Medical Use of Polycatecholamines + Oxidoreductases-Modified Curdlan Hydrogels—Perspectives
Abstract
:1. Introduction
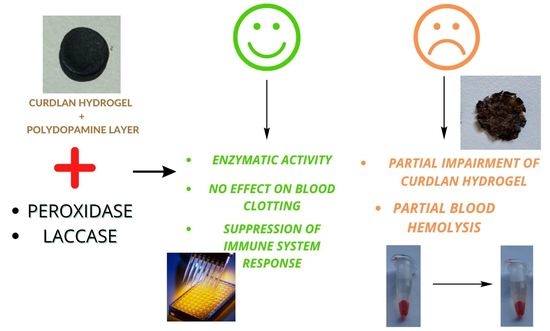
2. Results and Discussion
2.1. Laccase and Peroxidase Immobilization
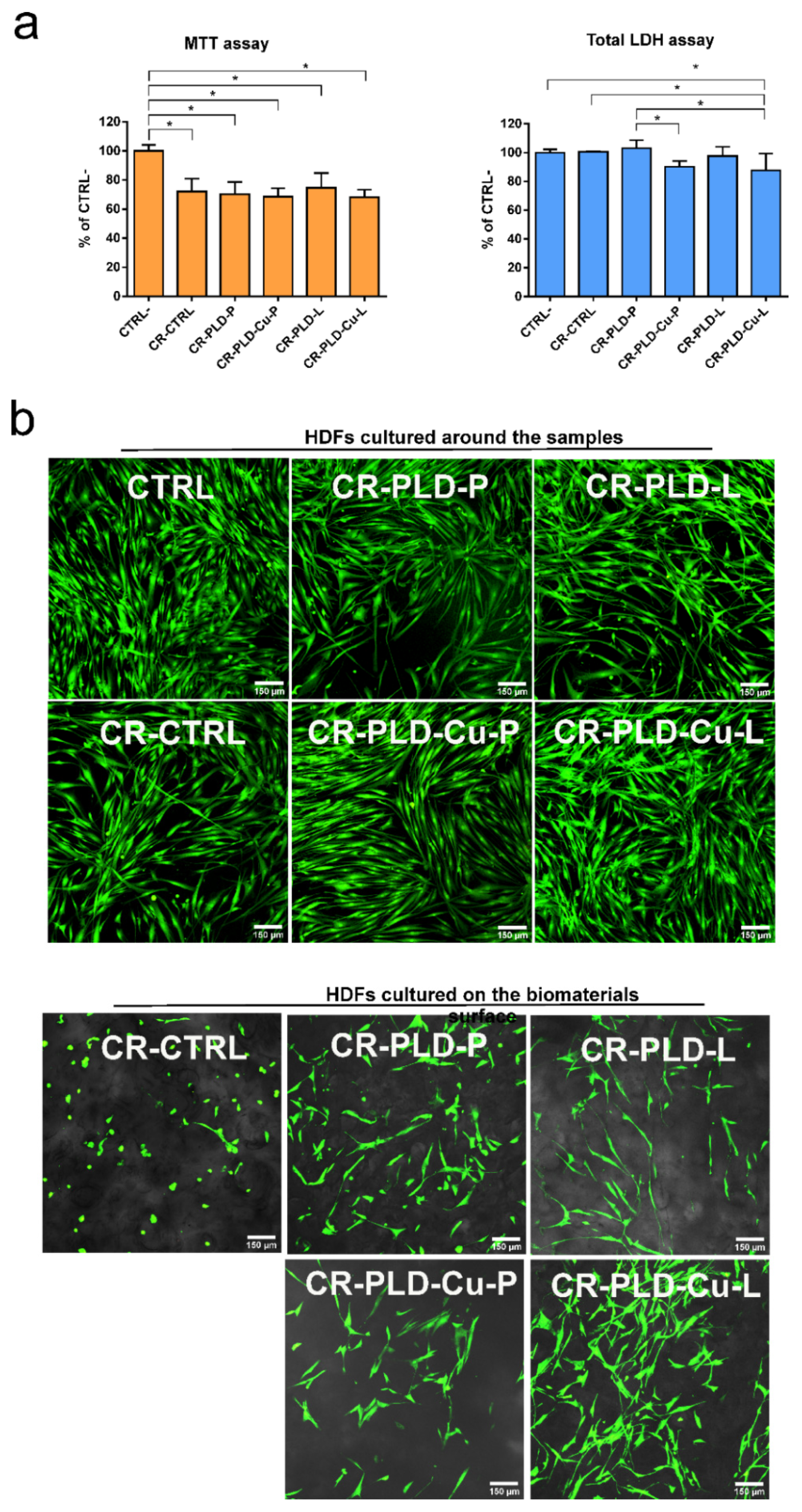
2.2. Cytotoxicity Tests
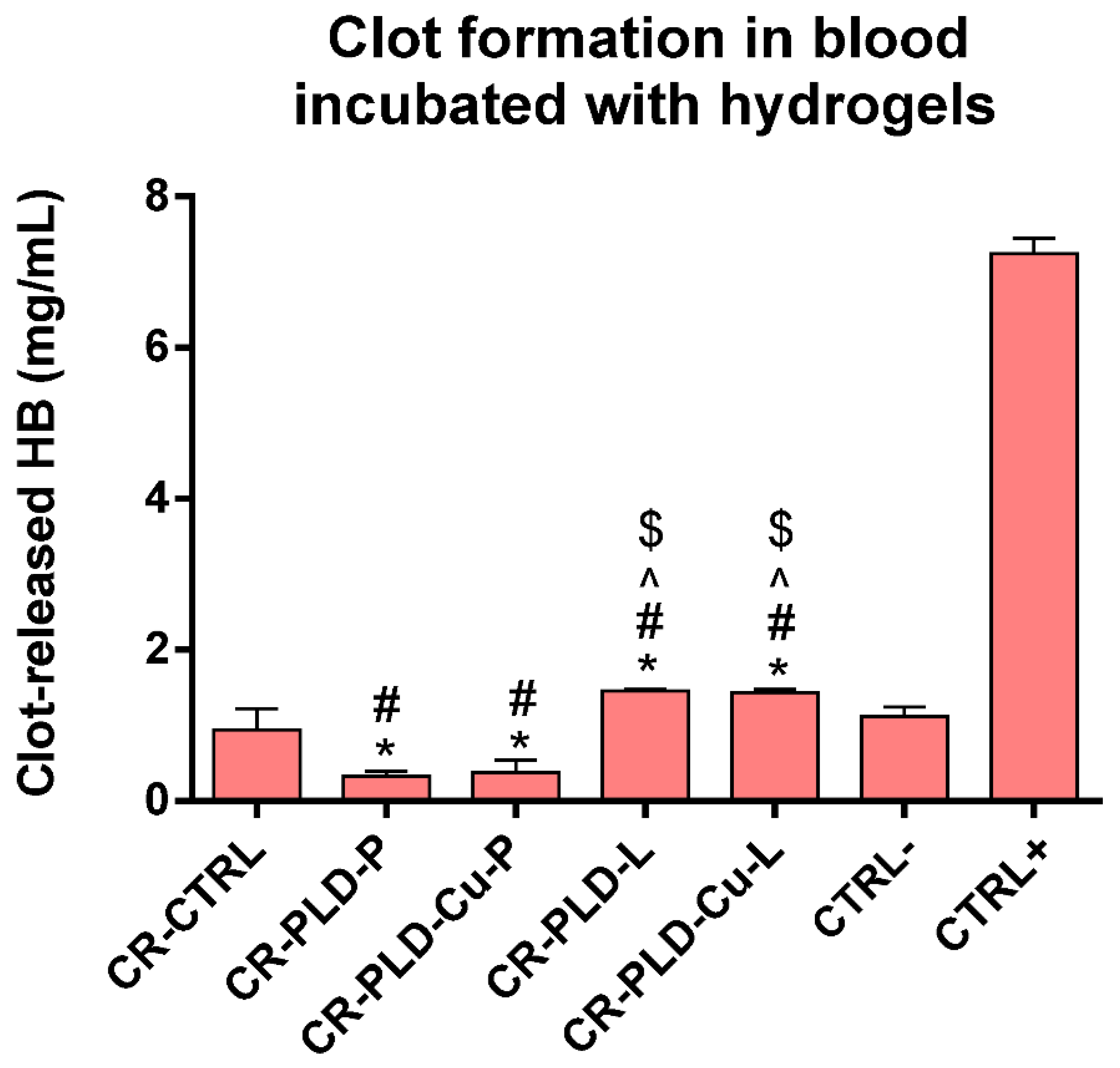
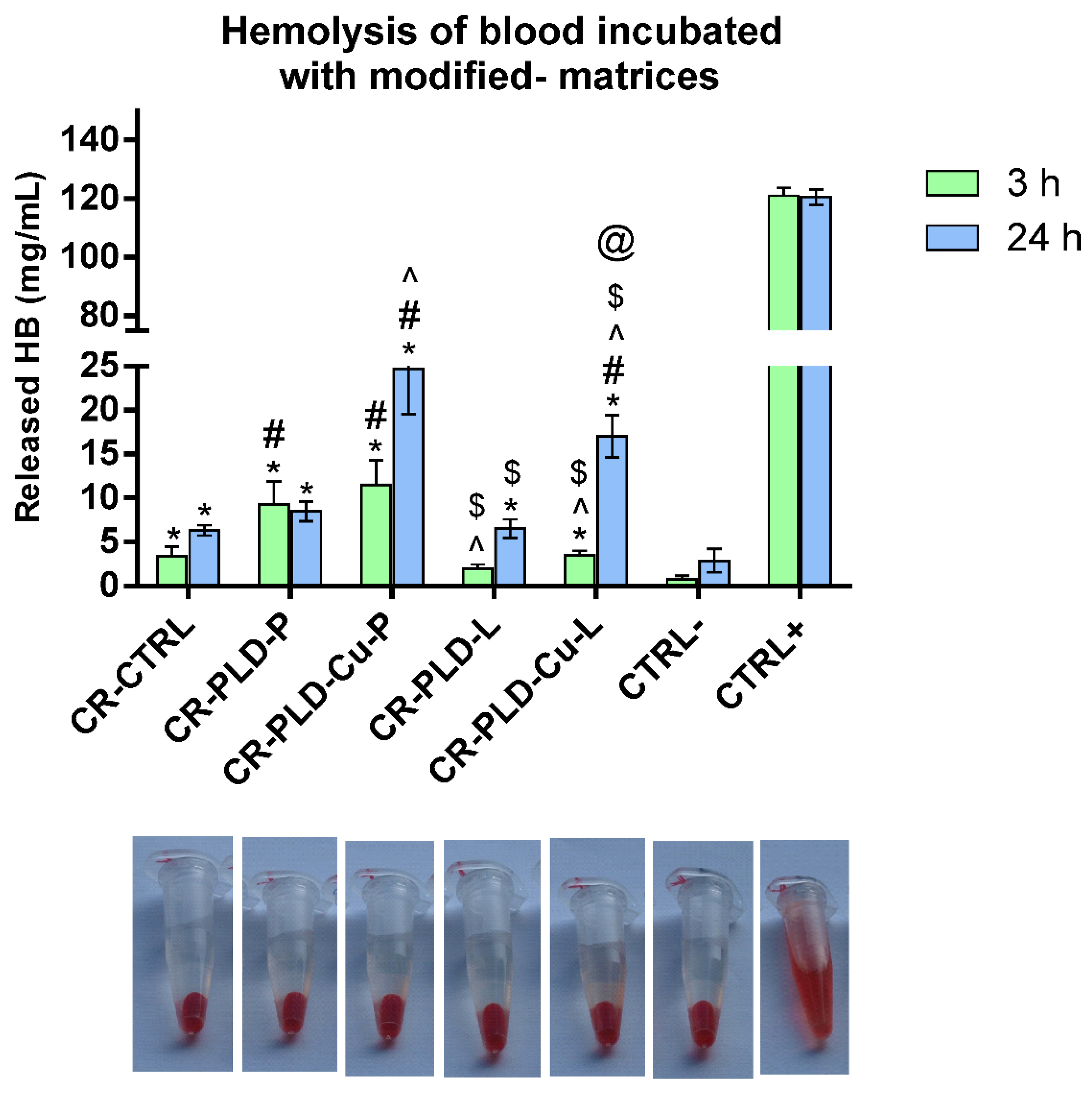
2.3. Interactions with Human Blood
2.4. Mechanical Properties
3. Materials and Methods
3.1. Laccase Production and Purification
3.2. Synthesis of Polycatecholamine-Coated Curdlan Hydrogels
3.3. Enzymes Immobilization and Quantitative Analysis
3.4. Laccase and Horseradish Peroxidase Assay
3.5. Cell Culture Tests
3.6. Cytotoxicity Tests on Human Dermal Fibroblasts
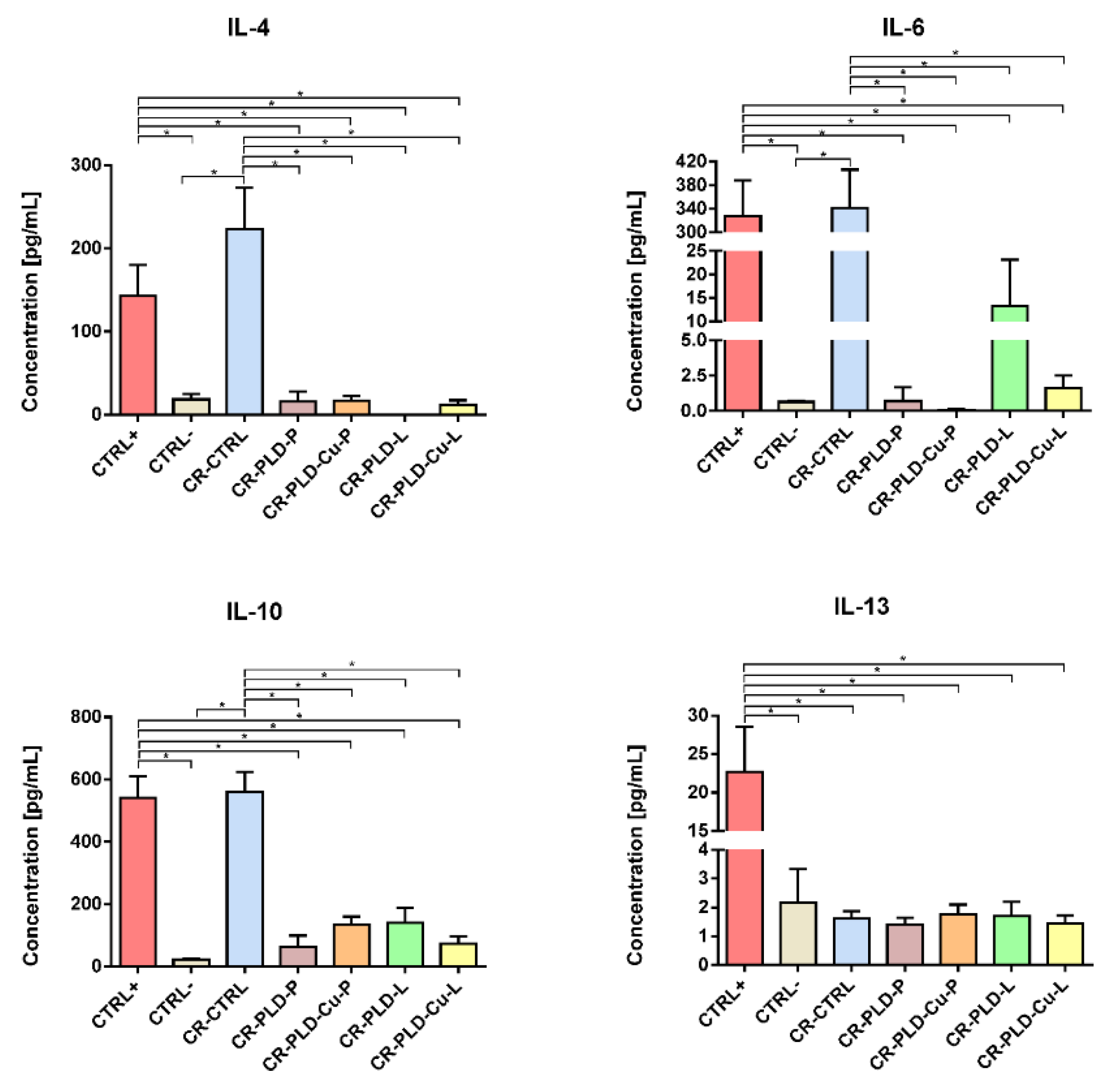
3.7. Cytokine Release by Monocytes
3.8. Blood Compatibility Tests
3.9. Mechanical Parameters Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, D.D.; Zhang, R.Y.; Zhang, G.Q.; Wang, H.X.; Ng, T.B. A Laccase with Antiproliferative Activity against Tumor Cells from an Edible Mushroom, White Common Agrocybe Cylindracea. Phytomedicine 2011, 18, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Jasim, A. Medicinal Properties of Laccase from Basidiomycetes Mushroom: A Review. Adv. Life Sci. Technol. 2017, 54, 99–109. [Google Scholar]
- Ho Wong, J.; Bun Ng, T.; Jiang, Y.; Liu, F.; Cho Wing Sze, S.; Yanbo Zhang, K. Purification and Characterization of a Laccase with Inhibitory Activity Toward HIV-1 Reverse Transcriptase and Tumor Cells from an Edible Mushroom (Pleurotus Cornucopiae). Protein Pept. Lett. 2010, 17, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New Bioactive Fungal Molecules with High Antioxidant and Antimicrobial Capacity Isolated from Cerrena Unicolor Idiophasic Cultures. Biomed Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Lele, S.S. Laccases-Properties and Applications. Bioresources 2009, 4, 1694–1717. [Google Scholar] [CrossRef]
