Abstract
The corticotropin-releasing hormone receptor 2 (CRHR2) gene encodes CRHR2, contributing to the hypothalamic–pituitary–adrenal stress response and to hyperglycemia and insulin resistance. CRHR2−/− mice are hypersensitive to stress, and the CRHR2 locus has been linked to type 2 diabetes and depression. While CRHR2 variants confer risk for mood disorders, MDD, and type 2 diabetes, they have not been investigated in familial T2D and MDD. In 212 Italian families with type 2 diabetes and depression, we tested 17 CRHR2 single nucleotide polymorphisms (SNPs), using two-point parametric-linkage and linkage-disequilibrium (i.e., association) analysis (models: dominant-complete-penetrance-D1, dominant-incomplete-penetrance-D2, recessive-complete-penetrance-R1, recessive-incomplete-penetrance-R2). We detected novel linkage/linkage-disequilibrium/association to/with depression (3 SNPs/D1, 2 SNPs/D2, 3 SNPs/R1, 3 SNPs/R2) and type 2 diabetes (3 SNPs/D1, 2 SNPs/D2, 2 SNPs/R1, 1 SNP/R2). All detected risk variants are novel. Two depression-risk variants within one linkage-disequilibrium block replicate each other. Two independent novel SNPs were comorbid while the most significant conferred either depression- or type 2 diabetes-risk. Although the families were primarily ascertained for type 2 diabetes, depression-risk variants showed higher significance than type 2 diabetes-risk variants, implying CRHR2 has a stronger role in depression-risk than type 2 diabetes-risk. In silico analysis predicted variants’ dysfunction. CRHR2 is for the first time linked to/in linkage-disequilibrium/association with depression-type 2 diabetes comorbidity and may underlie the shared genetic pathogenesis via pleiotropy.
1. Introduction
The hypothalamic–pituitary–adrenal axis (HPA) plays very important roles in humans’ physiologic response in stressful conditions []. The HPA is activated by the release of corticotropin-releasing hormone (CRH) from the hypothalamus, which leads to the secretion of adrenocorticotropin-releasing hormone (ACTH) from the pituitary gland []. The secretion of ACTH stimulates the production of cortisol from the adrenal glands, which then acts as a multisystemic stress signal []. Cortisol release is associated with insulin resistance, hyperglycemia, and type 2 diabetes (T2D) []. Chronic elevation in cortisol level can lead to structural changes in the brain associated with major depressive disorder (MDD) and anxiety []. Studies have shown that these physiopathological changes indicate mood disorders (i.e., MDD), and insulin resistance (i.e., T2D) can occur concomitantly [,,,]. The comorbidity of T2D and MDD [,,,] can be explained by environmental, iatrogenic (e.g., antipsychotics), hormonal, or genetic factors []. However, MDD per se confers 60% increased risk for T2D in drug-naïve patients []; thus, an underlying genetic comorbidity may link the two disorders, at least in a subset of patients.
CRH effects start by binding to one of its receptors. Currently, two main CRH receptors have been identified: CRHR1 and CRHR2. Both receptors are expressed in the brain and adrenal gland, but CRHR2 is more widely distributed, with expression in the pancreas, skeletal muscles, and adipose tissue []. Considering the important role that the HPA axis plays in stress response and glucose metabolism, variants in CRHR1 and CRHR2 genes may lead to abnormal psychological and/or T2D traits and account at least in part for the comorbidity of T2D and MDD []. CRHR2−/− mice were found to have anxiety-like behaviors and be hypersensitive to stress [], and the CRHR2 locus in humans (7p21-p15) has been linked to T2D and MDD [,,]. Risk variants in the CRHR2 gene have been reported in patients with MDD [], bipolar disorder [], and post-traumatic stress disorder (PTSD) []; and one risk variant has been reported in T2D []; however, variants in comorbid MDD-T2D patients have not been studied. Considering the pleiotropic role of CRHR2 gene in both mental and metabolic disorders, we hypothesized that risk variants in CRHR2 gene can predispose to MDD-T2D co-morbidity. Thus, we aimed to investigate in families CRHR2-variants linkage and/or linkage disequilibrium (LD, i.e., association) with/to MDD and/or T2D pathogenesis, and the potential CRHR2-variants contribution to the genetic comorbidity of MDD and T2D.
