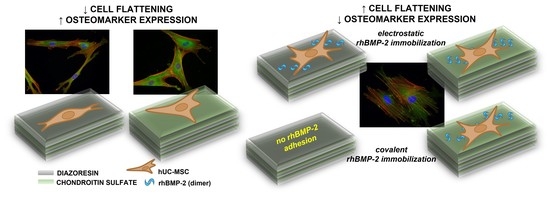
The Effect of the Topmost Layer and the Type of Bone Morphogenetic Protein-2 Immobilization on the Mesenchymal Stem Cell Response
Abstract
:1. Introduction
2. Results and Discussion
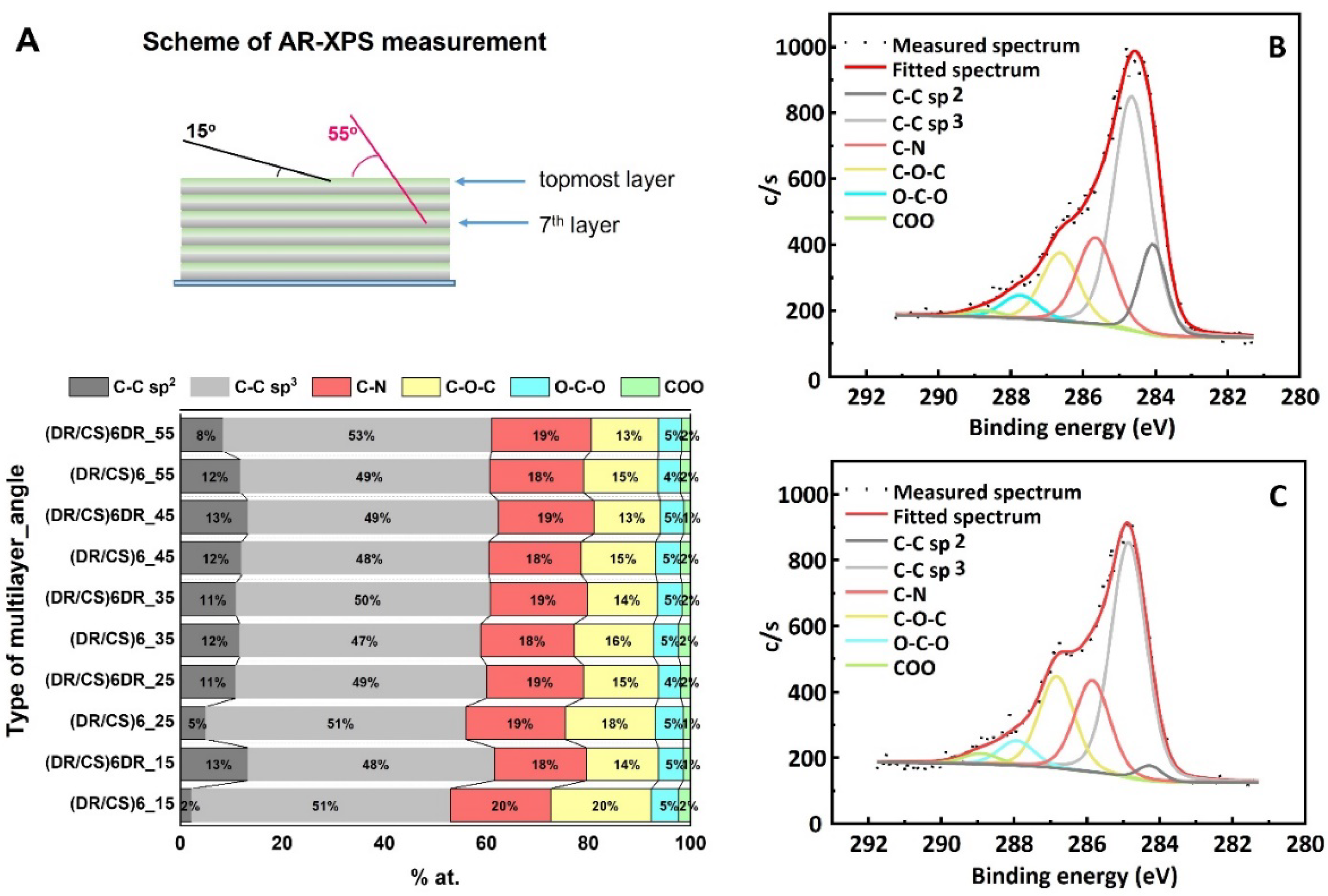
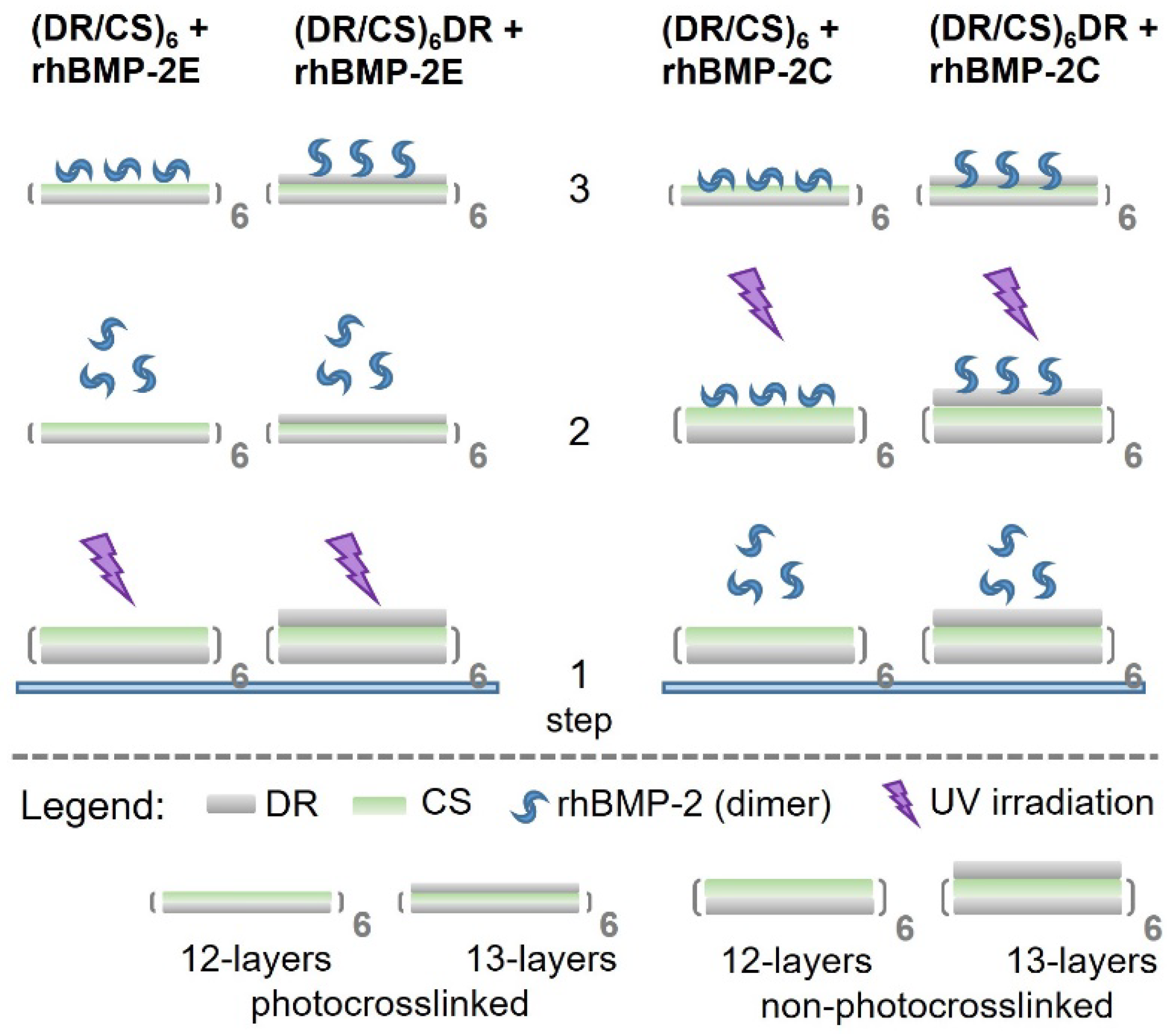
2.1. Characterization of Polymeric Films
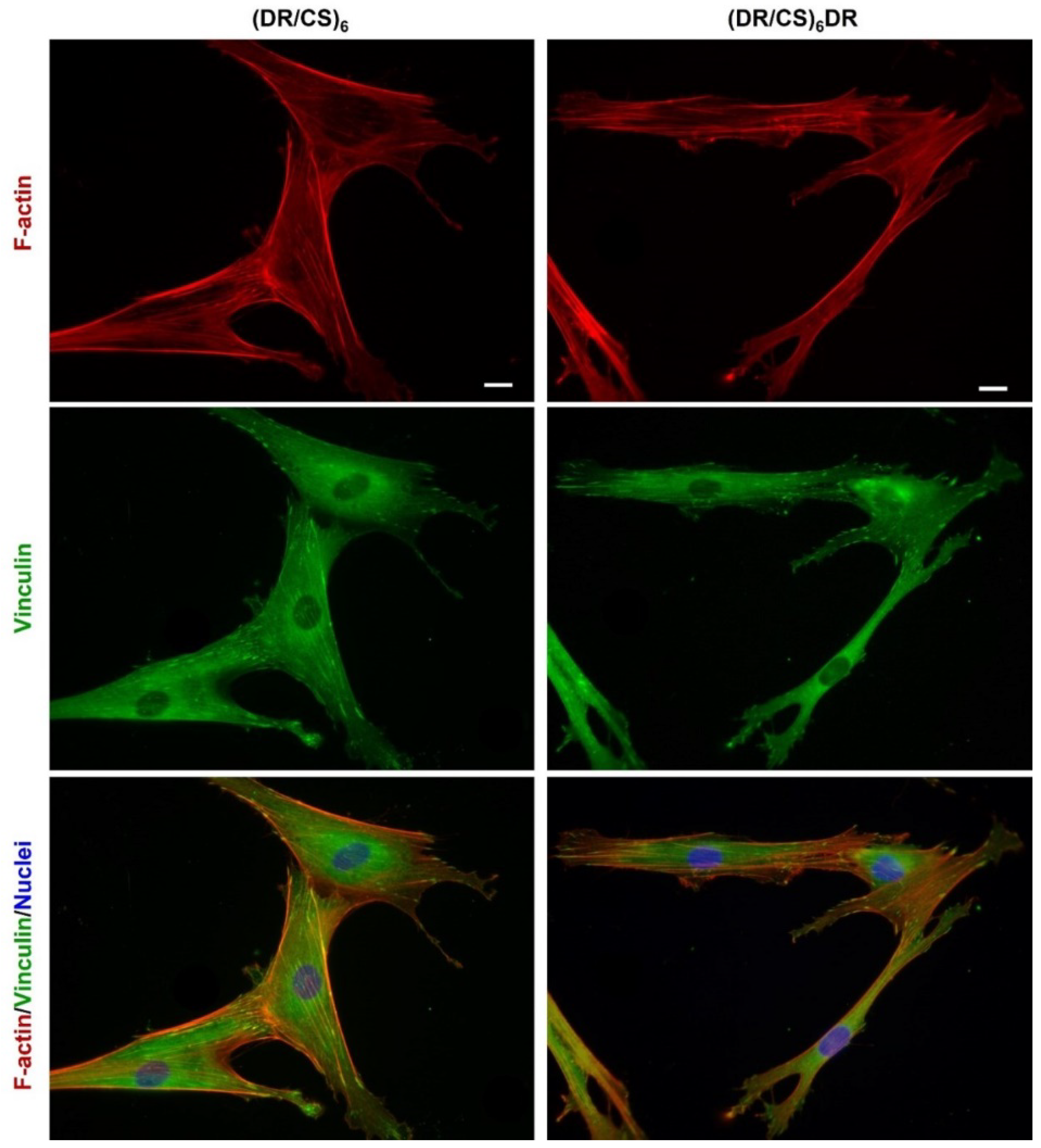
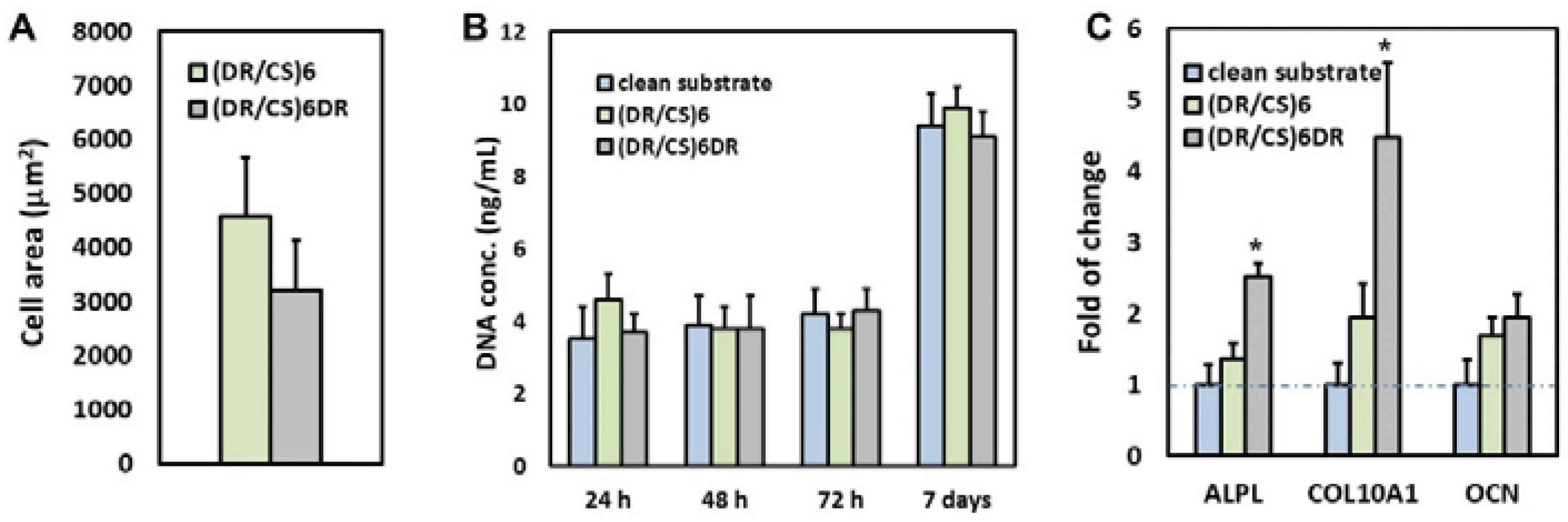
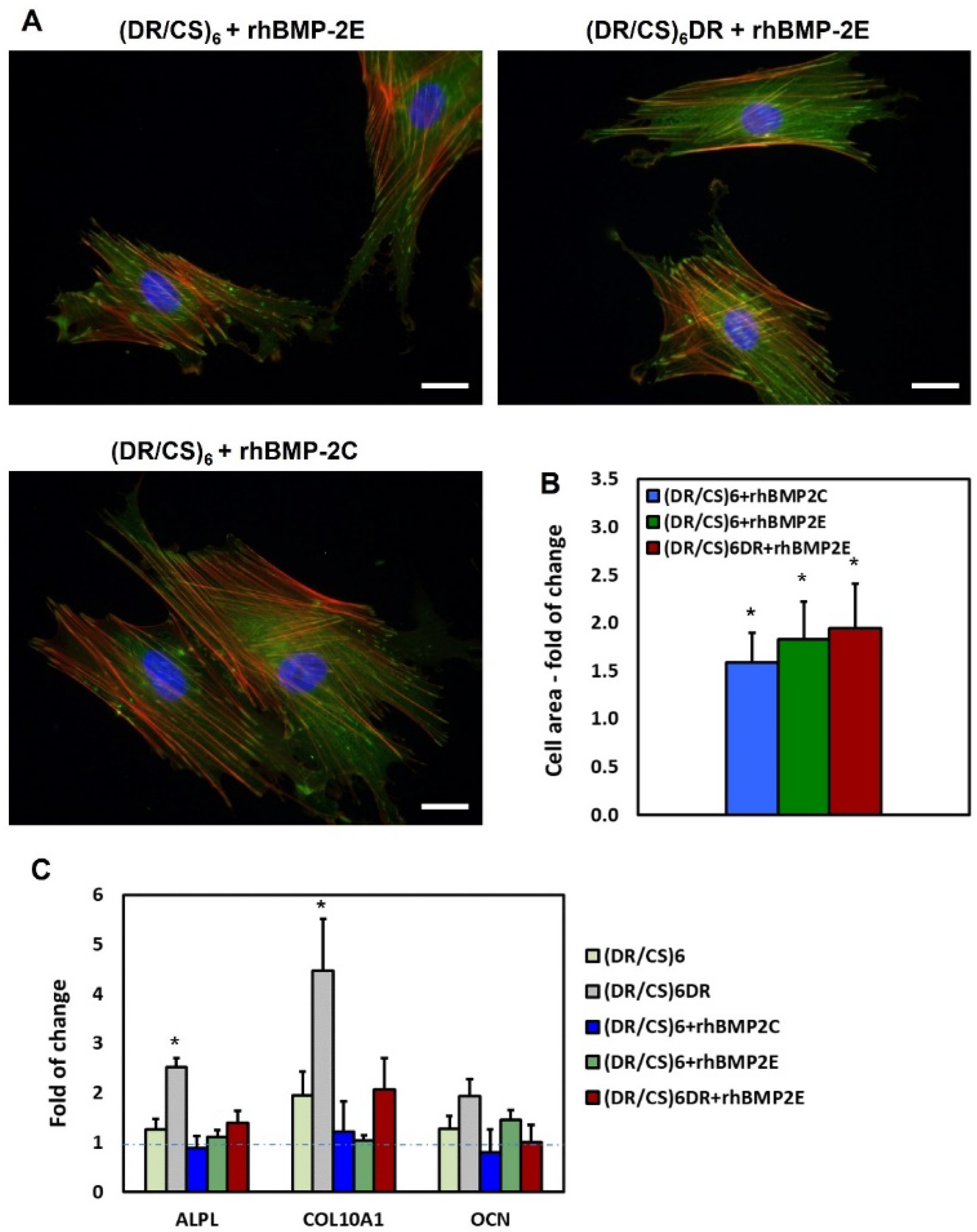
2.2. Response of hUC-MSCs Cultured on the Different Topmost Layers
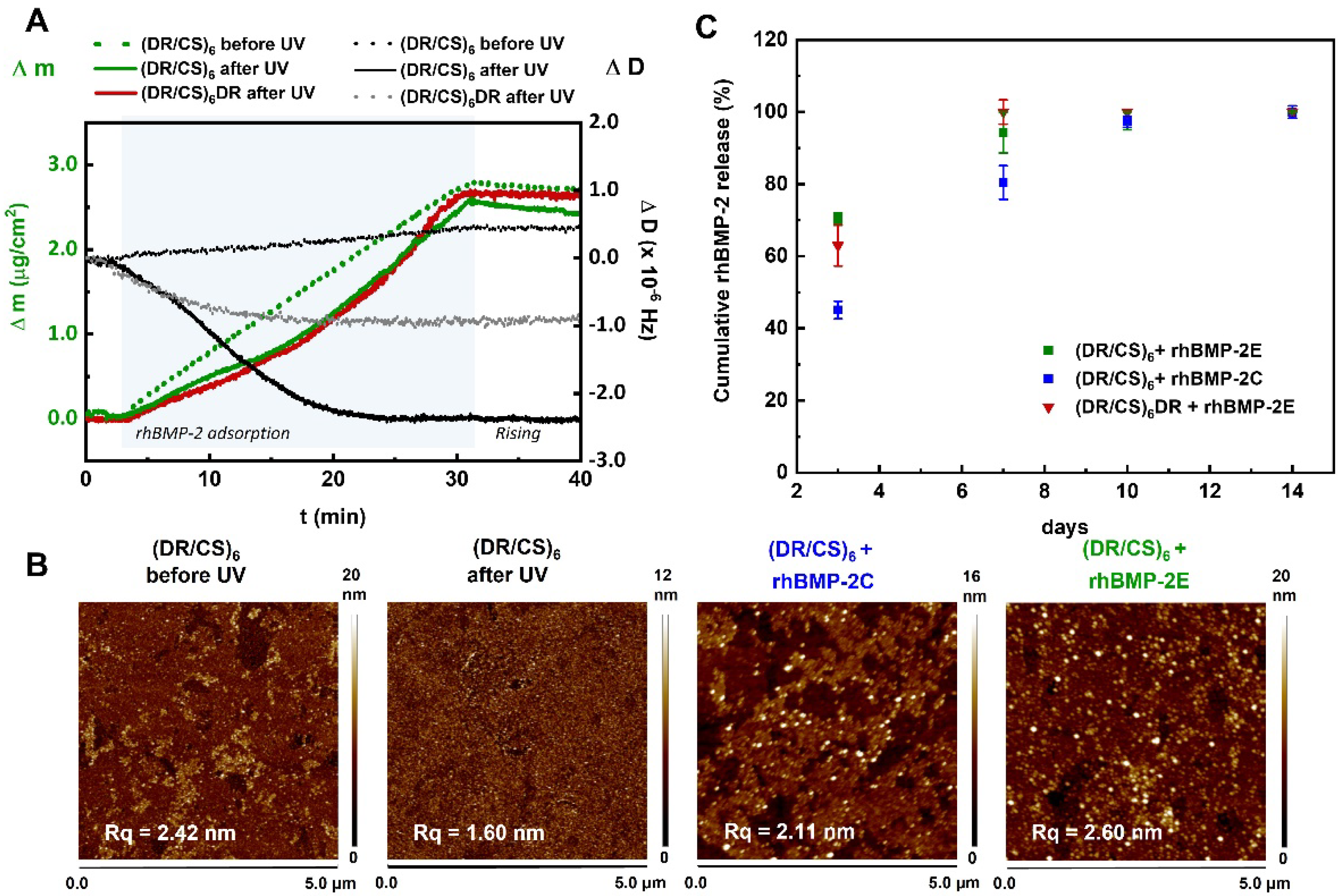
2.3. Interaction of the Topmost Layers with Immobilized rhBMP-2
2.4. Surface-rhBMP-2 Interaction in an Aqueous Environment
2.5. hUC-MSCs Response to rhBMP-2 Immobilized on the Surface
3. Material and Methods
3.1. Materials
3.2. Diazoresin Synthesis and Multilayer Preparation
3.3. X-ray Photoelectron Spectroscopy Measurements
3.4. Culture of hUC-MSCs
3.5. Cell Flattening, Focal Adhesion, and Actin Cytoskeleton Analysis
3.6. Proliferation Test
3.7. Induction of Osteogenic Differentiation of hUC-MSCs Cultured on Multilayer Polymeric Films
3.8. Protein Immobilization
3.9. Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) Measurements
3.10. Multivariate TOF-SIMS Analysis with PCA
3.11. QCM-D Assembly Study
3.12. Atomic Force Microscopy (AFM) Measurements
3.13. In Vitro Release of the rhBMP-2–ELISA Assay
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Muraglia, A.; Komlev, V.; Peyrin, F.; Rustichelli, F.; Crovace, A.; Cancedda, R. Tissue engineering of bone: Search for a better scaffold. Orthod. Craniofac. Res. 2005, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.; Kauffmann, P.; von Hahn, N.; Schirmer, U.; Liefeith, K.; Schliephake, H. Collagen-Based Osteogenic Nanocoating of Microrough Titanium Surfaces. Int. J. Mol. Sci. 2022, 23, 7803. