Stage-Specific Genetic Interaction between FgYCK1 and FgBNI4 during Vegetative Growth and Conidiation in Fusarium graminearum
Abstract
:1. Introduction
2. Results
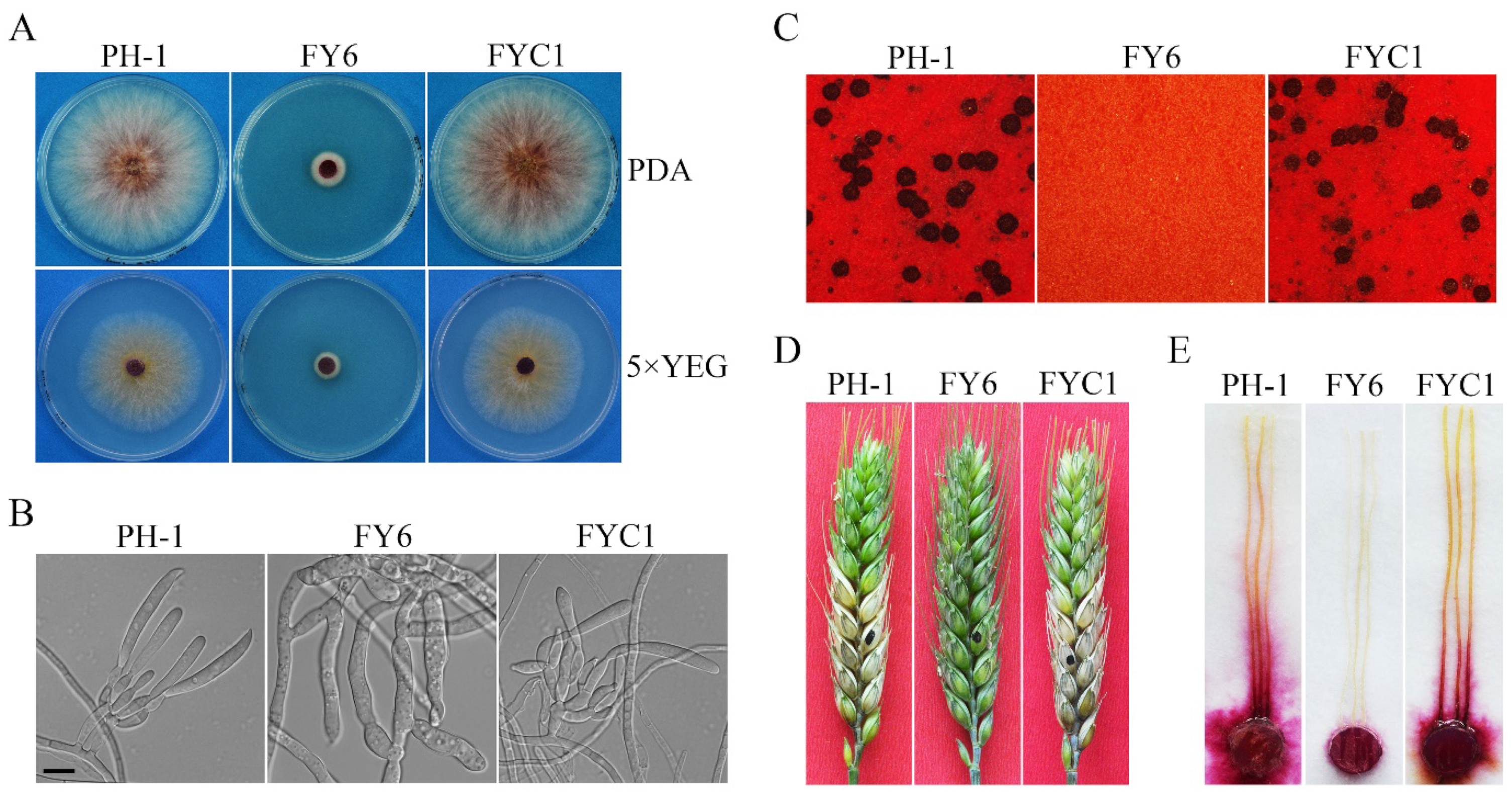
2.1. FgYCK1 Is Important for Vegetative Growth, Sexual/Asexual Development, and Pathogenesis
2.2. Spontaneous Suppressors of Fgyck1 Are Partially Recovered in Growth Rate and Conidiation
2.3. Identification of Mutations in Spontaneous Suppressor Strains of Fgyck1

2.4. The R699C Mutation in FgBNI4 Is Verified for Its Suppressive Effect on Fgyck1
2.5. Deletion of Entire FgBNI4 and Truncation of Its CCT Region Have the Same Suppressive Effect on the Fgyck1 Mutant
2.6. FgBNI4 Is Involved in Cell Wall Integrity and Hyphal Growth

2.7. The Fgyck1 Mutant Is Defective in Maintaining Polarized Growth
2.8. The Defect of Fgyck1 in Polarized Growth Is Alleviated by Deletion of FgBNI4
2.9. Deletion of FgBNI4 Alleviates the Defects of Fgyck1 in FgRho1 Internalization and Vacuolar Fusion
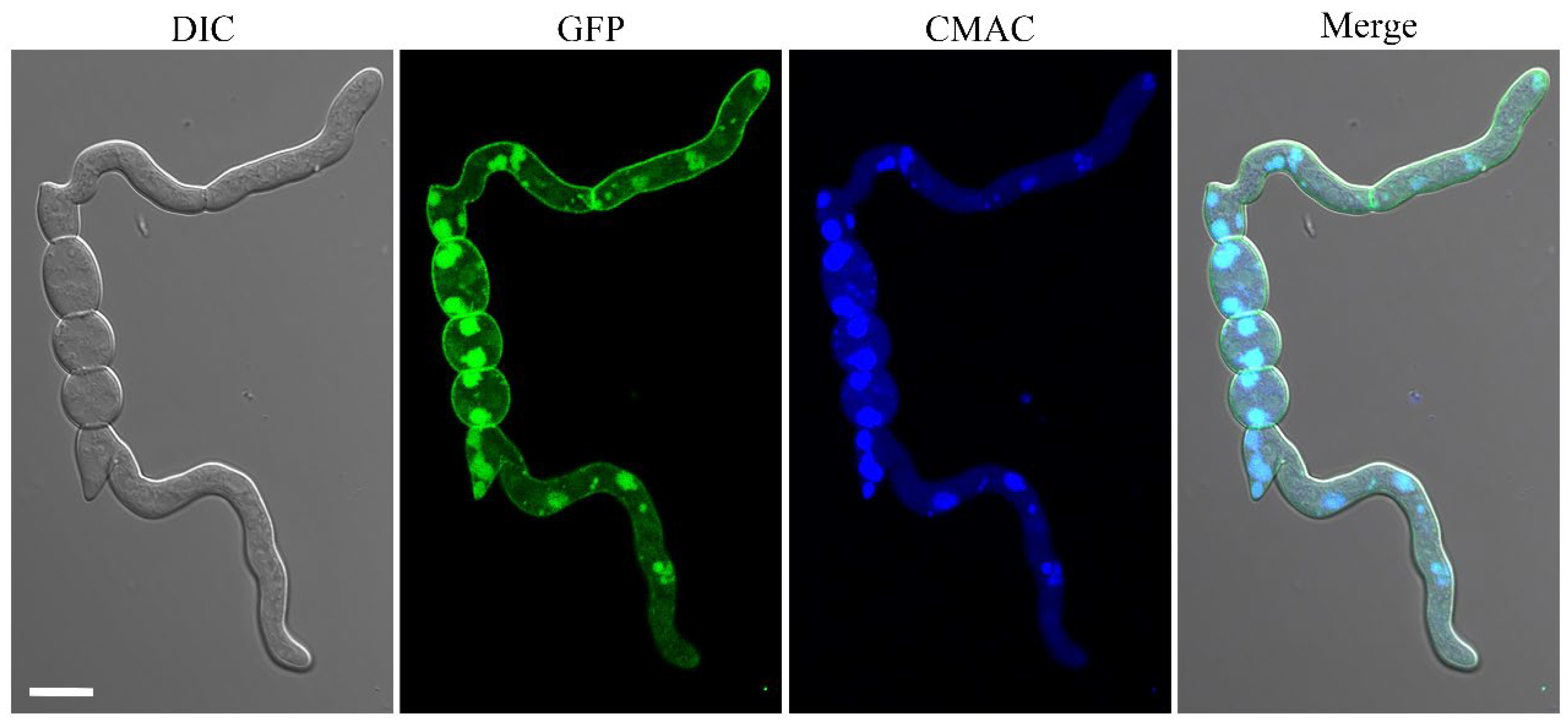
2.10. The FgYck1 Localizes to Plasma Membrane and Vacuolar Lumen
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Trail, F.; Gaffoor, I.; Vogel, S. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fuarium graminearum). Fungal Genet. Biol. 2005, 42, 528–533. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Broz, K.L.; Purvine, S.O.; Chrisler, W.B.; Nicora, C.D.; Connolly, L.R.; Freitag, M.; Baker, S.E.; Kistler, H.C. Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Sci. Rep. 2017, 7, 44296. [Google Scholar] [CrossRef]
- Lozowicka, B.; Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Kuldybayev, N.; Dutbayev, Y. Impact of diversified chemical and biostimulator protection on yield, health status, mycotoxin level, and economic profitability in spring wheat (Triticum aestivum L.) cultivation. Agronomy 2022, 12, 258. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Hou, R.; Zhao, Z.; Zheng, Q.; Xu, Q.; Zheng, D.; Wang, G.; Liu, H.; Gao, X.; et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog. 2011, 7, e1002460. [Google Scholar] [CrossRef]
- Li, B.; Dong, X.; Li, X.; Chen, H.; Zhang, H.; Zheng, X.; Zhang, Z. A subunit of the HOPS endocytic tethering complex, FgVps41, is important for fungal development and plant infection in Fusarium graminearum. Environ. Microbiol. 2018, 20, 1436–1451. [Google Scholar] [CrossRef]
- Tuazon, P.T.; Traugh, J.A. Casein kinase I and II-multipotential serine protein kinases: Structure, function, and regulation. Adv. Second. Messenger Phosphoprot. Res. 1991, 23, 123–164. [Google Scholar]
- Guerra, B.; Issinger, O.G. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 1999, 20, 391–408. [Google Scholar] [CrossRef]
- Cheong, J.K.; Virshup, D.M. Casein kinase 1: Complexity in the family. Int. J. Biochem. Cell Biol. 2011, 43, 465–469. [Google Scholar] [CrossRef]
