Peripheral Blood B-Lymphocytes Are Involved in Lymphocystis Disease Virus Infection in Flounder (Paralichthys olivaceus) via Cellular Receptor-Mediated Mechanism
Abstract
:1. Introduction
2. Results
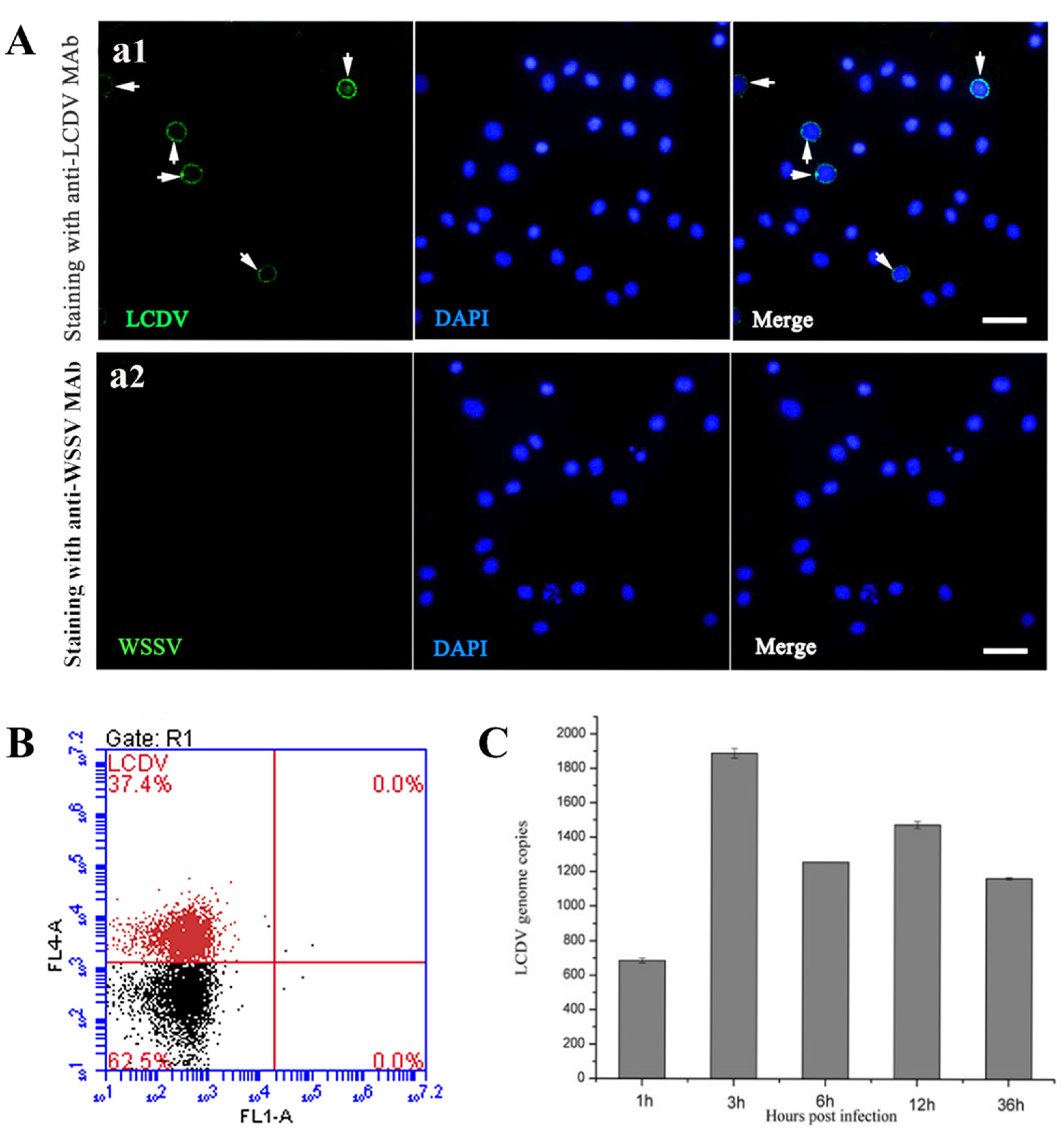
2.1. LCDV Infects the PBLs in Flounder In Vivo
2.2. LCDV Replicates in PBLs In Vitro
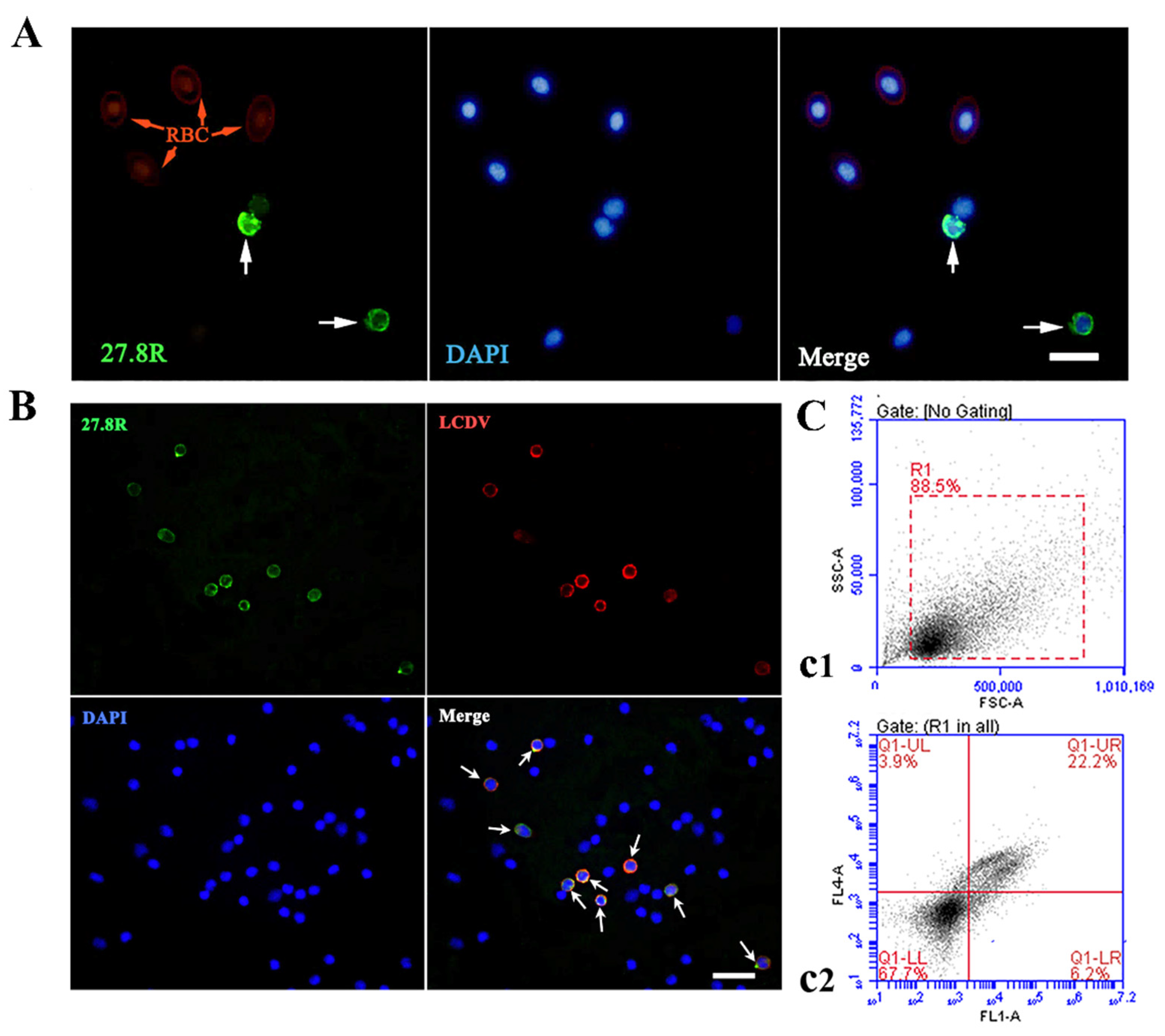
2.3. LCDV Infects the PBLs Expressing the 27.8R
2.4. Anti-27.8R MAb Blocks LCDV Infection of PBLs
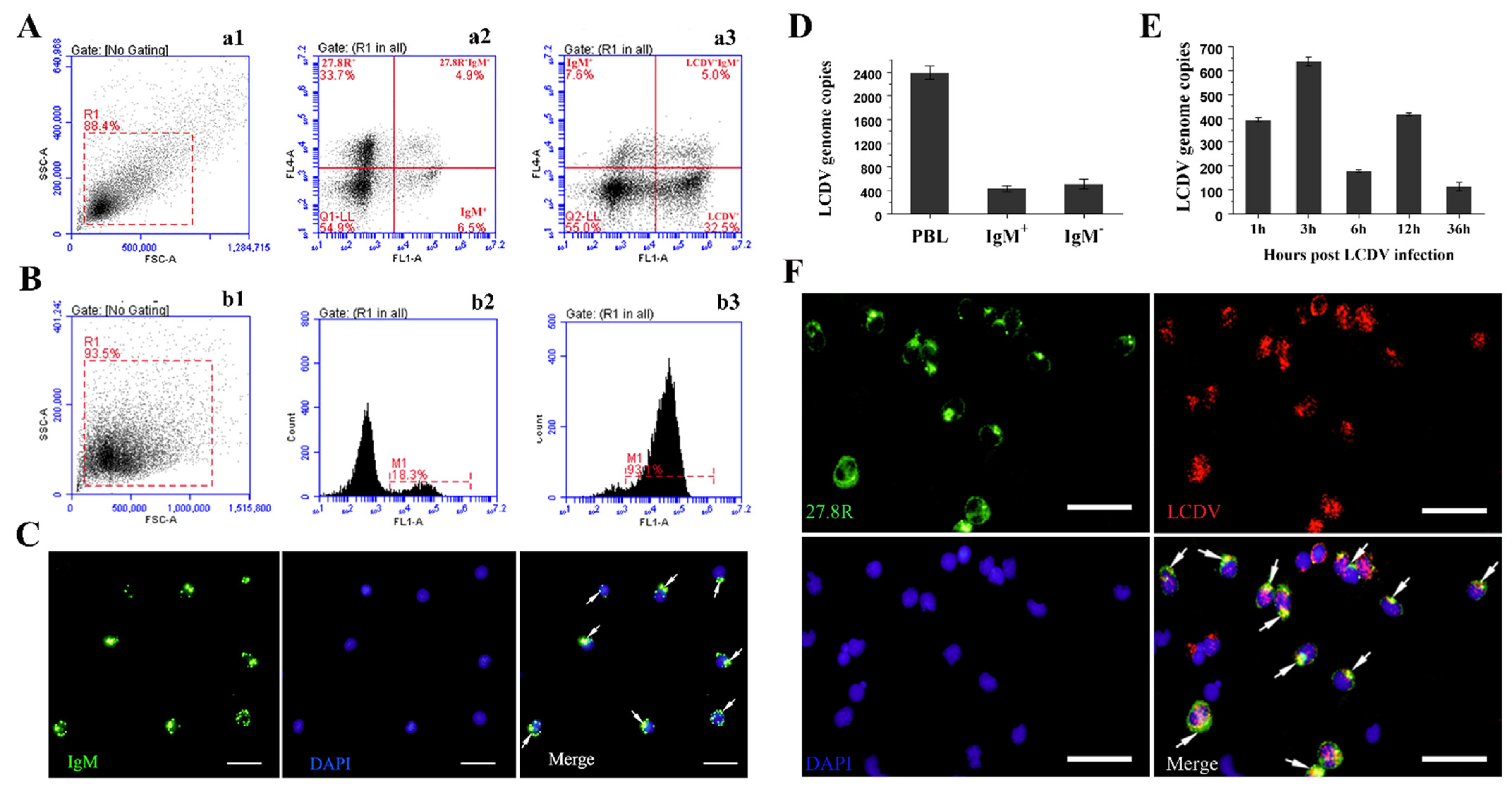
2.5. IgM+ and IgD+ B-Lymphocyte Subsets Involved in LCDV Infection
2.6. Co-Localization of LCDV and the 27.8R on the Sorted IgM+ B-Lymphocytes
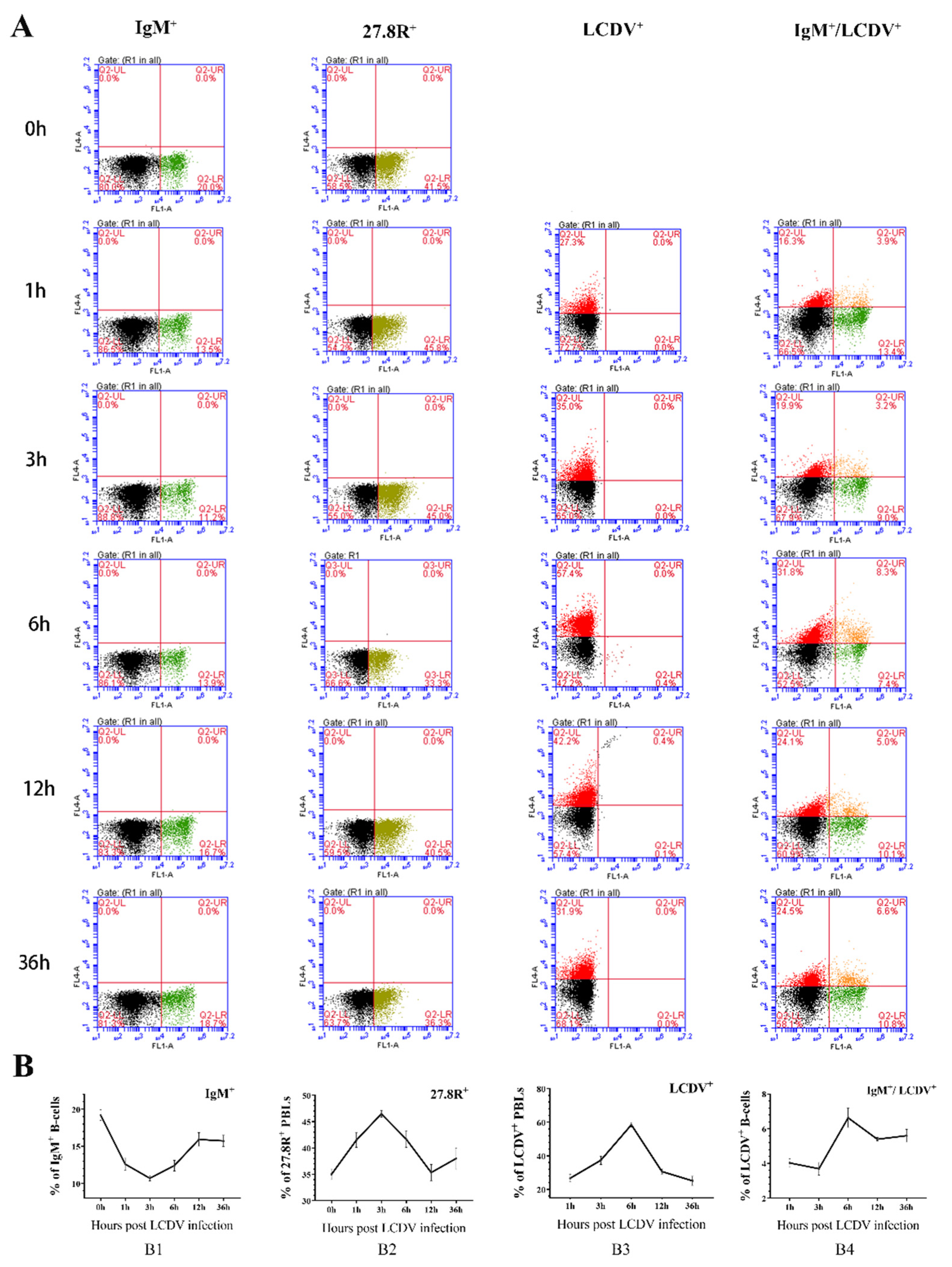
2.7. Kinetics of IgM+, 27.8R+, LCDV+, and LCDV+/IgM+ PBLs during Early Phase of LCDV Infection
3. Discussions
4. Materials and Methods
4.1. Ethics Statement
4.2. Fish, Virus and Antibodies
4.3. In Vivo LCDV Infection and Blood Cell Sampling
4.4. Indirect Immunofluorescence Assay
4.5. Flow Cytometry Analysis
4.6. Real-Time Quantitative PCR
4.7. In Vitro LCDV Infection of PBLs and Detection of Viral Replication
4.8. Co-Staining of LCDV with the 27.8R on PBLs
4.9. Immunogold Electron Microscopy
4.10. In Vitro Competition Assay between LCDV and Anti-27.8R MAb
4.11. Multicolor Fluorescence Microscopy
4.12. Magnetic Absorption Cell Sorting
4.13. Detection of IgM+, 27.8R+, LCDV+, and LCDV+/IgM+ PBLs at Different Time Points
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, F. The classification and nomenclature of viruses: Summary of results of meetings of the international committee on taxonomy of viruses in sendai, september 1984. Intervirology 1986, 25, 141–143. [Google Scholar] [CrossRef]
- Iwamoto, R.; Hasegawa, O.; LaPatra, S.; Yoshimizu, M. Isolation and characterization of the Japanese flounder (Paralichthys olivaceus) lymphocystis disease virus. J. Aquat. Anim. Health 2002, 14, 114–123. [Google Scholar] [CrossRef]
- Wolf, K. Fish Viruses and Fish Viral Diseases; Cornell University Press: New York, NY, USA, 1988. [Google Scholar]