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wyman, I.; Zhang, G.; Lin, J.; Liu, Z.; Wang, Y.; Hu, H. Preparation of superamphiphobic polymer-based coatings via spray- and dip-coating strategies. Prog. Org. Coat. 2016, 90, 463–471. [Google Scholar] [CrossRef]
- Wytrwal-Sarna, M.; Knobloch, P.; Lasota, S.; Michalik, M.; Nowakowska, M.; Kepczynski, M. Effect of polycation nanostructures on cell membrane permeability and toxicity. Environ. Sci. Nano 2022, 9, 702–713. [Google Scholar] [CrossRef]
- Wytrwal, M.; Koczurkiewicz, P.; Zrubek, K.; Niemiec, W.; Michalik, M.; Kozik, B.; Szneler, E.; Bernasik, A.; Madeja, Z.; Nowakowska, M.; et al. Growth and motility of human skin fibroblasts on multilayer strong polyelectrolyte films. J. Colloid Interface Sci. 2016, 461, 305–316. [Google Scholar] [CrossRef]
- Sekula, M.; Domalik-Pyzik, P.; Morawska-Chochol, A.; Bobis-Wozowicz, S.; Karnas, E.; Noga, S.; Boruczkowski, D.; Adamiak, M.; Madeja, Z.; Chlopek, J.; et al. Polylactide- and polycaprolactone-based substrates enhance angiogenic potential of human umbilical cord-derived mesenchymal stem cells in vitro—Implications for cardiovascular repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 521–533. [Google Scholar] [CrossRef]
- Stevens, B.; Yang, Y.; Mohandas, A.; Stucker, B.; Nguyen, K.T. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 573–582. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Zhang, Z.-Y.; Lv, F.-Y.; Chen, X.; Wang, Z.; Shen, Y.-Q.; Cong, H.