- Knippschild, U.; Gocht, A.; Wolff, S.; Huber, N.; Löhler, J.; Stöter, M. The casein kinase 1 family: Participation in multiple cellular processes in eukaryotes. Cell Signal 2005, 17, 675–689. [Google Scholar] [CrossRef]
- Sun, B.; Chen, L.; Cao, W.; Roth, A.F.; Davis, N.G. The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXϕ adaptin sorting signal. Mol. Biol. Cell 2004, 15, 1397–1406. [Google Scholar] [CrossRef]
- Peng, Y.; Grassart, A.; Lu, R.; Wong, C.C.; Yates III, J.; Barnes, G.; Drubin, D.G. Casein kinase 1 promotes initiation of clathrin-mediated endocytosis. Dev. Cell 2015, 32, 231–240. [Google Scholar] [CrossRef]
- Lord, C.; Bhandari, D.; Menon, S.; Ghassemian, M.; Nycz, D.; Hay, J.; Ghosh, P.; Ferro-Novick, S. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 2011, 473, 181–186. [Google Scholar] [CrossRef]
- Hoekstra, M.F.; Liskay, R.M.; Ou, A.C.; DeMaggio, A.J.; Burbee, D.G.; Heffron, F. HRR25, a putative protein kinase from budding yeast: Association with repair of damaged DNA. Science 1991, 253, 1031–1034. [Google Scholar] [CrossRef]
- Tanaka, C.; Tan, L.J.; Mochida, K.; Kirisako, H.; Koizumi, M.; Asai, E.; Sakoh-Nakatogawa, M.; Ohsumi, Y.; Nakatogawa, H. Hrr25 triggers selective autophagy–related pathways by phosphorylating receptor proteins. J. Cell Biol. 2014, 207, 91–105. [Google Scholar] [CrossRef]
- Wang, J.; Davis, S.; Menon, S.; Zhang, J.; Ding, J.; Cervantes, S.; Miller, E.; Jiang, Y.; Ferro-Novick, S. Ypt1/Rab1 regulates Hrr25/CK1δ kinase activity in ER–Golgi traffic and macroautophagy. J. Cell Biol. 2015, 210, 273–285. [Google Scholar] [CrossRef] [PubMed]
- De Assis, L.J.; Ulas, M.; Ries, L.N.A.; El Ramli, N.A.M.; Sarikaya-Bayram, O.; Braus, G.H.; Bayram, O.; Goldman, G.H. Regulation of Aspergillus nidulans CreA-mediated catabolite repression by the F-box proteins Fbx23 and Fbx47. Mbio 2018, 9, e00840-18. [Google Scholar] [CrossRef]
- Apostolaki, A.; Harispe, L.; Calcagno-Pizarelli, A.M.; Vangelatos, I.; Sophianopoulou, V.; Arst, H.N., Jr.; Peñalva, M.A.; Amillis, S.; Scazzocchio, C. Aspergillus nidulans CkiA is an essential casein kinase I required for delivery of amino acid transporters to the plasma membrane. Mol. Microbiol. 2012, 84, 530–549. [Google Scholar] [CrossRef]
- De Souza, C.P.; Hashmi, S.B.; Osmani, A.H.; Andrews, P.; Ringelberg, C.S.; Dunlap, J.C.; Osmani, S.A. Functional analysis of the Aspergillus nidulans kinome. PLoS ONE 2013, 8, e58008. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Chen, N.; Zhu, X.M.; Su, Z.Z.; Wang, J.Y.; Lu, J.P.; Liu, X.H.; Lin, F.C. The casein kinase MoYck1 regulates development, autophagy, and virulence in the rice blast fungus. Virulence 2019, 10, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.C.; Bradley, C.; Bryan, J.D.; Jerome, A.; Kweon, Y.; Panek, H.R. The Yck2 yeast casein kinase 1 isoform shows cell cycle-specific localization to sites of polarized growth and is required for proper septin organization. Mol. Biol. Cell 1999, 10, 1077–1092. [Google Scholar] [CrossRef]