- Pontejo, S.M.; Sánchez, C.; Martín, R.; Mulero, V.; Alcami, A.; Alejo, A. An orphan viral TNF receptor superfamily member identified in lymphocystis disease virus. Virol. J. 2013, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, Y.; Xu, L.; Wei, S.; Ouyang, Z.; Feng, J.; Qin, Q. Identification and characterization of a novel lymphocystis disease virus isolate from cultured grouper in China. J. Fish Dis. 2015, 38, 379–387. [Google Scholar] [CrossRef]
- Cherif, N.; Amdouni, F.; Maatoug, K.; Zaafran, S. Case Report: First occurrence of Lymphocystis disease virus 3 (LCDV-Sa) in wild marine fish in Tunisia. Ann. Mar. Sci. 2020, 4, 24–29. [Google Scholar]
- Lam, C.; Khairunissa, I.; Damayanti, L.; Kurobe, T.; The, S.J.; Pfahl, H.; Rapi, S.; Janetski, N.; Dolores, V.B. Detection of a new strain of lymphocystis disease virus (LCDV) in captive-bred clownfish Amphiprion percula in South Sulawesi, Indonesia. Aquacult. Int. 2020, 28, 2121–2137. [Google Scholar] [CrossRef]
- Borrego, J.J.; Valverde, E.J.; Labella, A.M.; Castro, D. Lymphocystis disease virus: Its importance in aquaculture. Rev. Aquac. 2017, 9, 179–193. [Google Scholar] [CrossRef]
- Paperna, I.; Sabnai, I.; Colorni, A. An outbreak of lymphocystis in Sparus aurata L. in the Gulf of Aqaba, Red Sea. J. Fish Dis. 1982, 5, 433–437. [Google Scholar] [CrossRef]
- Alonso, M.C.; Cano, I.; Garcia-Rosado, E.; Castro, D.; Lamas, J.; Barja, J.L.; Borreg, J.J. Isolation of lymphocystis disease virus from sole, Solea senegalensis Kaup, and blackspot sea bream, Pagellus bogaraveo (Brünnich). J. Fish Dis. 2005, 28, 221–228. [Google Scholar] [CrossRef]
- Jang, H.B.; Kim, Y.R.; Cha, I.S.; Noh, S.W.; Park, S.B.; Ohtani, M.; Hikima, J.; Aoki, T.; Jung, T.S. Detection of antigenic proteins expressed by lymphocystis virus as vaccine candidates in olive flounder, Paralichthys olivaceus (Temminck & Schlegel). J. Fish Dis. 2011, 34, 555–562. [Google Scholar]
