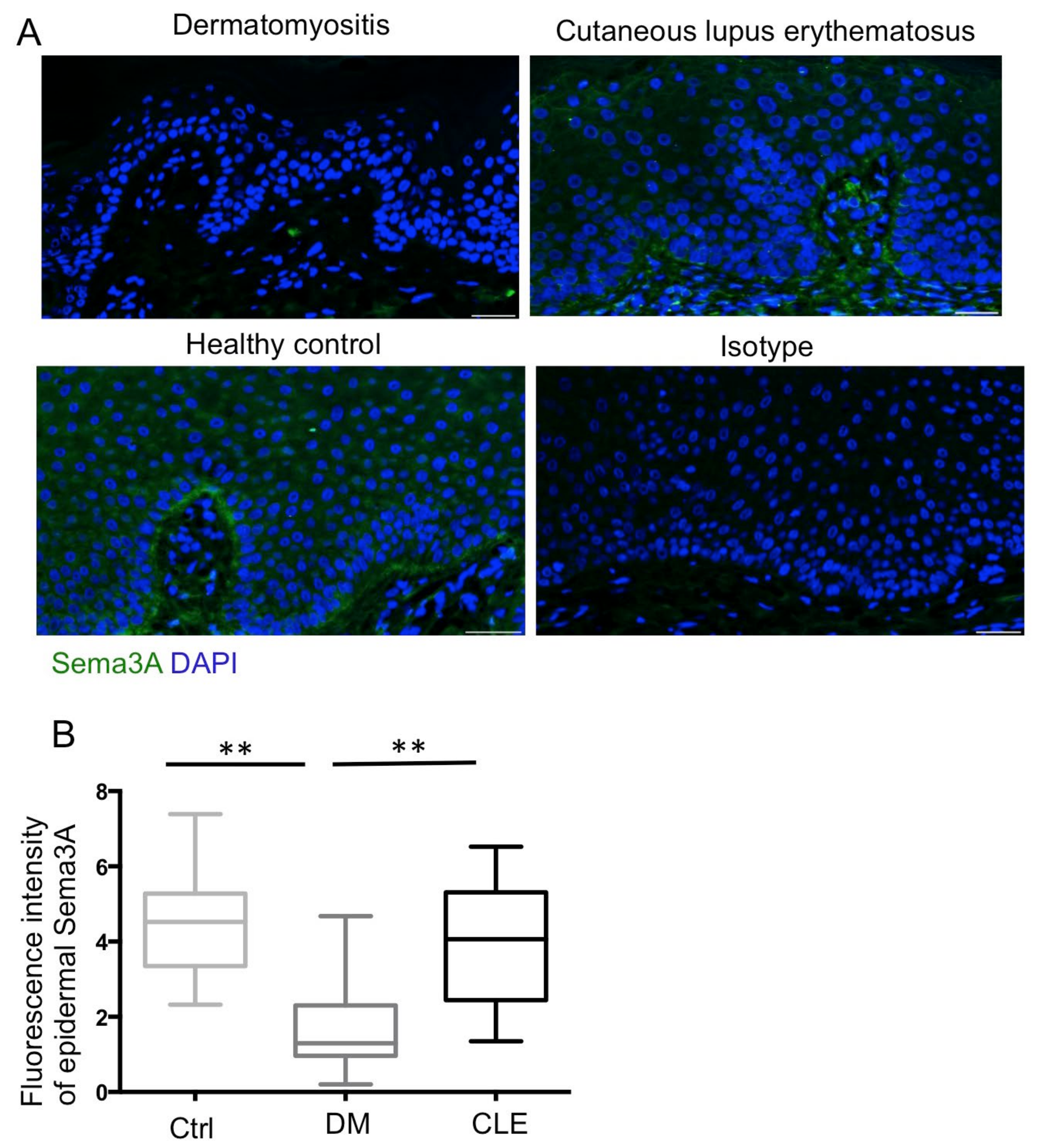
2.4. Reduced Expression of Epidermal Sema3A in Patients with DM
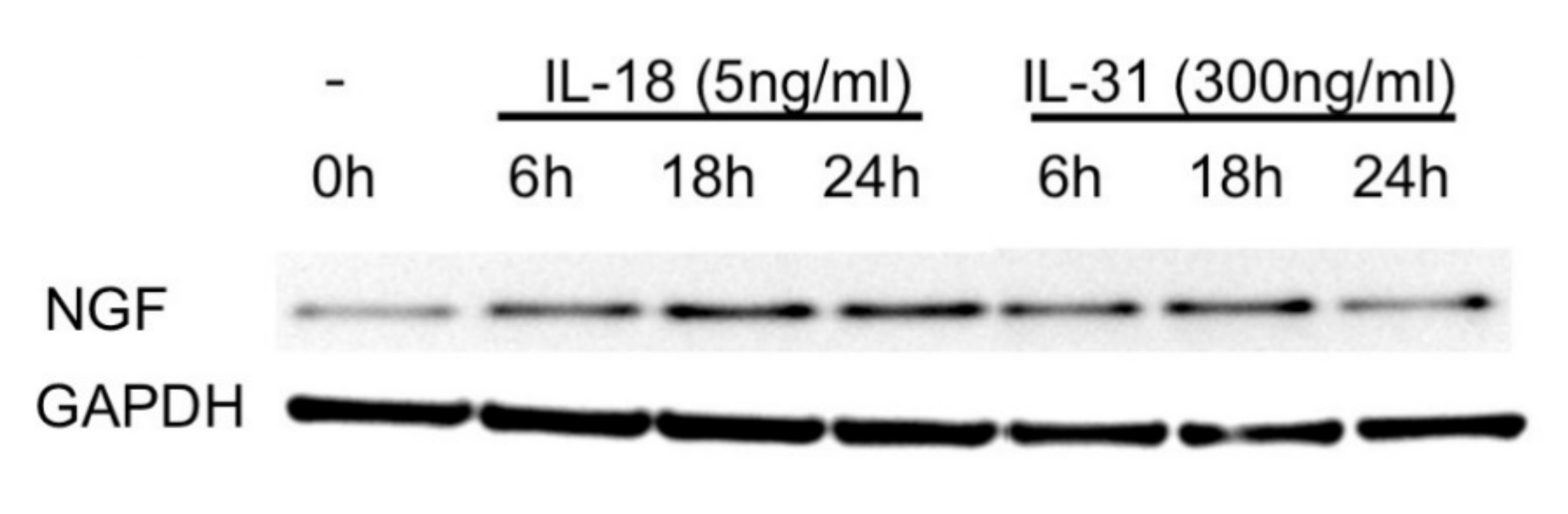
2.5. IL-18 and IL-31 Both Induced NGF Expression in Cultured Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Skin Samples
4.2. Histological Analysis
4.3. Antibodies
4.4. IENFD and Semi-Quantitative Immunofluorescence Measurements
4.5. Cell Culture
4.6. Western Blot Analysis
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, M.P.; Wiegmann, H.; Agelopoulos, K.; Ständer, S. Neuropathic itch: Routes to clinical diagnosis. Front. Med. 2021, 8, 641746. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Tan, A.; LoSicco, K.; Manabat, C.G.; Kannagra, A.; Carroll, C.; Chan, Y.H.; Ng, P.; Jorizzo, J. A comparative study of clinical characteristics, work-up, treatment, and association to malignancy in dermatomyositis between two tertiary skin centers in the USA and singapore. Int. J. Dermatol. 2013, 52, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Gourier, G.; Théréné, C.; Mazeas, M.; Abasq-Thomas, C.; Brenaut, E.; Huet, F.; Sonbol, H.; Campillo, E.; Lemerle, J.; Pasquier, E.; et al. Clinical characteristics of pruritus in systemic sclerosis vary according to the autoimmune subtype. Acta Derm. Venereol. 2018, 98, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Samotij, D.; Szczęch, J.; Kushner, C.J.; Mowla, M.R.; Dańczak-Pazdrowska, A.; Antiga, E.; Chasset, F.; Furukawa, F.; Hasegawa, M.; Hashizume, H.; et al. Prevalence of pruritus in cutaneous lupus erythematosus: Brief report of a multicenter, multinational cross-sectional study. BioMed Res. Int. 2018, 2018, 3491798. [Google Scholar] [CrossRef]
- Samotij, D.; Szczęch, J.; Antiga, E.; Bonciani, D.; Caproni, M.; Chasset, F.; Dańczak-Pazdrowska, A.; Furukawa, F.; Hasegawa, M.; Hashizume, H.; et al. Clinical characteristics of itch in cutaneous lupus erythematosus: A prospective, multicenter, multinational, cross-sectional study. Lupus 2021, 30, 1385–1393. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Sensitization of itch signaling: Itch sensitization-nerve growth factor, semaphorins. In Itch: Mechanisms and Treatment; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef]