- Kunamneni, A.; Camarero, S.; García-Burgos, C.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Engineering and Applications of Fungal Laccases for Organic Synthesis. Microb. Cell Fact. 2008, 7, 32. [Google Scholar] [CrossRef]
- Botta, L.; Brunori, F.; Tulimieri, A.; Piccinino, D.; Meschini, R.; Saladino, R. Laccase-Mediated Enhancement of the Antioxidant Activity of Propolis and Poplar Bud Exudates. ACS Omega 2017, 2, 2515–2523. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-Inflammatory and Anti-Oxidant Properties of Laccase-Synthesized Phenolic-O-Carboxymethyl Chitosan Hydrogels. N. Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef]
- Williamson, P.R. Role of Laccase in the Virulence of Talaromyces Marneffei: A Common Link between AIDS-Related Fungal Pathogens? Virulence 2016, 7, 627–629. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Humer, D.; Furlanetto, V.; Schruef, A.K.; Wlodarczyk, A.; Kuttke, M.; Divne, C.; Spadiut, O. Potential of Unglycosylated Horseradish Peroxidase Variants for Enzyme Prodrug Cancer Therapy. Biomed. Pharmacother. 2021, 142, 112037. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Thi, P.L.; Park, K.D. A Comparative Study of Enzyme-Mediated Crosslinking of Catechol- and Phenol-Functionalized Tetronic Hydrogels. Macromol. Res. 2022, 30, 190–197. [Google Scholar] [CrossRef]
- Courtois, P. Oral Peroxidases: From Antimicrobial Agents to Ecological Actors (Review). Mol. Med. Rep. 2021, 24, 500. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.; Pospiech, D.; Allertz, P.J.; Müller, M.; Salchert, K.; Hommel, R. Chemical Design of Hydrogels with Immobilized Laccase for the Reduction of Persistent Trace Compounds in Wastewater. ACS Appl. Polym. Mater. 2021, 3, 2823–2834. [Google Scholar] [CrossRef]
- Piao, M.; Zou, D.; Yang, Y.; Ren, X.; Qin, C.; Piao, Y. Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol A. Materials 2019, 12, 704. [Google Scholar] [CrossRef]