- Roth, A.F.; Papanayotou, I.; Davis, N.G. The yeast kinase Yck2 has a tripartite palmitoylation signal. Mol. Biol. Cell 2011, 22, 2702–2715. [Google Scholar] [CrossRef]
- Audhya, A.; Emr, S.D. Regulation of PI4,5P2 synthesis by nuclear-cytoplasmic shuttling of the Mss4 lipid kinase. EMBO J. 2003, 22, 4223–4236. [Google Scholar] [CrossRef]
- Jung, S.I.; Rodriguez, N.; Irrizary, J.; Liboro, K.; Bogarin, T.; Macias, M.; Eivers, E.; Porter, E.; Filler, S.G.; Park, H. Yeast casein kinase 2 governs morphology, biofilm formation, cell wall integrity, and host cell damage of Candida albicans. PLoS ONE 2017, 12, e0187721. [Google Scholar]
- Görl, M.; Merrow, M.; Huttner, B.; Johnson, J.; Roenneberg, T.; Brunner, M. A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J. 2001, 20, 7074–7084. [Google Scholar] [CrossRef]
- Liu, X.; Chen, A.; Caicedo-Casso, A.; Cui, G.; Du, M.; He, Q.; Lim, S.; Kim, H.J.; Hong, C.I.; Liu, Y. FRQ-CK1 interaction determines the period of circadian rhythms in Neurospora. Nat. Commun. 2019, 10, 4352. [Google Scholar] [CrossRef]
- DeMarini, D.J.; Adams, A.E.; Fares, H.; De Virgilio, C.; Valle, G.; Chuang, J.S.; Pringle, J.R. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 1997, 139, 75–93. [Google Scholar] [CrossRef]
- Larson, J.R.; Bharucha, J.P.; Ceaser, S.; Salamon, J.; Richardson, C.J.; Rivera, S.M.; Tatchell, K. Protein phosphatase type 1 directs chitin synthesis at the bud neck in Saccharomyces cerevisiae. Mol. Biol. Cell 2008, 19, 3040–3051. [Google Scholar] [CrossRef]
- Kozubowski, L.; Panek, H.; Rosenthal, A.; Bloecher, A.; DeMarini, D.J.; Tatchell, K. A Bni4-Glc7 phosphatase complex that recruits chitin synthase to the site of bud emergence. Mol. Biol. Cell 2003, 14, 26–39. [Google Scholar] [CrossRef]
- Bloecher, A.; Tatchell, K. Dynamic localization of protein phosphatase type 1 in the mitotic cell cycle of Saccharomyces cerevisiae. J. Cell Biol. 2000, 149, 125–140. [Google Scholar] [CrossRef]
- Lesage, G.; Shapiro, J.; Specht, C.A.; Sdicu, A.M.; Ménard, P.; Hussein, S.; Tong, A.H.; Boone, C.; Bussey, H. An interactional network of genes involved in chitin synthesis in Saccharomyces cerevisiae. BMC Genet. 2005, 6, 8. [Google Scholar] [CrossRef]
- Rowbottom, L.; Munro, C.A.; Gow, N.A. Candida albicans mutants in the BNI4 gene have reduced cell-wall chitin and alterations in morphogenesis. Microbiology 2004, 150, 3243–3252. [Google Scholar] [CrossRef]
- Levin, D.E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Richthammer, C.; Enseleit, M.; Sanchez-Leon, E.; März, S.; Heilig, Y.; Riquelme, M.; Seiler, S. RHO1 and RHO2 share partially overlapping functions in the regulation of cell wall integrity and hyphal polarity in Neurospora crassa. Mol. Microbiol. 2012, 85, 716–733. [Google Scholar] [CrossRef]
- Eitzen, G.; Thorngren, N.; Wickner, W. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J. 2001, 20, 5650–5656. [Google Scholar] [CrossRef]