2. Results
2.1. Linkage, Linkage Disequilibrium (LD, i.e., Association) Analysis, and LD among Single Nucleotide Polymorphisms (SNPs)
In this paragraph, we present the most significant SNPs of linkage/LD tests for MDD and T2D; the statistical results of all tests and models for MDD are presented in Figure 1 and for T2D in Figure 2. We detected novel significant (p ≤ 0.05) linkage to and/or LD/association with MDD for: 3 SNPs/D1 (rs2284220, within LD-block Set 01, rs10271601, and rs255114); 2 SNPs/D2 (rs2284220 and rs1003929, both within LD-block Set 01); 3 SNPs/R1 (rs117157639, rs10271601, and rs255114); and 3 SNPs/R2 (rs2284220 and rs1003929, both within LD-block Set 01, and rs10271601). The MDD risk variants are all novel. We detected novel significant (p ≤ 0.05) linkage to and/or LD/association with T2D for: 3 SNPs/D1 (rs77113016, rs7812133, and rs10271601); 2 SNPs/D2 (rs77113016 and rs8192498); 2 SNPs/R1 (rs7812133 and rs255114); and 1 SNP/R2 (rs255114). The T2D risk variants are all novel. Two independent SNPs (rs10271601, rs255114) were comorbid and novel. The MDD-risk SNPs were in an MDD-specific LD block (Set01 containing 2 risk variants) or independent, and the T2D-risk SNPs were all independent. Specifics of the significant SNPs are provided in Table 1. The CRHR2-risk SNPs in MDD and T2D overlapping the parametric models are illustrated in Figure 3 and Figure 4.

Figure 1.
Major depressive disorder (MDD) CRHR2-Risk Single Nucleotide Polymorphisms (SNPs). For each CRHR2-risk SNPs in MDD, we present the −log10(P) as a function of each test statistic [Linkage, linkage disequilibrium (LD)|Linkage, LD|NoLinkage, Linkage|LD, and LD + Linkage] and per inheritance model: D1: dominant, complete penetrance, D2: dominant, incomplete penetrance, R1: recessive, complete penetrance, R2: recessive, incomplete penetrance.

Figure 2.
Type 2 Diabetes (T2D) CRHR2-Risk Single Nucleotide Polymorphisms (SNPs). For each CRHR2-risk SNPs in T2D, we present the −log10(P) as a function of each test statistic [Linkage, linkage disequilibrium (LD)|Linkage, LD|NoLinkage, Linkage|LD, and LD + Linkage] and per inheritance model: D1: dominant, complete penetrance, D2: dominant, incomplete penetrance, R1: recessive, complete penetrance, R2: recessive, incomplete penetrance.

Table 1.
CRHR2-Risk Single Nucleotide Polymorphisms (SNPs) for Major Depressive Disorder (MDD) and Type 2 Diabetes (T2D).

Figure 3.
Major Depressive Disorder (MDD) Overlapping Risk Single Nucleotide Polymorphisms (SNPs). Overlapping models for CRHR2-risk SNPs in MDD using a Venn diagram.
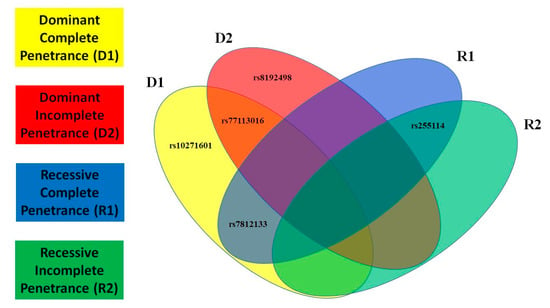
Figure 4.
Type 2 Diabetes (T2D) Overlapping Risk Single Nucleotide Polymorphisms (SNPs). Overlapping models for CRHR2-risk SNPs in T2D using a Venn diagram.
2.2. In-Silico Functional Predictions
For pathogenicity predictions, the coding variants were analyzed using Sorting Intolerant From Tolerant (SIFT) [], Polymorphism Phenotyping v2 [] (PolyPhen-2), and MutationTaster [] tools. The non-coding variants were analysed using tools that predict splicing (SpliceAI) [], transcription-factor (TF) binding (SNPnexus) [], SNP Function Prediction [], regulatory potential (RegulomeDB) [], and miRNA binding []. The non-risk allele (A) of the coding SNP rs77113016 (c.1312G > A, p.Val438Met) was predicted to be deleterious using SIFT; probably damaging using PolyPhen; and disease causing using MutationTaster. However, the novel risk allele (G) was predicted to create a binding site for a miRNA (hsa-miR-377-3p) that is also predicted to regulate genes involved in neuronal metabolic and oxidative stress []. The novel SNP rs255114 (MDD/T2D-comorbid risk) is located in a DNAase-hypersensitive site and a TF-binding site []. The novel risk allele (C) of SNP rs1003929 (MDD-risk) was predicted to affect the histone modification (H3K27me3) in neural tissue, causing an epigenetic modification of histone H3 associated with gene down-regulation [] (Figure 5).