- Harguindeguy, M.; Antonelli, C.; Belleville, M.P.; Sanchez-Marcano, J.; Pochat-Bohatier, C. Gelatin Supports with Immobilized Laccase as Sustainable Biocatalysts for Water Treatment. J. Appl. Polym. Sci. 2021, 138, 49669. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.; Huang, W.; Zhang, S. Immobilization of Laccase in a Sponge-like Hydrogel for Enhanced Durability in Enzymatic Degradation of Dye Pollutants. J. Colloid Interface Sci. 2015, 450, 353–360. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Benotti, M.; Darlington, R.; Lalgudi, R. Enhanced Biodegradation of Sediment-Bound Heavily Weathered Crude Oil with Ligninolytic Enzymes Encapsulated in Calcium-Alginate Beads. J. Hazard. Mater. 2018, 357, 498–505. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Wang, Y.; Zhang, M.; Liu, Y. Immobilization of a Laccase/2,2′-Azino-Bis-(3-Ethylbenzothiazoline)-6-Sulfonic Acid System to Layered Double Hydroxide/Alginate Biohybrid Beads for Biodegradation of Malachite Green Dye. Biomed Res. Int. 2018, 2018, 5471961. [Google Scholar] [CrossRef]
- Sampaio, L.M.P.; Padrão, J.; Faria, J.; Silva, J.P.; Silva, C.J.; Dourado, F.; Zille, A. Laccase Immobilization on Bacterial Nanocellulose Membranes: Antimicrobial, Kinetic and Stability Properties. Carbohydr. Polym. 2016, 145, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F. Review on the Preparation, Biological Activities and Applications of Curdlan and Its Derivatives. Eur. Polym. J. 2020, 141, 110096. [Google Scholar] [CrossRef]
- Chaudhari, V.; Buttar, H.S.; Bagwe-Parab, S.; Tuli, H.S.; Vora, A.; Kaur, G. Therapeutic and Industrial Applications of Curdlan With Overview on Its Recent Patents. Front. Nutr. 2021, 8, 646988. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Kazimierczak, P.; Vivcharenko, V.; Koziol, M.; Przekora, A. Effect of Vitamin c/Hydrocortisone Immobilization within Curdlan-Based Wound Dressings on in Vitro Cellular Response in Context of the Management of Chronic and Burn Wounds. Int. J. Mol. Sci. 2021, 22, 11474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A Hydrogen-Bonded Antibacterial Curdlan-Tannic Acid Hydrogel with an Antioxidant and Hemostatic Function for Wound Healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Li, Y.; Lv, M.; Li, P.; Xu, H.; Wang, L. Preparation and Characterization of Novel Curdlan/Chitosan Blending Membranes for Antibacterial Applications. Carbohydr. Polym. 2011, 84, 952–959. [Google Scholar] [CrossRef]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and Modified (1→3)-β-D-Glucans in Health Promotion and Disease Alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. In Vitro Evaluation of the Risk of Inflammatory Response after Chitosan/HA and Chitosan/β-1,3-Glucan/HA Bone Scaffold Implantation. Mater. Sci. Eng. C 2016, 61, 355–361. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.G.; Kang, E.T.; Fu, G.D. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Dong, Z.; Zhang, F.; Jin, J. Interface Chemistry Engineering for Stable Cycling of Reduced GO/SnO2 Nanocomposites for Lithium Ion Battery. Nano Lett. 2013, 13, 1711–1716. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Odusote, G.; Qu, F.; Priestley, R.D. Core-Shell Fe3O4 Polydopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Interfaces 2013, 5, 9167–9171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Lu, C.H.; Guo, X.C.; Chen, F.R.; Yang, H.H.; Wang, X.R. Mussel-Inspired Molecularly Imprinted Polymer Coating Superparamagnetic Nanoparticles for Protein Recognition. J. Mater. Chem. 2010, 20, 880–883. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, C.; Liu, Y.; Chen, F.; Zheng, Z.; Wang, X. Enzyme-/Redox-Responsive Mesoporous Silica Nanoparticles Based on Functionalized Dopamine as Nanocarriers for Cancer Therapy. ACS Omega 2019, 4, 6097–6105. [Google Scholar] [CrossRef]