- Logan, M.R.; Jones, L.; Eitzen, G. Cdc42p and Rho1p are sequentially activated and mechanistically linked to vacuole membrane fusion. Biochem. Biophys. Res. Commun. 2010, 394, 64–69. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Gueldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef]
- Liang, J.; Fu, X.; Hao, C.; Bian, Z.; Liu, H.; Xu, J.R.; Wang, G. FgBUD14 is important for ascosporogenesis and involves both stage-specific alternative splicing and RNA editing during sexual reproduction. Environ. Microbiol. 2021, 23, 5052–5068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xia, A.; Ye, M.; Ren, J.; Li, D.; Liu, H.; Wang, Q.; Lu, P.; Wu, C.; Xu, J.R.; et al. Opposing functions of Fng1 and the Rpd3 HDAC complex in H4 acetylation in Fusarium graminearum. PLoS Genet. 2020, 16, e1009185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Hu, S.; Liu, H.; Xu, J.R. PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. PLoS Genet. 2017, 13, e1006954. [Google Scholar] [CrossRef]
- Wendland, J.; Philippsen, P. Determination of cell polarity in germinated spores and hyphal tips of the filamentous ascomycete Ashbya gossypii requires a rhoGAP homolog. J. Cell Sci. 2000, 113, 1611–1621. [Google Scholar] [CrossRef]
- Brent Heath, I.; Bonham, M.; Akram, A.; Gupta, G.D. The interrelationships of actin and hyphal tip growth in the ascomycete Geotrichum candidum. Fungal Genet. Biol. 2003, 38, 85–97. [Google Scholar] [CrossRef]
- Steinberg, G. Hyphal growth: A tale of motors, lipids, and the Spitzenkorper. Eukaryot. Cell 2007, 6, 351–360. [Google Scholar] [CrossRef]
- Meyer, V.; Arentshorst, M.; van den Hondel, C.A.; Ram, A.F. The polarisome component SpaA localises to hyphal tips of Aspergillus niger and is important for polar growth. Fungal Genet. Biol. 2008, 45, 152–164. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhou, W.; Chen, X.L.; Huang, J.G.; Cheng, Z.H.; Zhao, W.S.; Zhang, Y.; Peng, Y.L. A spindle pole antigen gene MoSPA2 is important for polar cell growth of vegetative hyphae and conidia, but is dispensable for pathogenicity in Magnaporthe oryzae. Curr. Genet. 2014, 60, 255–263. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Yu, Z.; Yuan, Y.; Zheng, Q.; Xie, Q.; Li, G.; Abubakar, Y.S.; Zhou, J.; Wang, Z.; et al. FgSpa2 recruits FgMsb3, a Rab8 GAP, to the polarisome to regulate polarized trafficking, growth and pathogenicity in Fusarium graminearum. New Phytol. 2021, 229, 1665–1683. [Google Scholar] [CrossRef]
- Hou, Z.; Xue, C.; Peng, Y.; Katan, T.; Kistler, H.C.; Xu, J.R. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant Microbe Interact. 2002, 15, 1119–1127. [Google Scholar] [CrossRef]
- Arkowitz, R.A.; Bassilana, M. Regulation of hyphal morphogenesis by Ras and Rho small GTPases. Fungal Biol. Rev. 2015, 29, 7–19. [Google Scholar] [CrossRef]