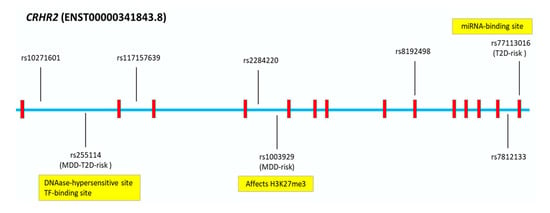
Figure 5.
Major Depressive Disorder (MDD) and/or Type 2 Diabetes (T2D) CRHR2-Single Nucleotide Polymorphisms (SNPs) and In Silico Analysis. In silico functional predictions of CRHR2-risk variants for MDD and/or T2D.
3. Discussion
Depression and T2D are both associated with high morbidity and impaired quality of life []. They share many etiological mechanisms and intersecting pathways, including the HPA-axis [] and insulin resistance []. Our novel findings demonstrate that in families with T2D, the CRHR2 gene contributes to both disorders via pleiotropism and underlies the shared genetic pathogenesis of their comorbidity. Within CRHR2, we detected five novel unique SNPs that were significantly linked to or in LD (i.e., association) with MDD; five novel unique SNPs that were linked to or in LD (i.e., association) with T2D; and two novel SNPs that were linked to both diseases across different modes of inheritance. All detected variants are novel both in MDD and T2D. One variant (rs7812133) has been previously studied in 66 MDD cases and 29 control subjects in relation to cortisol level and severity of depression []; however, the association was statistically non-significant, possibly due to the limited study size. In our study, the novel rs7812133 variant was significantly found in linkage to and LD/association with T2D (p = 0.04), and, as reported in the previous study [], does not contribute to depression. The novel T2D-risk rs8192498 variant detected within our study was previously found to be associated with resting metabolic rate and energy expenditure [], but was not found to be associated with suicide in traumatized children [] or panic disorder [], consistent with our negative finding in depression. The SNP rs77113016 identified to be a novel T2D-risk variant in our familial dataset was previously found in a severely obese child with hyperphagia, but it did not segregate with obesity in other family members [].
Interestingly, the variants linked to or in LD (i.e., association) with MDD had generally higher statistical significance level than the variants linked to or in LD (i.e., association) with T2D, despite the fact that the families were primarily ascertained for T2D, indicating that CRHR2 variants may confer a risk for MDD more strongly than for T2D. However, the most significant SNPs detected are either MDD-risk or T2D-risk variants. Of note, some of the MDD-risk variants were found within a novel LD block (Set01) which was not shared by the T2D-risk variants. The novel Set01 specific LD block related to MDD allows us to infer that a specific region in CRHR2 gene is linked to MDD and that the presence of other independent variants may confer additional risk for MDD and/or T2D. These results align with the concept that sequential stressors or chronic stress in the setting of genetic predisposition to HPA-axis hyperactivation may maintain chronic hypercortisolemia and lead primarily to depression [] via the mediated serotonin down-regulation [] and secondarily to T2D potentially via the long-term effects of chronic hypercortisolemia. In fact, CRHR2-knockout in mice led to stress hyper-responsiveness [], and CRHR2-blockade in rats abolished stress-induced neuronal serotonin synthesis []. In addition, CRHR2-knockout mice developed high blood pressure [], increased feeding [], and impaired glucose tolerance [], all traits related to T2D and metabolic syndrome []. In humans, the CRHR2 locus 7p21-p15 has been linked to depression [], bipolar disorder [,], obesity [], T2D [], and increased HDL and triglyceride levels []. In addition, CRHR2 antagonists attenuated the effect of brain-derived neurotrophic factor (BDNF) on reducing food intake and body weight []. Among previous human studies, the association of CRHR2 gene variants with MDD was not consistent. A rare CRHR2 variant (rs3779250-risk allele-C) has been reported in Japanese patients with MDD [], and a previous study in Belgium reported an MDD-risk variant with borderline significance (p = 0.04) []. However, a later study in Denmark [] reported no positive findings, probably due to ethnic differences. In addition, epigenetic changes in the CRHR2 gene were associated with MDD in female adolescents []. CRHR2 variants have also been reported in patients with bipolar disorder (rs8192492-risk allele-A) [], PTSD (rs2267715-risk allele-A) [], and T2D (rs917195 T2D-risk allele-C) []; and certain haplotypes have been associated with suicidal risk in bipolar patients [].