- Bobko, S.; Zeidler, C.; Osada, N.; Riepe, C.; Pfleiderer, B.; Pogatzki-Zahn, E.; Lvov, A.; Ständer, S. Intraepidermal nerve fibre density is decreased in lesional and inter-lesional prurigo nodularis and reconstitutes on healing of lesions. Acta Derm. Venereol. 2016, 96, 404–406. [Google Scholar] [CrossRef]
- Tekatas, A.; Tekatas, D.D.; Solmaz, V.; Karaca, T.; Pamuk, O.N. Small fiber neuropathy and related factors in patients with systemic lupus erythematosus; the results of cutaneous silent period and skin biopsy. Adv. Rheumatol. 2020, 60, 31. [Google Scholar] [CrossRef]
- Hurliman, E.; Groth, D.; Wendelschafer-Crabb, G.; Kennedy, W.; Kavand, S.; Ericson, M.; Hordinsky, M. Small-fibre neuropathy in a patient with dermatomyositis and severe scalp pruritus. Br. J. Dermatol. 2016, 176, 209–211. [Google Scholar] [CrossRef]
- Wong, L.-S.; Yen, Y.-T.; Lee, C.-H. The implications of pruritogens in the pathogenesis of atopic dermatitis. Int. J. Mol. Sci. 2021, 22, 7227. [Google Scholar] [CrossRef]
- Obreja, O.; Rukwied, R.; Nagler, L.; Schmidt, M.; Schmelz, M.; Namer, B. Nerve growth factor locally sensitizes nociceptors in human skin. Pain 2017, 159, 416–426. [Google Scholar] [CrossRef]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. Ngf and its receptors in the regulation of inflammatory response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef]
- Gono, T.; Kawaguchi, Y.; Sugiura, T.; Ichida, H.; Takagi, K.; Katsumata, Y.; Hanaoka, M.; Okamoto, Y.; Ota, Y.; Yamanaka, H. Interleukin-18 is a key mediator in dermatomyositis: Potential contribution to development of interstitial lung disease. Rheumatology 2010, 49, 1878–1881. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Gharaee-Kermani, M.; Berthier, C.C.; Nault, T.; Hile, G.A.; Estadt, S.N.; Patrick, M.T.; Wasikowski, R.; Billi, A.C.; Lowe, L.; et al. IL18-containing 5-gene signature distinguishes histologically identical dermatomyositis and lupus erythematosus skin lesions. JCI Insight 2020, 5, e139558. [Google Scholar] [CrossRef]