- Wang, M.; Sheng, X.Z.; Xing, J.; Tang, X.Q.; Zhan, W.-B. Identification of a 27.8 kDa protein from flounder gill cells involved in lymphocystis disease virus binding and infection. Dis. Aquat. Organ. 2011, 94, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cano, I.; Valverde, E.J.; Garcia-Rosado, E.; Alonso, M.C.; Lopez-Jimena, B.; Ortiz-Delgado, J.B.; Borrego, J.J.; Sarasquete, C.; Castro, D. Transmission of lymphocystis disease virus to cultured gilthead seabream, Sparus aurata L.; larvae. J. Fish Dis. 2013, 36, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Z.; Wang, M.; Xing, J.; Zhan, W.B. Monoclonal antibodies against 27.8 kDa protein receptor efficiently block lymphocystis disease virus infection in flounder Paralichthys olivaceus gill cells. Dis. Aquat. Organ. 2012, 100, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Z.; Zhong, Y.; Zeng, J.; Tang, X.Q.; Xing, J.; Chi, H.; Zhan, W.B. Lymphocystis disease virus (Iridoviridae) enters flounder (Paralichthys olivaceus) gill cells via a caveolae-mediated endocytosis mechanism facilitated by viral receptors. Int. J. Mol. Sci. 2020, 21, 4722. [Google Scholar] [CrossRef]
- Tang, X.Q.; Diao, J.; Zhan, W.B.; Sheng, X.Z. Identification of a 37.6 kDa membrane protein from flounder gill cells involved in binding and infection to lymphocystis disease virus. Aquaculture 2012, 358, 132–138. [Google Scholar] [CrossRef]
- Wu, R.H.; Sheng, X.Z.; Tang, X.Q.; Xing, J.; Zhan, W.B. Transcriptome analysis of flounder (Paralichthys olivaceus) gill in response to lymphocystis disease virus (LCDV) infection: Novel insights into fish defense mechanisms. Int. J. Mol. Sci. 2018, 19, 160. [Google Scholar] [CrossRef]
- Wu, R.H.; Tang, X.Q.; Sheng, X.Z.; Zhan, W.B. Relationship between expression of cellular receptor-27.8 kDa and lymphocystis disease virus (LCDV) infection. PLoS ONE 2015, 10, e0127940. [Google Scholar]
- Zhong, Y.; Fei, C.J.; Tang, X.Q.; Zhan, W.B.; Sheng, X.Z. A 32 kDa viral attachment protein of lymphocystis disease virus (LCDV) specifically interacts with a 27.8 kDa cellular receptor from flounder (Paralichthys olivaceus). J. Gen. Virol. 2017, 98, 1477–1488. [Google Scholar] [CrossRef]