In our study, the novel SNPs rs2284220, rs1003929, and rs117157639 in LD/association with MDD, novel rs7812133 in LD/association with T2D, and novel rs255114 in LD/association with both disorders are likely the most relevant in the disorders’ pathogenesis. Large-scale case–control studies are needed to replicate these findings. However, as rs2284220 and rs1003929 lie within the same LD block, each one is a potential replicate of the other. Further, the two novel independent MDD-T2D comorbid SNPs, rs10271601 and rs255114, might underlie the pleiotropic effects of CRHR2 on mood and metabolic disturbances. The in silico functional analysis for the significantly linked SNPs predicted overall regulatory gene down-regulation (rs2284220, rs255114, rs1003929, rs117157639, rs77113016, and rs7812133) and perhaps lower CRHR2 density. CRHR2 down-regulation may be associated with the same effects seen in the CRHR2-knockout mice which manifested stressed and anxiety-like behavior []. CRHR2 down-regulation may also lead to hypercortisolemia via an inappropriately high-normal CRH and ACTH, via CRHR1 pituitary activation, and simultaneously drive central and peripheral catecholamine secretion, which can further mediate the anxiety-causing and hyperglycemic effects [].
4. Methods and Materials
We used the dataset of 212 families descended from at least 3 generations of Caucasian Italians originating from the Italian peninsula. Both sexes were included. Families with identical twins and siblings with uncertain paternity were excluded. The families had familial T2D [,] and were phenotyped for the presence or absence of MDD using DSM-IV diagnostic criteria []. All subjects and data were fully deidentified. Families were previously recruited in Italy, following the Helsinki declaration guidelines, and provided written informed consent prior to participation. The study was institutionally approved by the Jefferson Ethical Committee.
In the family subjects, we amplified 17 CRHR2 single nucleotide polymorphisms (SNPs) by microarray. We performed genotyping and Mendelian error exclusion using PLINK []. Using Pseudomarker [], we analyzed the 17 SNPs in CRHR2 for 2-point parametric-linkage to and LD (i.e., association) with T2D and MDD using the following models: dominant with complete penetrance (D1), dominant with incomplete penetrance (D2), recessive with complete penetrance (R1), and recessive with incomplete penetrance (R2). Pseudomarker [] uses statistical algorithms to perform parametric testing of both linkage and LD in affected and non-affected individuals. It provides 5 test statistics: Linkage, LD|Linkage, LD|NoLinkage, Linkage|LD, and LD + Linkage. The probability of an allele being linked or in LD to the phenotype is expressed in p values. In our study, we used (p ≤ 0.05) as cut off for statistical significance. To test the presence or absence of LD blocks within the variants showing statistically significant results in T2D or MDD (p ≤ 0.05), we computed LD correlations via LD matrix among the SNPs available in the Toscani Italian population from the 1000 Genomes Project (https://www.internationalgenome.org/data-portal/population/TSI) (accessed on 28 September 2021) []. The SNPs that significantly correlated (r2 ≥ 0.9) with other SNPs were considered within the same LD block and labeled based on that unique LD block (e.g., Set 01, Set 02). All SNPs designated as “Independent” were not correlated with any other SNPs.
5. Conclusions
This is the first study detecting CRHR2 gene pleiotropism underlying genetic comorbidity of MDD and T2D in T2D families. The relatively higher genetic homogeneity of the Italian families derived from a peninsula-based population may have increased the detection power of our analysis []. However, this study has limitations. The relationship between the CRHR2 SNPs and the metabolic disturbances and MDD can be indirect if the SNP is in linkage but not in LD, that is association, with the phenotype, and thus it is not in LD with the putative risk SNP. Some of the SNPs we detected were in LD with T2D and/or MDD as well as both in linkage and LD with T2D and/or MDD. However, to prove which SNP risk allele produces biological effects, in vitro studies are need. In fact, the SNP in LD with MDD and/or T2D might be in LD with another SNP which is the one having the major biological effects. Disentangling biological effects from risk SNPs in LD is not trivial. The predicted functional variants reported in our study should be experimentally validated; hence functional studies are needed. Our study is limited to one homogeneous ethnic group; therefore, the findings should be replicated in other ethnicities in order to draw a better picture of the role of the CRHR2 gene in these complex diseases.