-L.; Yu, B. Self-assembled covalent capillary coating of diazoresin/sodium polystyrene sulfonate for analysis of proteins by capillary electrophoresis. Ferroelectrics 2019, 546, 188–196. [Google Scholar] [CrossRef]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Structure, biological function and therapeutic applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. Morphogenetic messages are in the extracellular matrix: Biotechnology from bench to bedside. Biochem. Soc. Trans. 2000, 28, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Bessho, K.; Fujimura, K.; Konishi, Y.; Kusumoto, K.; Ogawa, Y.; Iizuka, T. Osteoinduction by recombinant human bone morphogenetic protein-2 at intramuscular, intermuscular, subcutaneous and intrafatty sites. Int. J. Oral Maxillofac. Surg. 2000, 29, 62–66. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Liu, C. Biomaterial-guided immobilization and osteoactivity of bone morphogenetic protein-2. Appl. Mater. Today 2020, 19, 100599. [Google Scholar] [CrossRef]
- Posa, F.; Baha-Schwab, E.H.; Wei, Q.; Di Benedetto, A.; Neubauer, S.; Reichart, F.; Kessler, H.; Spatz, J.P.; Albiges-Rizo, C.; Mori, G.; et al. Surface Co-presentation of BMP-2 and integrin selective ligands at the nanoscale favors α5β1 integrin-mediated adhesion. Biomaterials 2021, 267, 120484. [Google Scholar] [CrossRef]
- Sefkow-Werner, J.; Machillot, P.; Sales, A.; Castro-Ramirez, E.; Degardin, M.; Boturyn, D.; Cavalcanti-Adam, E.A.; Albiges-Rizo, C.; Picart, C.; Migliorini, E. Heparan sulfate co-immobilized with cRGD ligands and BMP2 on biomimetic platforms promotes BMP2-mediated osteogenic differentiation. Acta Biomater. 2020, 114, 90–103. [Google Scholar] [CrossRef]