- Wu, R.H.; Tang, X.Q.; Sheng, X.Z.; Zhan, W.B. Tissue distribution of the 27.8 kDa receptor and its dynamic expression in response to lymphocystis disease virus infection in flounder (Paralichthys olivaceus). J. Comp. Pathol. 2015, 153, 324–332. [Google Scholar] [CrossRef]
- Sheng, X.Z.; Wu, R.H.; Tang, X.Q.; Xing, J.; Zhan, W.B. Tissue localization of lymphocystis disease virus (LCDV) receptor-27.8 kDa and its expression kinetics induced by the viral infection in turbot (Scophthalmus maximus). Int. J. Mol. Sci. 2015, 16, 26506–26519. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, X.Q.; Sheng, X.Z.; Xing, J.; Zhan, W.B. Voltage-dependent anion channel protein 2 (VDAC2) and receptor of activated protein C kinase 1 (RACK1) act as functional receptors for lymphocystis disease virus infection. J. Virol. 2019, 93, e00122-19. [Google Scholar] [CrossRef] [PubMed]
- Colorni, A.; Diamant, A. Splenic and cardiac lymphocystis in the red drum, Sciaenops ocellatus (L.). J. Fish Dis. 1995, 18, 467–471. [Google Scholar] [CrossRef]
- García-Rosado, E.; Castro, D.; Cano, I.; Pérez-Prieto, S.I.; Borrego, J.J. Serological techniques for detection of lymphocystis virus in fish. Aquat. Living Resour. 2002, 15, 179–185. [Google Scholar] [CrossRef]
- Flint, M.; Tscherne, D.M. Cellular receptors and HCV entry. Methods Mol. Biol. 2009, 510, 265–277. [Google Scholar] [PubMed]
- Bek, E.J.; McMinn, P.C. The pathogenesis and prevention of encephalitis due to human enterovirus 71. Curr. Infect. Dis. Rep. 2012, 14, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Turk, S.M.; Hutt Fletcher, L.M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 1995, 69, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- Molesworth, S.J.; Lake, C.M.; Borza, C.M.; Turk, S.M.; Hutt-Fletcher, L.M. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J. Virol. 2000, 74, 6324–6332. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Grundmann, S.; Royen, N.V.; Voskuil, M.; Schirmer, S.H.; Ulusans, S.; Bode, C.; Buschmann, I.R.; Piek, J.J. Leukocyte subpopulations and arteriogenesis: Specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 2005, 181, 285–293. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef]