- Kim, H.J.; Zeidi, M.; Bonciani, D.; Pena, S.M.; Tiao, J.; Sahu, S.; Werth, V.P. Itch in dermatomyositis: The role of increased skin interleukin-31. Br. J. Dermatol. 2018, 179, 669–678. [Google Scholar] [CrossRef]
- Kawakami, T.; Ishihara, M.; Mihara, M. Distribution density of intraepidermal nerve fibers in normal human skin. J. Dermatol. 2001, 28, 63–70. [Google Scholar] [CrossRef]
- Wang, L.; Hilliges, M.; Jernberg, T.; Wiegleb-Edström, D.; Johansson, O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res. 1990, 261, 26–33. [Google Scholar] [CrossRef]
- Raasing, L.R.M.; Vogels, O.J.M.; Veltkamp, M.; van Swol, C.F.P.; Grutters, J.C. Current view of diagnosing small fiber neuropathy. J. Neuromuscul. Dis. 2021, 8, 185–207. [Google Scholar] [CrossRef]
- Gøransson, L.G.; Tjensvoll, A.B.; Herigstad, A.; Mellgren, S.I.; Omdal, R. Small-diameter nerve fiber neuropathy in systemic lupus erythematosus. Arch. Neurol. 2006, 63, 401–404. [Google Scholar] [CrossRef]
- McGlasson, S.; Wiseman, S.; Wardlaw, J.; Dhaun, N.; Hunt, D.P.J. Neurological disease in lupus: Toward a personalized medicine approach. Front. Immunol. 2018, 9, 1146. [Google Scholar] [CrossRef]
- Nockher, W.A.; Renz, H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006, 117, 583–589. [Google Scholar] [CrossRef]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef]
- Raychaudhuri, S.P.; Jiang, W.-Y.; Raychaudhuri, S.K. Revisiting the koebner phenomenon. Am. J. Pathol. 2008, 172, 961–971. [Google Scholar] [CrossRef]
- Johansson, O.; Liang, Y.; Emtestam, L. Increased nerve growth factor- and tyrosine kinase a-like immunoreactivities in prurigo nodularis skin–an exploration of the cause of neurohyperplasia. Arch. Dermatol. Res. 2002, 293, 614–619. [Google Scholar] [CrossRef]
- Hirth, M.; Rukwied, R.; Gromann, A.; Turnquist, B.; Weinkauf, B.; Francke, K.; Albrecht, P.; Rice, F.; Hägglöf, B.; Ringkamp, M.; et al. Nerve growth factor induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain 2013, 154, 2500–2511. [Google Scholar] [CrossRef]