Author Contributions
C.G. conceived and supervised the project, including families’ recruitment, statistical analysis, and manuscript drafting. J.O. and D.G. helped with the statistical analysis and manuscript drafting. M.A. helped with the bioinformatic analysis and manuscript drafting. M.V., R.W. and T.T.P. helped with data interpretation and critically revised the manuscript. All authors have approved the final manuscript. C.G. is the guarantor for the work. All authors have read and agreed to the published version of the manuscript.
Funding
C.G., R.W., T.P., J.O., and D.G. were supported by NICHD 5R01HD086911 (PI Gragnoli). NICHD had no role in the study design, data collection, analysis, and data interpretation or manuscript writing.
Institutional Review Board Statement
The study was institutionally approved by the Jefferson Ethical Committee (OHR-19).
Informed Consent Statement
Families were recruited following the Helsinki declaration guidelines and provided written informed consent prior to participation. Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request. The data are not publicly available due to privacy restrictions, lacking specific patients’ consent.
Acknowledgments
We thank the families who participated in the study, and we thank Bios Biotech Multi-Diagnostic Health Center, Rome, Italy, for data access and for financial, medical, and laboratory staff support. An abstract related to this manuscript has been accepted at the 30th European Congress of Psychiatry (EPA), Budapest, Hungary, June 2022.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACTH | adrenocorticotropin-releasing hormone |
| CRH | corticotropin-releasing hormone |
| D1 | dominant with complete penetrance |
| D2 | dominant with incomplete penetrance |
| HPA | hypothalamic–pituitary–adrenal axis |
| LD | linkage disequilibrium |
| MDD | Major Depressive Disorder |
| PTSD | post-traumatic stress disorder |
| R1 | recessive with complete penetrance |
| R2 | recessive with incomplete penetrance |
| SNPs | single nucleotide polymorphisms |
| T2D | type 2 diabetes |
References
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, J.J.; Inda, C.; Refojo, D.; Holsboer, F.; Arzt, E.; Silberstein, S. The Corticotropin-Releasing Hormone Network and the Hypothalamic-Pituitary-Adrenal Axis: Molecular and Cellular Mechanisms Involved. Neuroendocrinology 2011, 94, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, Cortisol; StatPearls: Tampa, FL, USA, 2021. [Google Scholar]
- Ortiz, R.; Kluwe, B.; Odei, J.B.; Echouffo Tcheugui, J.B.; Sims, M.; Kalyani, R.R.; Bertoni, A.G.; Golden, S.H.; Joseph, J.J. The association of morning serum cortisol with glucose metabolism and diabetes: The Jackson Heart Study. Psychoneuroendocrinology 2019, 103, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.-D.; Rizak, J.; Feng, X.-L.; Yang, S.-C.; Lü, L.-B.; Pan, L.; Yin, Y.; Hu, X.-T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6, 30187. [Google Scholar] [CrossRef]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef]
- Demakakos, P.; Pierce, M.B.; Hardy, R. Depressive symptoms and risk of type 2 diabetes in a national sample of middle-aged and older adults: The English longitudinal study of aging. Diabetes Care 2010, 33, 792–797. [Google Scholar] [CrossRef]
- Pan, A.; Lucas, M.; Sun, Q.; Van Dam, R.M.; Franco, O.H.; Manson, J.A.E.; Willett, W.C.; Ascherio, A.; Hu, F.B. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch. Intern. Med. 2010, 170, 1884–1891. [Google Scholar] [CrossRef]
- Rustad, J.K.; Musselman, D.L.; Nemeroff, C.B. The relationship of depression and diabetes: Pathophysiological and treatment implications. Psychoneuroendocrinology 2011, 36, 1276–1286. [Google Scholar] [CrossRef]
- Mezuk, B.; Heh, V.; Prom-Wormley, E.; Kendler, K.S.; Pedersen, N.L. Association between major depression and type 2 diabetes in midlife: Findings from the screening across the Lifespan twin study. Psychosom. Med. 2015, 77, 559–566. [Google Scholar] [CrossRef]