- Edholm, E.S.; Bengten, E.; Stafford, J.L.; Sahoo, M.; Taylor, E.B.; Miller, N.W.; Wilson, M. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J. Immunol. 2010, 185, 4082–4094. [Google Scholar] [CrossRef]
- Tang, X.Q.; Liu, F.G.; Sheng, X.Z.; Xing, J.; Zhan, W.B. Production, characterization and application of monoclonal antibody against immunoglobulin D heavy chain of flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2017, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhan, W.B.; Xing, J.; Sheng, X.Z. Production, characterization and applicability of monoclonal antibodies to immunoglobulin of Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2007, 23, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tang, X.Q.; Zhan, W.B.; Xing, J.; Sheng, X.Z. Immunoglobulin tau heavy chain (IgT) in flounder, Paralichthys olivaceus: Molecular cloning, characterization, and expression analyses. Int. J. Mol. Sci. 2016, 17, 1571. [Google Scholar] [CrossRef]
- Babcock, G.J.; Decker, L.L.; Volk, M.; Thorley-Lawson, D.A. EBV persistence in memory B cells in vivo. Immunity 1998, 9, 395–404. [Google Scholar] [CrossRef]
- Borza, C.M.; Hutt-Fletcher, L.M. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 2002, 8, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Ersing, I.; Nobre, L.; Wang, L.W.; Soday, L.; Ma, Y.J.; Paulo, J.A.; Narita, Y.; Ashbaugh, C.W.; Jiang, C.; Grayson, N.E.; et al. A temporal proteomic map of Epstein-Barr virus lytic replication in B Cells. Cell Rep. 2017, 19, 1479–1493. [Google Scholar] [CrossRef]
- Sunil-Chandra, N.P.; Efstathiou, S.; Nash, A.A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 1992, 73, 3275–3279. [Google Scholar] [CrossRef]
- Hochberg, D.; Souza, T.; Catalina, M.; Sullivan, J.L.; Luzuriaga, K.; Thorley-Lawson, D.A. Acute infection with Epstein-Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J. Virol. 2004, 78, 5194–5204. [Google Scholar] [CrossRef]