- Migliorini, E.; Valat, A.; Picart, C.; Cavalcanti-Adam, E.A. Tuning cellular responses to BMP-2 with material surfaces. Cytokine Growth Factor Rev. 2016, 27, 43–54. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Wigmosta, T.B.; Popat, K.C.; Kipper, M.J. Bone morphogenetic protein-2 delivery from polyelectrolyte multilayers enhances osteogenic activity on nanostructured titania. J. Biomed. Mater. Res. A 2021, 109, 1173–1182. [Google Scholar] [CrossRef]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009, 20, 343–355. [Google Scholar] [CrossRef]
- Fourel, L.; Valat, A.; Faurobert, E.; Guillot, R.; Bourrin-Reynard, I.; Ren, K.; Lafanechère, L.; Planus, E.; Picart, C.; Albiges-Rizo, C. β3 integrin-mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. J. Cell Biol. 2016, 212, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.; Filipowska, J.; Osyczka, A.M.; Nowakowska, M.; Szczubialka, K. Osteoinductive activity of insulin-functionalized cell culture surfaces obtained using diazonium chemistry. Front. Chem. 2014, 2, 117. [Google Scholar] [CrossRef]
- Polčák, J.; Čechal, J.; Bábor, P.; Urbánek, M.; Průša, S.; Šikola, T. Angle-resolved XPS depth profiling of modeled structures: Testing and improvement of the method. Surf. Interface Anal. 2010, 42, 649–652. [Google Scholar] [CrossRef]
- Cumpson, P.J. Angle-resolved XPS depth-profiling strategies. Appl. Surf. Sci. 1999, 144–145, 16–20. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Major, G.H.; Linford, M.R.; Easton, C.D. Practical guides for x-ray photoelectron spectroscopy (XPS): Interpreting the carbon 1s spectrum. J. Vac. Sci. Technol. A 2021, 39, 013204. [Google Scholar] [CrossRef]
- Park, J.; Andrade, B.; Seo, Y.; Kim, M.J.; Zimmerman, S.C.; Kong, H. Engineering the Surface of Therapeutic “Living” Cells. Chem. Rev. 2018, 118, 1664–1690. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Yamaguchi, Y. Proteoglycans as modulators of growth factor activities. Cell 1991, 64, 867–869. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Ehlert, M.; Roszek, K.; Jędrzejewski, T.; Bartmański, M.; Radtke, A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity-Preliminary In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 5642. [Google Scholar] [CrossRef]