- Ji, H.-F.; Zhuang, Q.-S.; Shen, L.; Ji, H.-F.; Zhuang, Q.-S.; Shen, L. Genetic overlap between type 2 diabetes and major depressive disorder identified by bioinformatics analysis. Oncotarget 2016, 7, 17410–17414. [Google Scholar] [CrossRef]
- Amare, A.T.; Schubert, K.O.; Klingler-Hoffmann, M.; Cohen-Woods, S.; Baune, B.T. The genetic overlap between mood disorders and cardiometabolic diseases: A systematic review of genome wide and candidate gene studies. Transl. Psychiatry 2017, 7, e1007. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Mulugeta, A.; Wood, A.R.; Zhou, A.; Beaumont, R.N.; Tuke, M.A.; Jones, S.E.; Ruth, K.S.; Yaghootkar, H.; Sharp, S.; et al. Using genetics to understand the causal influence of higher BMI on depression. Int. J. Epidemiol. 2019, 48, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Berge, L.I.; Riise, T. Comorbidity between Type 2 Diabetes and Depression in the Adult Population: Directions of the Association and Its Possible Pathophysiological Mechanisms. Int. J. Endocrinol. 2015, 2015, 164760. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Gragnoli, C. Hypothesis of the neuroendocrine cortisol pathway gene role in the comorbidity of depression, type 2 diabetes, and metabolic syndrome. Appl. Clin. Genet. 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Bale, T.L.; Contarino, A.; Smith, G.W.; Chan, R.; Gold, L.H.; Sawchenko, P.E.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000, 24, 410–414. [Google Scholar] [CrossRef]
- Wiltshire, S.; Hattersley, A.T.; Hitman, G.A.; Walker, M.; Levy, J.C.; Sampson, M.; O’rahilly, S.; Frayling, T.M.; Bell, J.I.; Lathrop, G.M.; et al. A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (The diabetes UK Warren 2 repository): Analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am. J. Hum. Genet. 2001, 69, 553–569. [Google Scholar] [CrossRef]
- Camp, N.J.; Lowry, M.R.; Lynn Richards, R.; Plenk, A.M.; Carter, C.; Hensel, C.H.; Abkevich, V.; Skolnick, M.H.; Shattuck, D.; Rowe, K.G.; et al. Genome-wide linkage analyses of extended Utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am. J. Med. Genet.-Neuropsychiatr. Genet. 2005, 135B, 85–93. [Google Scholar] [CrossRef]
- Leak, T.S.; Langefeld, C.D.; Keene, K.L.; Gallagher, C.J.; Lu, L.; Mychaleckyj, J.C.; Rich, S.S.; Freedman, B.I.; Bowden, D.W.; Sale, M.M. Chromosome 7p linkage and association study for diabetes related traits and type 2 diabetes in an African-American population enriched for nephropathy. BMC Med. Genet. 2010, 11, 22. [Google Scholar] [CrossRef]
- Ishitobi, Y.; Nakayama, S.; Yamaguchi, K.; Kanehisa, M.; Higuma, H.; Maruyama, Y.; Ninomiya, T.; Okamoto, S.; Tanaka, Y.; Tsuru, J.; et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am. J. Med. Genetics. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2012, 159B, 429–436. [Google Scholar] [CrossRef]
- Cruceanu, C.; Schmouth, J.F.; Torres-Platas, S.G.; Lopez, J.P.; Ambalavanan, A.; Darcq, E.; Gross, F.; Breton, B.; Spiegelman, D.; Rochefort, D.; et al. Rare susceptibility variants for bipolar disorder suggest a role for G protein-coupled receptors. Mol. Psychiatry 2018, 23, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Li, G.; Cao, C.; Fang, R.; Liu, P.; Luo, S.; Zhang, X. Correlation between hypothalamic-pituitary-adrenal axis gene polymorphisms and posttraumatic stress disorder symptoms. Horm. Behav. 2020, 117, 104604. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N.; et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Schatzberg, A.F.; Keller, J.; Tennakoon, L.; Lembke, A.; Williams, G.; Kraemer, F.B.; Sarginson, J.E.; Lazzeroni, L.C.; Murphy, G.M. HPA Axis Genetic Variation, Cortisol, and Psychosis in Major Depression. Mol. Psychiatry 2014, 19, 220–227. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7–20. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Rödelsperger, C.; Schuelke, M.; Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 2010, 7, 575–576. [Google Scholar] [CrossRef]