- Shepherd, N.; Lan, J.; Li, W.; Rane, S.; Yu, Q. Primary human B cells at different differentiation and maturation stages exhibit distinct susceptibilities to vaccinia virus binding and infection. J. Virol. 2019, 93, e00973-19. [Google Scholar] [CrossRef]
- Upasani, V.; Vo, H.T.M.; Auerswald, H.; Laurent, D.; Heng, S.; Duong, V.; Rodenhuis-Zybert, I.A.; Dussart, P.; Cantaert, T. Direct infection of B cells by dengue virus modulates B Cell responses in a cambodian pediatric cohort. Front. Immunol. 2021, 11, 594813. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Kim, S.R.; Kitamura, S.I.; Kim, D.W.; Jung, S.J.; Nishizawa, T.; Yoshimizu, M.; Oh, M.J. Lymphocystis disease virus persists in the epidermal tissues of olive flounder, Paralichthys olivaceus (Temminch & Schlegel), at low temperatures. J. Fish Dis. 2009, 32, 699–703. [Google Scholar] [PubMed]
- Abós, B.; Castro, R.; González Granja, A.; Havixbeck, J.J.; Barreda, D.R.; Tafalla, C. Early activation of teleost B cells in response to rhabdovirus infection. J. Virol. 2015, 89, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barreda, D.R.; Zhang, Y.A.; Boshra, H.; Gelman, A.E.; Lapatra, S.; Tort, L.; Sunyer, J.O. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 2006, 7, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Øverland, H.S.; Pettersen, E.F.; Ronneseth, A.; Wergeland, H.I. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2009, 28, 193–204. [Google Scholar] [CrossRef]
- Tang, X.Q.; Yang, S.; Sheng, X.Z.; Xing, J.; Zhan, W.B. Transcriptome analysis of immune response of mIgM(+) B lymphocytes in Japanese flounder (Paralichthys olivaceus) to Lactococcus lactis in vitro revealed that IFN I-3 could enhance their phagocytosis. Front. Immunol. 2019, 10, 1622. [Google Scholar] [CrossRef]
- Hu, G.B.; Cong, R.S.; Fan, T.J.; Mei, X.G. Induction of apoptosis in a flounder gill cell line by lymphocystis disease virus infection. J. Fish Dis. 2004, 27, 657–662. [Google Scholar] [CrossRef]
- Essbauer, S.; Fischer, U.; Bergmann, S.; Ahne, W. Investigations on the ORF 167L of lymphocystis disease virus (Iridoviridae). Virus Genes 2004, 28, 19–39. [Google Scholar] [CrossRef]