- Vasilevich, A.S.; Vermeulen, S.; Kamphuis, M.; Roumans, N.; Eroumé, S.; Hebels, D.G.A.J.; van de Peppel, J.; Reihs, R.; Beijer, N.R.M.; Carlier, A.; et al. On the correlation between material-induced cell shape and phenotypical response of human mesenchymal stem cells. Sci. Rep. 2020, 10, 18988. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, F.F.B.; Papenburg, B.; Vasilevich, A.; Hulsman, M.; Zhao, Y.; Levers, M.; Fekete, N.; de Boer, M.; Yuan, H.; Singh, S.; et al. Mining for osteogenic surface topographies: In silico design to in vivo osseo-integration. Biomaterials 2017, 137, 49–60. [Google Scholar] [CrossRef]
- Monneau, Y.; Arenzana-Seisdedos, F.; Lortat-Jacob, H. The sweet spot: How GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 2016, 99, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing Cell Proliferation and Osteogenic Differentiation of MC3T3-E1 Pre-osteoblasts by BMP-2 Delivery in Graphene Oxide-Incorporated PLGA/HA Biodegradable Microcarriers. Sci. Rep. 2017, 7, 12549. [Google Scholar] [CrossRef] [PubMed]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Michel, R.; Castner, D.G. Advances in time-of-flight secondary ion mass spectrometry analysis of protein films. Surf. Interface Anal. 2006, 38, 1386–1392. [Google Scholar] [CrossRef]
- Gajos, K.; Awsiuk, K.; Budkowski, A. Controlling orientation, conformation, and biorecognition of proteins on silane monolayers, conjugate polymers, and thermo-responsive polymer brushes: Investigations using TOF-SIMS and principal component analysis. Colloid Polym. Sci. 2021, 299, 385–405. [Google Scholar] [CrossRef]