- Murphy, J.L.; Vollenweider, L.; Xu, F.; Lee, B.P. Adhesive Performance of Biomimetic Adhesive-Coated Biologic Scaffolds. Biomacromolecules 2010, 11, 2976–2984. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Michalicha, A.; Palka, K.; Roguska, A.; Pisarek, M.; Belcarz, A. Polydopamine-Coated Curdlan Hydrogel as a Potential Carrier of Free Amino Group-Containing Molecules. Carbohydr. Polym. 2021, 256, 117524. [Google Scholar] [CrossRef] [PubMed]
- Burzio, L.A.; Waite, J.H. Cross-Linking in Adhesive Quinoproteins: Studies with Model Decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- LaVoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine Covalently Modifies and Functionally Inactivates Parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef]
- Michalicha, A.; Roguska, A.; Przekora, A.; Budzyńska, B.; Belcarz, A. Poly(Levodopa)-Modified β -Glucan as a Candidate for Wound Dressings. Carbohydr. Polym. 2021, 272, 118485. [Google Scholar] [CrossRef]
- Michalicha, A.; Espona-Noguera, A.; Canal, C.; Budzyńska, B.; Pięt, M.; Przywara, S.; Pawelec, J.; Belcarz, A. Polycatecholamine and Gentamicin as Modifiers for Antibacterial and Blood-Biocompatible Polyester Vascular Prostheses. Mater. Sci. Eng. C 2022, 133, 112645. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Saha, A.; Noked, M.; Gedanken, A.; Margel, S. Mussel-Inspired Polynorepinephrine/MXene-Based Magnetic Nanohybrid for Electromagnetic Interference Shielding in X-Band and Strain-Sensing Performance. Langmuir 2022, 38, 3936–3950. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, P.; Różalska, S.; Wiktorska, M.; Brzezińska-Błaszczyk, E.; Agier, J. Curdlan Stimulates Tissue Mast Cells to Synthesize Pro-Inflammatory Mediators, Generate ROS, and Migrate via Dectin-1 Receptor. Cell. Immunol. 2020, 351, 104079. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.J.D.A.; Toniazzo, V.; Ruch, D. Dopamine-Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zheng, Y.; Xi, T.; Zhang, C.; Song, W.; Burugapalli, K.; Yang, H.; Ma, Y. Concentration-Dependent Cytotoxicity of Copper Ions on Mouse Fibroblasts in Vitro: Effects of Copper Ion Release from TCu380A vs TCu220C Intra-Uterine Devices. Biomed. Microdevices 2012, 14, 709–720. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus Cerrena Unicolor as an Effective Source of New Antiviral, Immunomodulatory, and Anticancer Compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Karp, M.; Jaszek, M.; Janusz, G.; Osińska-Jaroszuk, M.; Sulej, J.; Stefaniuk, D.; Tomczak, W.; Giannopoulos, K. Laccase Purified from Cerrena Unicolor Exerts Antitumor Activity against Leukemic Cells. Oncol. Lett. 2016, 11, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Driscoll, C.B.; Walters, P.R.; Limper, A.H.; Carmona, E.M. -Glucan-Activated Human B Lymphocytes Participate in Innate Immune Responses by Releasing Proinflammatory Cytokines and Stimulating Neutrophil Chemotaxis. J. Immunol. 2015, 195, 5318–5326. [Google Scholar] [CrossRef]
- Kataoka, K.; Muta, T.; Yamazaki, S.; Takeshige, K. Activation of Macrophages by Linear (1→3)-β-D-Glucans. Implications for the Recognition of Fungi by Innate Immunity. J. Biol. Chem. 2002, 277, 36825–36831. [Google Scholar] [CrossRef]
- Ratitong, B.; Marshall, M.; Pearlman, E. β-Glucan-Stimulated Neutrophil Secretion of IL-1α Is Independent of GSDMD and Mediated through Extracellular Vesicles. Cell Rep. 2021, 35, 109139. [Google Scholar] [CrossRef]
- Vlasova, I.I. Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. Molecules 2018, 23, 2561. [Google Scholar] [CrossRef]