- Iwakiri, S.; Song, J.Y.; Nakayama, K.; Oh, M.J.; Ishida, M.; Kitamura, S.I. Host responses of Japanese flounder Paralichthys olivaceus with lymphocystis cell formation. Fish Shellfish Immunol. 2014, 38, 406–411. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, X.H.; Zhang, J.; Gui, J.F.; Zhang, Q.Y. Subcellular localization and characterization of G protein-coupled receptor homolog from lymphocystis disease virus isolated in China. Viral Immunol. 2007, 20, 150–159. [Google Scholar] [CrossRef]
- De Pinto, V.; Messina, A.; Lane, D.J.R.; Lawen, A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 2010, 584, 1793–1799.53. [Google Scholar] [CrossRef]
- Fongsaran, C.; Phaonakrop, N.; Roytrakul, S.; Thepparit, C.; Kuadkitkan, A.; Smith, D.R. Voltage dependent anion channel is redistributed during Japanese Encephalitis virus infection of insect Cells. Sci. World J. 2014, 2014, 976015. [Google Scholar] [CrossRef] [PubMed]
- Cano, I.; Ferro, P.; Alonso, M.C.; Sarasquete, C.; Garcia-Rosado, E.; Borrego, J.J.; Castro, D. Application of in situ detection techniques to determine the systemic condition of lymphocystis disease virus infection in cultured gilt-head seabream, Sparus aurata L. J. Fish Dis. 2009, 32, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Valverde, E.J.; Borrego, J.J.; Sarasquete, M.C.; Ortiz-Delgado, J.B.; Castro, D. Target organs for lymphocystis disease virus replication in gilthead seabream (Sparus aurata). Vet. Res. 2017, 48, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.B.; Li, Y.Q.; Sheng, X.Z.; Xing, J.; Tang, X.Q. Detection of lymphocystis disease virus in Japanese flounder Paralichthys olivaceus and other marine teleosts from northern China. Chin. J. Oceanol. Limnol. 2010, 28, 1213–1220. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, X.Q.; Sheng, X.Z.; Xing, J.; Zhan, W.B. Development and characterization of monoclonal antibodies to the 32 kDa viral attachment protein of lymphocystis disease virus and their neutralizing ability in vitro. Int. J. Mol. Sci. 2018, 19, 2536. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Cheng, S.F.; Zhan, W.B.; Xing, J.; Sheng, X.Z. Development and characterization of monoclonal antibody to the lymphocystis disease virus of Japanese flounder Paralichthys olivaceus isolated from China. J. Virol. Methods 2006, 135, 173–180. [Google Scholar] [CrossRef]