- Quaas, B.; Burmeister, L.; Li, Z.; Satalov, A.; Behrens, P.; Hoffmann, A.; Rinas, U. Stability and Biological Activity of E. coli Derived Soluble and Precipitated Bone Morphogenetic Protein-2. Pharm. Res. 2019, 36, 184. [Google Scholar] [CrossRef]
- Rasi Ghaemi, S.; Delalat, B.; Ceto, X.; Harding, F.J.; Tuke, J.; Voelcker, N.H. Synergistic influence of collagen I and BMP 2 drives osteogenic differentiation of mesenchymal stem cells: A cell microarray analysis. Acta Biomater. 2016, 34, 41–52. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Schuster, L.; Ardjomandi, N.; Munz, M.; Umrath, F.; Klein, C.; Rupp, F.; Reinert, S.; Alexander, D. Establishment of Collagen: Hydroxyapatite/BMP-2 Mimetic Peptide Composites. Materials 2020, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Pomorska, A.; Wolski, K.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. Polymer brushes grafted from nanostructured zinc oxide layers—Spatially controlled decoration of nanorods. Eur. Polym. J. 2019, 112, 186–194. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. Analytical Techniques for the Characterization of Bioactive Coatings for Orthopaedic Implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef]
- Joyce, P.; Kempson, I.; Prestidge, C.A. Orientating lipase molecules through surface chemical control for enhanced activity: A QCM-D and ToF-SIMS investigation. Colloids Surf. B Biointerfaces 2016, 142, 173–181. [Google Scholar] [CrossRef]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.W.; Jang, E.C.; Son, T.I.; Lee, T.J.; Song, K.