- Jaganathan, K.; Kyriazopoulou Panagiotopoulou, S.; McRae, J.F.; Darbandi, S.F.; Knowles, D.; Li, Y.I.; Kosmicki, J.A.; Arbelaez, J.; Cui, W.; Schwartz, G.B.; et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell 2019, 176, 535–548.e24. [Google Scholar] [CrossRef]
- Dayem Ullah, A.Z.; Oscanoa, J.; Wang, J.; Nagano, A.; Lemoine, N.R.; Chelala, C. SNPnexus: Assessing the functional relevance of genetic variation to facilitate the promise of precision medicine. Nucleic Acids Res. 2018, 46, W109–W113. [Google Scholar] [CrossRef]
- Xu, Z.; Taylor, J.A. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009, 37 (Suppl. 2), W600–W605. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, F.; Li, T.; Lu, M.; Wang, L.; Yue, W.; Zhang, D. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genom. 2012, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Griss, J.; Viteri, G.; Sidiropoulos, K.; Nguyen, V.; Fabregat, A.; Hermjakob, H. ReactomeGSA-Efficient Multi-Omics Comparative Pathway Analysis. Mol. Cell. Proteom. 2020, 19, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, Y.; Loh, Y.P.; Tng, J.Q.; Lim, M.C.; Cao, Z.; Raju, A.; Lieberman Aiden, E.; Li, S.; Manikandan, L.; et al. H3K27me3-rich genomic regions can function as silencers to repress gene expression via chromatin interactions. Nat. Commun. 2021, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Goldney, R.D.; Phillips, P.J.; Fisher, L.J.; Wilson, D.H. Diabetes, Depression, and Quality of Life. Diabetes Care 2004, 27, 1066–1070. [Google Scholar] [CrossRef]
- Prestele, S.; Aldenhoff, J.; Reiff, J. The HPA-axis as a possible link between depression, diabetes mellitus and cognitive dysfunction. Fortschr. Neurol.-Psychiatr. 2003, 71, 24–36. [Google Scholar] [CrossRef]
- Lyra e Silva, N.d.M.; Lam, M.P.; Soares, C.N.; Munoz, D.P.; Milev, R.; De Felice, F.G. Insulin Resistance as a Shared Pathogenic Mechanism Between Depression and Type 2 Diabetes. Front. Psychiatry 2019, 10, 57. [Google Scholar] [CrossRef]
- Hellwege, J.N.; Edwards, D.R.V.; Acra, S.; Chen, K.; Buchowski, M.S.; Edwards, T.L. Association of gene coding variation and resting metabolic rate in a multi-ethnic sample of children and adults. BMC Obes. 2017, 4, 12. [Google Scholar] [CrossRef]
- Roy, A.; Hodgkinson, C.A.; DeLuca, V.; Goldman, D.; Enoch, M.A. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J. Psychiatr. Res. 2012, 46, 72–79. [Google Scholar] [CrossRef]
- Keck, M.E.; Kern, N.; Erhardt, A.; Unschuld, P.G.; Ising, M.; Salyakina, D.; Müller, M.B.; Knorr, C.C.; Lieb, R.; Hohoff, C.; et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147, 1196–1204. [Google Scholar] [CrossRef]
- Challis, B.G.; Luan, J.; Keogh, J.; Wareham, N.J.; Farooqi, I.S.; O’Rahilly, S. Genetic variation in the corticotrophin-releasing factor receptors: Identification of single-nucleotide polymorphisms and association studies with obesity in UK Caucasians. Int. J. Obes. 2004, 28, 442–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sukhareva, E.V. The role of the corticotropin-releasing hormone and its receptors in the regulation of stress response. Vavilovskii Zhurnal Genet. I Sel. 2021, 25, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ramos, O.A.; Lattig, M.C.; González Barrios, A.F. Modeling of the hypothalamic-pituitary-adrenal axis-mediated interaction between the serotonin regulation pathway and the stress response using a Boolean approximation: A novel study of depression. Theor. Biol. Med. Model. 2013, 10, 59. [Google Scholar] [CrossRef]
- Donner, N.C.; Siebler, P.H.; Johnson, D.T.; Villarreal, M.D.; Mani, S.; Matti, A.J.; Lowry, C.A. Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology 2016, 63, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Coste, S.C.; Kesterson, R.A.; Heldwein, K.A.; Stevens, S.L.; Heard, A.D.; Hollis, J.H.; Murray, S.E.; Hill, J.K.; Pantely, G.A.; Hohimer, A.R.; et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000, 24, 403–409. [Google Scholar] [CrossRef]