- Zhan, W.B.; Wang, Y.H.; Fryer, J.L.; Okubo, K.; Fukuda, H. Production of monoclonal antibodies (MAbs) against white spot syndrome virus (WSSV). J. Aquat. Anim. Health 1999, 11, 17–22. [Google Scholar] [CrossRef]
- Tang, X.Q.; Qin, Y.H.; Sheng, X.Z.; Xing, J.; Zhan, W.B. Characterization of CD3+ T lymphocytes of Japanese flounder (Paralichthys olivaceus) and its response after immunization with formalin-inactivated Edwardsiella tarda. Fish Shellfish Immunol. 2017, 63, 220–227. [Google Scholar] [CrossRef]
- Yoshimizu, M.; Yoshinaka, T.; Hatori, S.; Kasai, H. Survivability of fish pathogenic viruses in environmental water, and inactivation of fish viruses (special issue: International symposium on koi herpesvirus disease). Bull. Fish Res. Agency Suppl. 2005, 2, 47–54. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Zeng, J.; Zhong, Y.; Tang, X.; Xing, J.; Chi, H.; Zhan, W. Peripheral Blood B-Lymphocytes Are Involved in Lymphocystis Disease Virus Infection in Flounder (Paralichthys olivaceus) via Cellular Receptor-Mediated Mechanism. Int. J. Mol. Sci. 2022, 23, 9225. https://doi.org/10.3390/ijms23169225
Sheng X, Zeng J, Zhong Y, Tang X, Xing J, Chi H, Zhan W. Peripheral Blood B-Lymphocytes Are Involved in Lymphocystis Disease Virus Infection in Flounder (Paralichthys olivaceus) via Cellular Receptor-Mediated Mechanism. International Journal of Molecular Sciences. 2022; 23(16):9225. https://doi.org/10.3390/ijms23169225
Chicago/Turabian StyleSheng, Xiuzhen, Jing Zeng, Ying Zhong, Xiaoqian Tang, Jing Xing, Heng Chi, and Wenbin Zhan. 2022. "Peripheral Blood B-Lymphocytes Are Involved in Lymphocystis Disease Virus Infection in Flounder (Paralichthys olivaceus) via Cellular Receptor-Mediated Mechanism" International Journal of Molecular Sciences 23, no. 16: 9225. https://doi.org/10.3390/ijms23169225