-S. Photo-immobilization of bone morphogenetic protein-2 using azidophenyl gelatin on a collagen sheet enhances osteogenesis in a rat calvarial defect model. J. Ind. Eng. Chem. 2016, 40, 177–184. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.K.; Kim, P.J.; Jung, L.Y.; Lee, M. Design of hydrogels to stabilize and enhance bone morphogenetic protein activity by heparin mimetics. Acta Biomater. 2018, 72, 45–54. [Google Scholar] [CrossRef]
- Salvi, C.; Lyu, X.; Peterson, A.M. Effect of Assembly pH on Polyelectrolyte Multilayer Surface Properties and BMP-2 Release. Biomacromolecules 2016, 17, 1949–1958. [Google Scholar] [CrossRef]
- Monteiro, I.A.; Kollmetz, T.; Malmström, J. Engineered systems to study the synergistic signaling between integrin-mediated mechanotransduction and growth factors (Review). Biointerphases 2018, 13, 06D302. [Google Scholar] [CrossRef]
- Mesa-Restrepo, A.; Civantos, A.; Allain, J.P.; Patiño, E.; Alzate, J.F.; Balcázar, N.; Montes, R.; Pavón, J.J.; Rodríguez-Ortiz, J.A.; Torres, Y. Synergistic Effect of rhBMP-2 Protein and Nanotextured Titanium Alloy Surface to Improve Osteogenic Implant Properties. Metals 2021, 11, 464. [Google Scholar] [CrossRef]
- Sotobori, T.; Ueda, T.; Myoui, A.; Yoshioka, K.; Nakasaki, M.; Yoshikawa, H.; Itoh, K. Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin beta1 into lipid rafts. Exp. Cell Res. 2006, 312, 3927–3938. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Fourel, L.; Boudou, T.; Albigès-Rizo, C.; Picart, C. Presentation of BMP-2 from a Soft Biopolymeric Film Unveils its Activity on Cell Adhesion and Migration. Adv. Mater. 2011, 23, H111–H118. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.; Filipowska, J.; Osyczka, A.M.; Szuwarzynski, M.; Nowakowska, M.; Szczubialka, K. Photocrosslinked ultrathin anionic polysaccharide supports for accelerated growth of human mesenchymal stem cells. Cell Prolif. 