- Solinski, H.J.; Rukwied, R.; Schmelz, M. Microinjection of pruritogens in NGF-sensitized human skin. Sci. Rep. 2021, 11, 21490. [Google Scholar] [CrossRef]
- Goshima, Y.; Sasaki, Y.; Nakayama, T.; Ito, T.; Kimura, T. Functions of semaphorins in axon guidance and neuronal regeneration. Jpn. J. Pharmocol. 2000, 82, 273–279. [Google Scholar] [CrossRef]
- Vadasz, Z.; Toubi, E. Semaphorin3A: A potential therapeutic tool in immune-mediated diseases. Eur. J. Rheumatol. 2018, 5, 58–61. [Google Scholar] [CrossRef]
- Iragavarapu-Charyulu, V.; Wojcikiewicz, E.; Urdaneta, A. Semaphorins in angiogenesis and autoimmune diseases: Therapeutic targets? Front. Immunol. 2020, 11, 346. [Google Scholar] [CrossRef]
- Liu, L.-N.; Li, X.-M.; Ye, D.-Q.; Pan, H.-F. Emerging role of semaphorin-3A in autoimmune diseases. Inflammopharmacology 2018, 26, 655–665. [Google Scholar] [CrossRef]
- Vadasz, Z.; Ben-Izhak, O.; Bejar, J.; Sabo, E.; Kessel, A.; Storch, S.; Toubi, E. The involvement of immune semaphorins and neuropilin-1 in lupus nephritis. Lupus 2011, 20, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J. Immunol. 2010, 185, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Q.; Tanelian, D.L.; Smith, G.M. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 2004, 24, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Dontchev, V.; Letourneau, P.C. Nerve growth factor and semaphorin 3a signaling pathways interact in regulating sensory neuronal growth cone motility. J. Neurosci. 2002, 22, 6659–6669. [Google Scholar] [CrossRef]
- Tominaga, M.; Ogawa, H.; Takamori, K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br. J. Dermatol. 2008, 158, 842–844. [Google Scholar] [CrossRef]
- Hayashi, M.; Kamiya, Y.; Itoh, H.; Higashi, T.; Miyazaki, T.; Funakoshi, K.; Yamashita, N.; Goshima, Y.; Andoh, T.; Yamada, Y.; et al. Intrathecally administered sema3A protein attenuates neuropathic pain behavior in rats with chronic constriction injury of the sciatic nerve. Neurosci. Res. 2011, 69, 17–24. [Google Scholar] [CrossRef]