- Cugno, M.; Marzano, A.V.; Lorini, M.; Carbonelli, V.; Tedeschi, A. Enhanced Tissue Factor Expression by Blood Eosinophils from Patients with Hypereosinophilia: A Possible Link with Thrombosis. PLoS ONE 2014, 9, e111862. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Mira, M.L.; Azevedo, M.S.; Manso, C. Mechanisms of Hemolysis Induced by Copper. Free Radic. Res. 1988, 4, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, S.; Luo, Z.; Yu, H.; Zhu, W.; Mao, Q.; Zhang, X.; Liang, Z.; Dong, A. Laccase-Mediated In Situ Oxidation of Dopamine for Dyeing of Human Hair. Fibers Polym. 2021, 22, 141–148. [Google Scholar] [CrossRef]
- Sun, K.; Li, S.; Si, Y.; Huang, Q. Advances in Laccase-Triggered Anabolism for Biotechnology Applications. Crit. Rev. Biotechnol. 2021, 41, 969–993. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Lin, Y.; Yu, P.; Su, L.; Mao, L. Laccase-Catalyzed Oxidation and Intramolecular Cyclization of Dopamine: A New Method for Selective Determination of Dopamine with Laccase/Carbon Nanotube-Based Electrochemical Biosensors. Electrochim. Acta 2007, 52, 4144–4152. [Google Scholar] [CrossRef]
- Schacterle, G.R.; Pollack, R.L. Method of for the Quantitative in Biologic Assay Material of Small Amounts Protein The Commonly Employed Method for the Quantitative Assay of Proteins by Lowry et al. (1) Takes about 40 Min to Perform and Requires Three Reagents. The Modification of T. Anal. Chem. 1973, 51, 654–655. [Google Scholar]
- Leonowicz, A.; Grzywnowicz, K. Quantitative Estimation of Laccase Forms in Some White-Rot Fungi Using Syringaldazine as a Substrate. Enzyme Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Brioukhanov, A.L.; Durand, M.C.; Dolla, A.; Aubert, C. Response of Desulfovibrio Vulgaris Hildenborough to Hydrogen Peroxide: Enzymatic and Transcriptional Analyses. FEMS Microbiol. Lett. 2010, 310, 175–181. [Google Scholar] [CrossRef]
- Gallati, H.; Brodbeck, H. Horseradish Peroxidase: Kinetic Studies and Optimization of the Activity Determination with the Substrates H2O2 and o-Phenylenediamine. J. Clin. Chem. Clin. Biochem. 1982, 20, 221–225. [Google Scholar]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do Novel Cement-Type Biomaterials Reveal Ion Reactivity That Affects Cell Viability in Vitro? Cent. Eur. J. Biol. 2014, 9, 277–289. [Google Scholar] [CrossRef]