- Heinisch, J.J.; Rodicio, R. Protein kinase C in fungi-more than just cell wall integrity. FEMS Microbiol. Rev. 2018, 42, fux051. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rocha, A.L.; Roncero, M.I.; López-Ramirez, A.; Mariné, M.; Guarro, J.; Martínez-Cadena, G.; Di Pietro, A. Rho1 has distinct functions in morphogenesis, cell wall biosynthesis and virulence of Fusarium oxysporum. Cell Microbiol. 2008, 10, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Bourett, T.M.; Sweigard, J.A.; Czymmek, K.J.; Carroll, A.; Howard, R.J. Reef coral fluorescent proteins for visualizing fungal pathogens. Fungal Genet. Biol. 2002, 37, 211–220. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, C.; Li, Y.; Xu, J.R. Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2014, 5, 4518. [Google Scholar] [CrossRef]
- Gao, X.; Jin, Q.; Jiang, C.; Li, Y.; Li, C.; Liu, H.; Kang, Z.; Xu, J.R. FgPrp4 kinase is important for spliceosome B-complex activation and splicing efficiency in Fusarium graminearum. PLoS Genet. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Wang, H.; Chen, L.; Zhang, J.; Sun, M.; Xu, J.R.; Wang, C. The PKR regulatory subunit of protein kinase A (PKA) is involved in the regulation of growth, sexual and asexual development, and pathogenesis in Fusarium graminearum. Mol. Plant Pathol. 2018, 19, 909–921. [Google Scholar] [CrossRef]
- Chen, Z.; Zehraoui, E.; Atanasoff-Kardjalieff, A.K.; Strauss, J.; Studt, L.; Ponts, N. Effect of H2A.Z deletion is rescued by compensatory mutations in Fusarium graminearum. PLoS Genet. 2020, 16, e1009125. [Google Scholar] [CrossRef]
- Kelly, J.M. 13 The regulation of carbon metabolism in filamentous fungi. In Biochemistry and Molecular Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 321–340. [Google Scholar]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.S.; Wang, Z.; Lu, G. Carbon catabolite repression in filamentous fungi. Int. J. Mol. Sci. 2017, 19, 10048. [Google Scholar] [CrossRef]
- Snowdon, C.; Johnston, M. A novel role for yeast casein kinases in glucose sensing and signaling. Mol. Biol. Cell 2016, 27, 3369–3375. [Google Scholar] [CrossRef]
- Zou, J.; Friesen, H.; Larson, J.; Huang, D.; Cox, M.; Tatchell, K.; Andrews, B. Regulation of cell polarity through phosphorylation of Bni4 by Pho85 G1 cyclin-dependent kinases in Saccharomyces cerevisiae. Mol. Biol. Cell 2009, 20, 3239–3250. [Google Scholar] [CrossRef]
- Pérez, J.; Arcones, I.; Gómez, A.; Casquero, V.; Roncero, C. Phosphorylation of Bni4 by MAP kinases contributes to septum assembly during yeast cytokinesis. FEMS Yeast Res. 2016, 16, fow060. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Wang, Y.; Li, C.; Bian, Z.; Zhang, X.; Liu, H.; Xu, J.R.; Jiang, C. Deletion of all three MAP kinase genes results in severe defects in stress responses and pathogenesis in Fusarium graminearum. Stress Biol. 2022, 2, 6. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, J.; Han, X.; Turšić-Wunder, A.; Toh, J.D.; Hong, W.; Gao, Y.G.; Miao, Y. Polarisome scaffolder Spa2-mediated macromolecular condensation of Aip5 for actin polymerization. Nat. Commun. 2019, 10, 5078. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, H.J.; Lee, J.; Kim, K.W.; Yun, S.H.; Shim, W.B.; Lee, Y.W. Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr. Genet. 2009, 55, 449–459. [Google Scholar] [CrossRef]