- Shadrach, J.L.; Pierchala, B.A. Semaphorin3a signaling is dispensable for motor axon reinnervation of the adult neuromuscular junction. eNeuro 2018, 5, ENEURO.0155–17.2018. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Nakanishi, K. Unique action of interleukin-18 on t cells and other immune cells. Front. Immunol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Hawro, T.; Saluja, R.; Weller, K.; Altrichter, S.; Metz, M.; Maurer, M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy 2014, 69, 113–117. [Google Scholar] [CrossRef]
- Pereira, M.P.; Mühl, S.; Pogatzki-Zahn, E.M.; Agelopoulos, K.; Ständer, S. Intraepidermal nerve fiber density: Diagnostic and therapeutic relevance in the management of chronic pruritus: A review. Dermatol. Ther. 2016, 6, 509–517. [Google Scholar] [CrossRef]
- Lee, C.H.; Hong, C.H.; Yu, W.T.; Chuang, H.Y.; Huang, S.K.; Chen, G.S.; Yoshioka, T.; Sakata, M.; Liao, W.T.; Ko, Y.C.; et al. Mechanistic correlations between two itch biomarkers, cytokine interleukin-31 and neuropeptide β-endorphin, via STAT3/calcium axis in atopic dermatitis. Br. J. Dermatol. 2012, 167, 794–803. [Google Scholar] [CrossRef]

| Healthy Control (n = 23) | DM (n = 35) | CLE (n = 29) | |
|---|---|---|---|
| Face IENF (/mm) | 47.1 +/− 21.3 (n = 6) | 45.7 +/− 21.5 (n = 5) | 11.5 +/− 7.3 * (n = 19) |
| Trunk IENF (/mm) | 34.2 +/− 9.2 (n = 13) | 37.2 +/− 9.9 (n = 10) | 9.5 +/− 12.2 * (n = 4) |
| Limbs IENF (/mm) | 34.7 +/− 7.7 (n = 4) | 42.6 +/− 12.9 (n = 20) | 9.9 +/− 6.1 * (n = 6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, L.-S.; Lee, C.-H.; Yen, Y.-T. Increased Epidermal Nerve Growth Factor without Small-Fiber Neuropathy in Dermatomyositis. Int. J. Mol. Sci. 2022, 23, 9030. https://doi.org/10.3390/ijms23169030
Wong L-S, Lee C-H, Yen Y-T. Increased Epidermal Nerve Growth Factor without Small-Fiber Neuropathy in Dermatomyositis. International Journal of Molecular Sciences. 2022; 23(16):9030. https://doi.org/10.3390/ijms23169030
Chicago/Turabian StyleWong, Lai-San, Chih-Hung Lee, and Yu-Ta Yen. 2022. "Increased Epidermal Nerve Growth Factor without Small-Fiber Neuropathy in Dermatomyositis" International Journal of Molecular Sciences 23, no. 16: 9030. https://doi.org/10.3390/ijms23169030