| Amount of Immobilized Enzyme (µg/g Dry Hydrogel) | Type of Coating on Curdlan Hydrogel | ||||
|---|---|---|---|---|---|
| Polydopamine (PDA) | Poly(L-DOPA) (PLD) | None | |||
| – | +Cu+2 | – | +Cu+2 | ||
| laccase | 1625 | 1815 | 2520 | 2755 | 0 |
| peroxidase | 2386 | 3072 | 3430 | 3608 | 0 |
| Activity of immobilized enzyme (U/g dry hydrogel) | poly(L-DOPA) (PLD) | none | |||
| – | +Cu+2 | ||||
| laccase | 9.375 | 57.437 | 0 | ||
| peroxidase | 5.312 | 12.812 | 0 | ||
| Sample Code | Sample Description | Sample Image |
|---|---|---|
| CR-CTRL | curdlan hydrogel |  |
| CR-PLD-P | curdlan hydrogel coated with poly(L-DOPA) + peroxidase |  |
| CR-PLD-Cu-P | curdlan hydrogel coated with poly(L-DOPA) with Cu+2 ions + peroxidase |  |
| CR-PLD-L | curdlan hydrogel coated with poly(L-DOPA) + laccase |  |
| CR-PLD-Cu-L | curdlan hydrogel coated with poly(L-DOPA) with Cu+2 ions + laccase |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalicha, A.; Przekora, A.; Stefaniuk, D.; Jaszek, M.; Matuszewska, A.; Belcarz, A. Medical Use of Polycatecholamines + Oxidoreductases-Modified Curdlan Hydrogels—Perspectives. Int. J. Mol. Sci. 2022, 23, 10084. https://doi.org/10.3390/ijms231710084
Michalicha A, Przekora A, Stefaniuk D, Jaszek M, Matuszewska A, Belcarz A. Medical Use of Polycatecholamines + Oxidoreductases-Modified Curdlan Hydrogels—Perspectives. International Journal of Molecular Sciences. 2022; 23(17):10084. https://doi.org/10.3390/ijms231710084
Chicago/Turabian StyleMichalicha, Anna, Agata Przekora, Dawid Stefaniuk, Magdalena Jaszek, Anna Matuszewska, and Anna Belcarz. 2022. "Medical Use of Polycatecholamines + Oxidoreductases-Modified Curdlan Hydrogels—Perspectives" International Journal of Molecular Sciences 23, no. 17: 10084. https://doi.org/10.3390/ijms231710084