- Fernandes, C.; Gow, N.A.; Gonçalves, T. The importance of subclasses of chitin synthase enzymes with myosin-like domains for the fitness of fungi. Fungal Biol. Rev. 2016, 30, 1–14. [Google Scholar] [CrossRef]
- Yamamoto, W.; Wada, S.; Nagano, M.; Aoshima, K.; Siekhaus, D.E.; Toshima, J.Y.; Toshima, J. Distinct roles for plasma membrane PtdIns(4)P and PtdIns(4,5)P2 during receptor-mediated endocytosis in yeast. J. Cell Sci. 2018, 131, jcs207696. [Google Scholar] [CrossRef]
- Audhya, A.; Loewith, R.; Parsons, A.B.; Gao, L.; Tabuchi, M.; Zhou, H.; Boone, C.; Hall, M.N.; Emr, S.D. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J. 2004, 23, 3747–3757. [Google Scholar] [CrossRef]
- Zhou, X.; Heyer, C.; Choi, Y.E.; Mehrabi, R.; Xu, J.R. The CID1 cyclin C-like gene is important for plant infection in Fusarium graminearum. Fungal Genet. Biol. 2010, 47, 143–151. [Google Scholar] [CrossRef]
- Cavinder, B.; Sikhakolli, U.; Fellows, K.M.; Trail, F. Sexual development and ascospore discharge in Fusarium graminearum. J. Vis. Exp. 2012, 61, e3895. [Google Scholar] [CrossRef]
- Xu, H.; Ye, M.; Xia, A.; Jiang, H.; Huang, P.; Liu, H.; Hou, R.; Wang, Q.; Li, D.; Xu, J.R.; et al. The Fng3 ING protein regulates H3 acetylation and H4 deacetylation by interacting with two distinct histone modifying complexes. New Phytol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Du, J.; Chi, M.; Sun, X.; Liang, W.; Huang, J.; Li, B. The Y137H mutation in the cytochrome P450 FgCYP51B protein confers reduced sensitivity to tebuconazole in Fusarium graminearum. Pest Manag. Sci. 2018, 74, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Mehrabi, R.; Koten, C.; Kang, Z.; Wei, Y.; Seong, K.; Kistler, H.C.; Xu, J.R. Transducin beta-like gene FTL1 is essential for pathogenesis in Fusarium graminearum. Eukaryot. Cell 2009, 8, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.; Hou, Z.; Tracy, M.; Kistler, H.C.; Xu, J.R. Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum. Phytopathology 2005, 95, 744–750. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Wang, Q.; Wu, C.; Jiang, C.; Xu, J.R. The tri-snRNP specific protein FgSnu66 is functionally related to FgPrp4 kinase in Fusarium graminearum. Mol. Microbiol. 2018, 109, 494–508. [Google Scholar] [CrossRef]
- Jiang, C.; Cao, S.; Wang, Z.; Xu, H.; Liang, J.; Liu, H.; Wang, G.; Ding, M.; Wang, Q.; Gong, C.; et al. An expanded subfamily of G-protein-coupled receptor genes in Fusarium graminearum required for wheat infection. Nat. Microbiol. 2019, 4, 1582–1591. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- McLaren, W.; Pritchard, B.; Rios, D.; Chen, Y.; Flicek, P.; Cunningham, F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 2010, 26, 2069–2070. [Google Scholar] [CrossRef]