- Simpson, S.J.S.; Smith, L.I.F.; Jones, P.M.; Bowe, J.E. UCN2: A new candidate influencing pancreatic β-cell adaptations in pregnancy. J. Endocrinol. 2020, 245, 247–257. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Hamshere, M.L.; Schulze, T.G.; Schumacher, J.; Corvin, A.; Owen, M.J.; Jamra, R.A.; Propping, P.; Maier, W.; Orozco Y Diaz, G.; Mayoral, F.; et al. Mood-incongruent psychosis in bipolar disorder: Conditional linkage analysis shows genome-wide suggestive linkage at 1q32.3, 7p13 and 20q13.31. Bipolar Disord. 2009, 11, 610–620. [Google Scholar] [CrossRef]
- Kremeyer, B.; García, J.; Müller, H.; Burley, M.W.; Herzberg, I.; Parra, M.V.; Duque, C.; Vega, J.; Montoya, P.; López, M.C.; et al. Genome-wide linkage scan of bipolar disorder in a Colombian population isolate replicates loci on chromosomes 7p21-22, 1p31, 16p12 and 21q21-22 and identifies a novel locus on chromosome 12q. Hum. Hered. 2011, 70, 255–268. [Google Scholar] [CrossRef]
- Pérusse, L.; Rankinen, T.; Zuberi, A.; Chagnon, Y.C.; Weisnagel, S.J.; Argyropoulos, G.; Walts, B.; Snyder, E.E.; Bouchard, C. The human obesity gene map: The 2004 update. Obes. Res. 2005, 13, 381–490. [Google Scholar] [CrossRef]
- Tam, C.H.; Lam, V.K.; So, W.Y.; Ma, R.C.; Chan, J.C.; Ng, M.C. Genome-wide linkage scan for factors of metabolic syndrome in a Chinese population. BMC Genet. 2010, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Toriya, M.; Maekawa, F.; Maejima, Y.; Onaka, T.; Fujiwara, K.; Nakagawa, T.; Nakata, M.; Yada, T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2010, 22, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, S.M.; Del-Favero, J.; Adolfsson, R.; Souery, D.; Massat, I.; Mendlewicz, J.; Van Broeckhoven, C.; Claes, S. Gene-based SNP genetic association study of the corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am. J. Med. Genet. 2002, 114, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Buttenschon, H.N.; Krogh, J.; Nielsen, M.N.; Kaerlev, L.; Nordentoft, M.; Mors, O. Association analyses of depression and genes in the hypothalamus-pituitary-adrenal axis. Acta Neuropsychiatr. 2017, 29, 59–64. [Google Scholar] [CrossRef]
- Humphreys, K.L.; Moore, S.R.; Davis, E.G.; MacIsaac, J.L.; Lin, D.T.S.; Kobor, M.S.; Gotlib, I.H. DNA methylation of HPA-axis genes and the onset of major depressive disorder in adolescent girls: A prospective analysis. Transl. Psychiatry 2019, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Tharmalingam, S.; Kennedy, J.L. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2007, 22, 282–287. [Google Scholar] [CrossRef]
- McCann, S.M.; Antunes-Rodrigues, J.; Franci, C.R.; Anselmo-Franci, J.A.; Karanth, S.; Rettori, V. Role of the hypothalamic pituitary adrenal axis in the control of the response to stress and infection. Braz. J. Med. Biol. Res. 2000, 33, 1121–1131. [Google Scholar] [CrossRef]
- Gragnoli, C. Proteasome Modulator 9 and Macrovascular Pathology of T2D. Cardiovasc. Diabetol. 2011, 10, 32. [Google Scholar] [CrossRef]
- Gragnoli, C. Proteasome modulator 9 SNPs are linked to hypertension in type 2 diabetes families. Cardiovasc. Diabetol. 2011, 10, 77. [Google Scholar] [CrossRef]
- Amerian Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; Amerian Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Hiekkalinna, T.; Schaffer, A.A.; Lambert, B.; Norrgrann, P.; Goring, H.H.; Terwilliger, J.D. Pseudomarker: A powerful program for joint linkage and/or linkage disequilibrium analysis on mixtures of singletons and related individuals. Hum. Hered. 2011, 71, 256–266. [Google Scholar] [CrossRef] [PubMed]
- LDmatrix Function-RDocumentation. Available online: https://www.rdocumentation.org/packages/LDlinkR/versions/1.1.2/topics/LDmatrix (accessed on 28 September 2021).
- Gordon, D.; Finch, S.J.; Kim, W. Heterogeneity in Statistical Genetics; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).