2014, 47, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Pomorska, A.; Nattich-Rak, M.; Wytrwal-Sarna, M.; Bernasik, A. Protein adsorption mechanisms at rough surfaces: Serum albumin at a gold substrate. J. Colloid Interface Sci. 2018, 530, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef]
- Wytrwal, M.; Leduc, C.; Sarna, M.; Goncalves, C.; Kepczynski, M.; Midoux, P.; Nowakowska, M.; Pichon, C. Gene delivery efficiency and intracellular trafficking of novel poly(allylamine) derivatives. Int. J. Pharm. 2015, 478, 372–382. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wytrwal-Sarna, M.; Sekuła-Stryjewska, M.; Pomorska, A.; Ocłoń, E.; Gajos, K.; Sarna, M.; Zuba-Surma, E.; Bernasik, A.; Szczubiałka, K. The Effect of the Topmost Layer and the Type of Bone Morphogenetic Protein-2 Immobilization on the Mesenchymal Stem Cell Response. Int. J. Mol. Sci. 2022, 23, 9287. https://doi.org/10.3390/ijms23169287
Wytrwal-Sarna M, Sekuła-Stryjewska M, Pomorska A, Ocłoń E, Gajos K, Sarna M, Zuba-Surma E, Bernasik A, Szczubiałka K. The Effect of the Topmost Layer and the Type of Bone Morphogenetic Protein-2 Immobilization on the Mesenchymal Stem Cell Response. International Journal of Molecular Sciences. 2022; 23(16):9287. https://doi.org/10.3390/ijms23169287
Chicago/Turabian StyleWytrwal-Sarna, Magdalena, Małgorzata Sekuła-Stryjewska, Agata Pomorska, Ewa Ocłoń, Katarzyna Gajos, Michal Sarna, Ewa Zuba-Surma, Andrzej Bernasik, and Krzysztof Szczubiałka. 2022. "The Effect of the Topmost Layer and the Type of Bone Morphogenetic Protein-2 Immobilization on the Mesenchymal Stem Cell Response" International Journal of Molecular Sciences 23, no. 16: 9287. https://doi.org/10.3390/ijms23169287