- Catlett, N.L.; Lee, B.N.; Yoder, O.C.; Turgeon, B.G. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl. 2003, 50, 9–11. [Google Scholar] [CrossRef]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007, 2, 924–932. [Google Scholar] [CrossRef]
- Urban, A.; Neukirchen, S.; Jaeger, K.E. A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res. 1997, 25, 2227–2228. [Google Scholar] [CrossRef]
- Zhou, X.; Li, G.; Xu, J.R. Efficient approaches for generating GFP fusion and epitope-tagging constructs in filamentous fungi. Methods Mol. Biol. 2011, 722, 199–212. [Google Scholar]
- Zheng, W.; Zhao, X.; Xie, Q.; Huang, Q.; Zhang, C.; Zhai, H.; Xu, L.; Lu, G.; Shim, W.B.; Wang, Z. A conserved homeobox transcription factor Htf1 is required for phialide development and conidiogenesis in Fusarium species. PLoS ONE 2012, 7, e45432. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, S.; Zhou, X.; Wang, C.; Xiang, P.; Zheng, Q.; Xu, J.R. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS ONE 2012, 7, e49495. [Google Scholar] [CrossRef]
- Wang, G.; Sun, P.; Gong, Z.; Gu, L.; Lou, Y.; Fang, W.; Zhang, L.; Su, L.; Yang, T.; Wang, B.; et al. Srk1 kinase, a SR protein-specific kinase, is important for sexual reproduction, plant infection and pre-mRNA processing in Fusarium graminearum. Environ. Microbiol. 2018, 20, 3261–3277. [Google Scholar] [CrossRef]
- Ren, J.; Li, C.; Gao, C.; Xu, J.R.; Jiang, C.; Wang, G. Deletion of FgHOG1 is suppressive to the mgv1 mutant by stimulating Gpmk1 activation and avoiding intracellular turgor elevation in Fusarium graminearum. Front. Microbiol. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 2020, pdb-prot102269. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Li, Y.; Li, G.; Xu, J.R. Expression of HopAI interferes with MAP kinase signalling in Magnaporthe oryzae. Environ. Microbiol. 2017, 19, 4190–4204. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Yang, C.; Huang, J.; Chen, X.; Zhou, J.; Li, G.; Norvienyeku, J.; Wang, Z. A HOPS protein, MoVps41, is crucially important for vacuolar morphogenesis, vegetative growth, reproduction and virulence in Magnaporthe oryzae. Front. Plant Sci. 2017, 8, 1091. [Google Scholar] [CrossRef]
- Li, C.; Melesse, M.; Zhang, S.; Hao, C.; Wang, C.; Zhang, H.; Hall, M.C.; Xu, J.R. FgCDC14 regulates cytokinesis, morphogenesis, and pathogenesis in Fusarium graminearum. Mol. Microbiol. 2015, 98, 770–786. [Google Scholar] [CrossRef]




| Strain | Brief Description | Reference |
|---|---|---|
| PH-1 | Wild type | [40] |
| FY6 | Fgyck1 deletion mutant of PH-1 | [7] |
| PHLA1 | Transformant of PH-1 expressing LifeAct-GFP | [41] |
| FYC1 | Complementary strain of Fgyck1 mutant FY6 | This study |
| RGY3, RGY5 | GFP-FgYCK1 transformant of PH-1 | This study |
| S1-S25 | Spontaneous suppressors of FY6 | This study |
| FB5 | Fgbni4 deletion mutant of PH-1 | This study |
| FB18 | Fgbni4 deletion mutant of PH-1 | This study |
| FBC9 | complementary strain of Fgbni4 mutant FB5 | This study |
| RC11 | FgBNI4R699C transformant of PH-1 | This study |
| LF41 | FgBNI4ΔCT transformant of PH-1 | This study |
| RCY14 | Fgyck1 FgBNI4R699C mutant | This study |
| LFY5 | Fgyck1 FgBNI4ΔCT mutant | This study |
| DK21, DK41 | Fgyck1 Fgbni4 double mutants | This study |
| FYLA1, FYLA3 | LifeAct-GFP transformants of FY6 | This study |
| PHSP26 | FgSPA2-GFP transformant of PH-1 | This study |
| FYSP21, FYSP22 | FgSPA2-GFP transformants of FY6 | This study |
| PHR12, PHR19 | Transformants of PH-1 expressing GFP-FgRHO1 | This study |
| FYR2 | GFP-FgRHO1 transformant of FY6 | This study |
| DKR1, DKR2 | GFP-FgRHO1 transformants of DK21 | This study |
| Strain | Growth Rate (mm/Day) a | Conidiation (×104 Conidia/mL) b | Disease Index c |
|---|---|---|---|
| PH-1 (WT) | 11.3 ± 0.5 A* | 123.8 ± 7.7 A | 8.8 ± 1.6 A |
| FY6 (Fgyck1) | 1.8 ± 0.0 C | 0 | 0 |
| FYC1 (Fgyck1/FgYCK1) | 11.2 ± 0.2 A | 129.1 ± 8.7 A | 8.8 ± 1.5 A |
| RCY14 (Fgyck1 FgBNI4R699C) | 3.0 ± 0.1 B | 45.2 ± 9.6 B | 0 |
| LFY5 (Fgyck1 FgBNI4ΔCT) | 3.0 ± 0.1 B | 46.4 ± 8.0 B | 0 |
| DK21 (Fgyck1 Fgbni4-21) | 3.2 ± 0.0 B | 45.8 ± 5.8 B | 0 |
| DK41 (Fgyck1 Fgbni4-41) | 3.1 ± 0.0 B | 46.6 ± 5.8 B | 0 |
| Suppressor | Predicted Gene | Yeast Homolog | Nucleotide Change | Amino Acid Changes |
|---|---|---|---|---|
| S8 | FGRRES_10104 | IES4 | C568AA to TAA | Q190 * |
| S12 | FGRRES_07218 | BNI4 | G889−1T to AT (intron 1) | S297 fs IR |
| FGRRES_17518 | MSS4 | A1058 to C | N353T | |
| S21 | FGRRES_03646 | TNA1 | G238AT to AAT | D80N |
| FGRRES_17597 | TOK1 | G160GT to CGT | G54R | |
| S22 | FGRRES_06258 | none | ΔG1473A1474 | N492 fs |
| S23 | FGRRES_07218 | BNI4 | ΔC2134T2135 | L712 fs |
| S24 | FGRRES_03646 | TNA1 | G238AT to AAT | D80N |
| FGRRES_09715 | MIG1 | ΔCTC920–922 | DP307 | |
| S25 | FGRRES_03646 | TNA1 | G238AT to AAT | D80N |
| FGRRES_03242 | none | ΔCAT1113–1115 | DI371 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Hu, D.; Liu, Q.; Hou, R.; Xu, J.-R.; Wang, G. Stage-Specific Genetic Interaction between FgYCK1 and FgBNI4 during Vegetative Growth and Conidiation in Fusarium graminearum. Int. J. Mol. Sci. 2022, 23, 9106. https://doi.org/10.3390/ijms23169106
Zhu J, Hu D, Liu Q, Hou R, Xu J-R, Wang G. Stage-Specific Genetic Interaction between FgYCK1 and FgBNI4 during Vegetative Growth and Conidiation in Fusarium graminearum. International Journal of Molecular Sciences. 2022; 23(16):9106. https://doi.org/10.3390/ijms23169106
Chicago/Turabian StyleZhu, Jindong, Denghui Hu, Qianqian Liu, Rui Hou, Jin-Rong Xu, and Guanghui Wang. 2022. "Stage-Specific Genetic Interaction between FgYCK1 and FgBNI4 during Vegetative Growth and Conidiation in Fusarium graminearum" International Journal of Molecular Sciences 23, no. 16: 9106. https://doi.org/10.3390/ijms23169106






