Gain-of-Function Dynamin-2 Mutations Linked to Centronuclear Myopathy Impair Ca2+-Induced Exocytosis in Human Myoblasts
Abstract
:1. Introduction
2. Results
2.1. pHluorin Signals in Human C25 Myoblasts
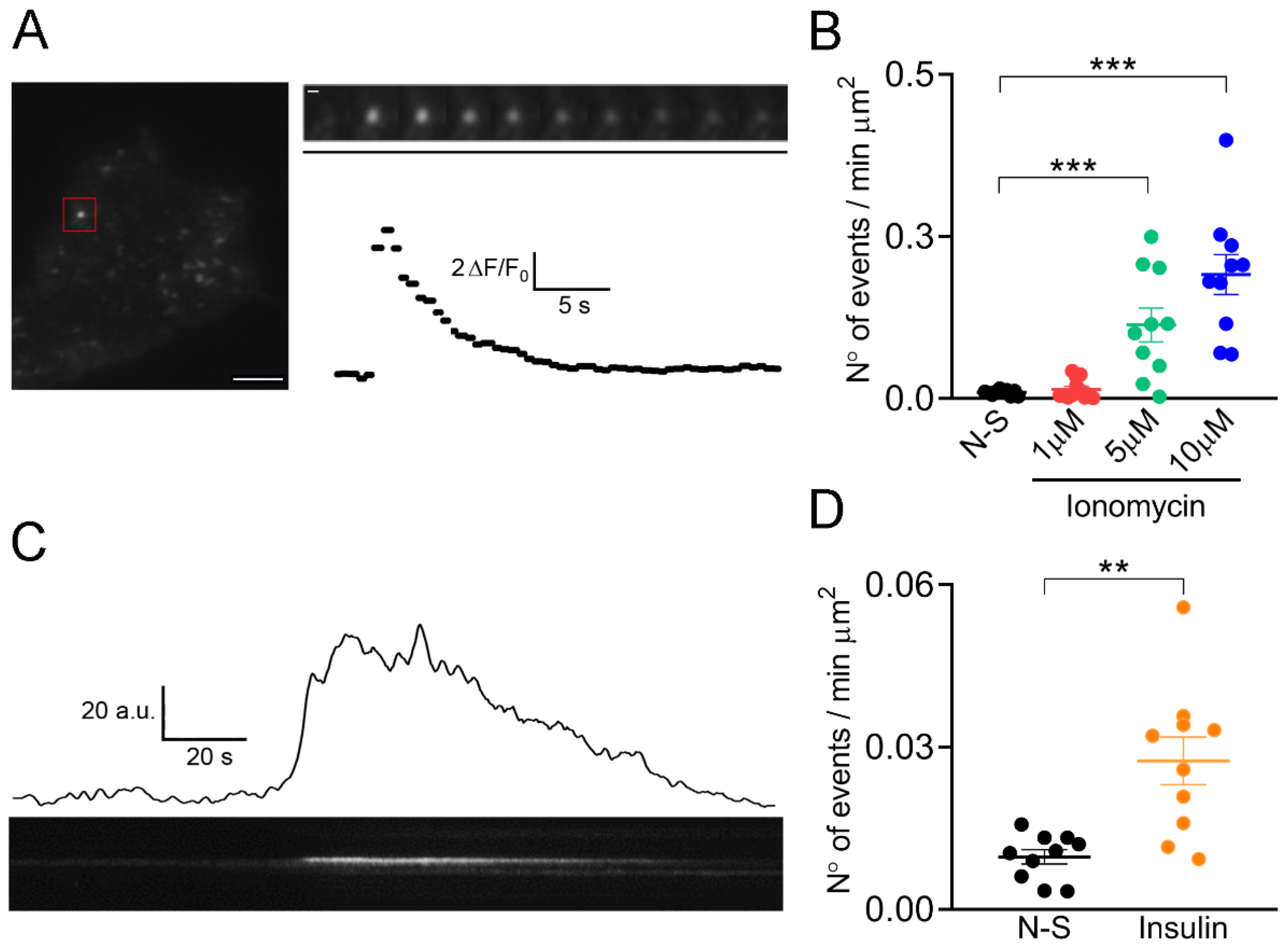
2.2. Kinetics of the IRAP-pHluorin Signals in C25 Myoblasts Depends on the Stimulus
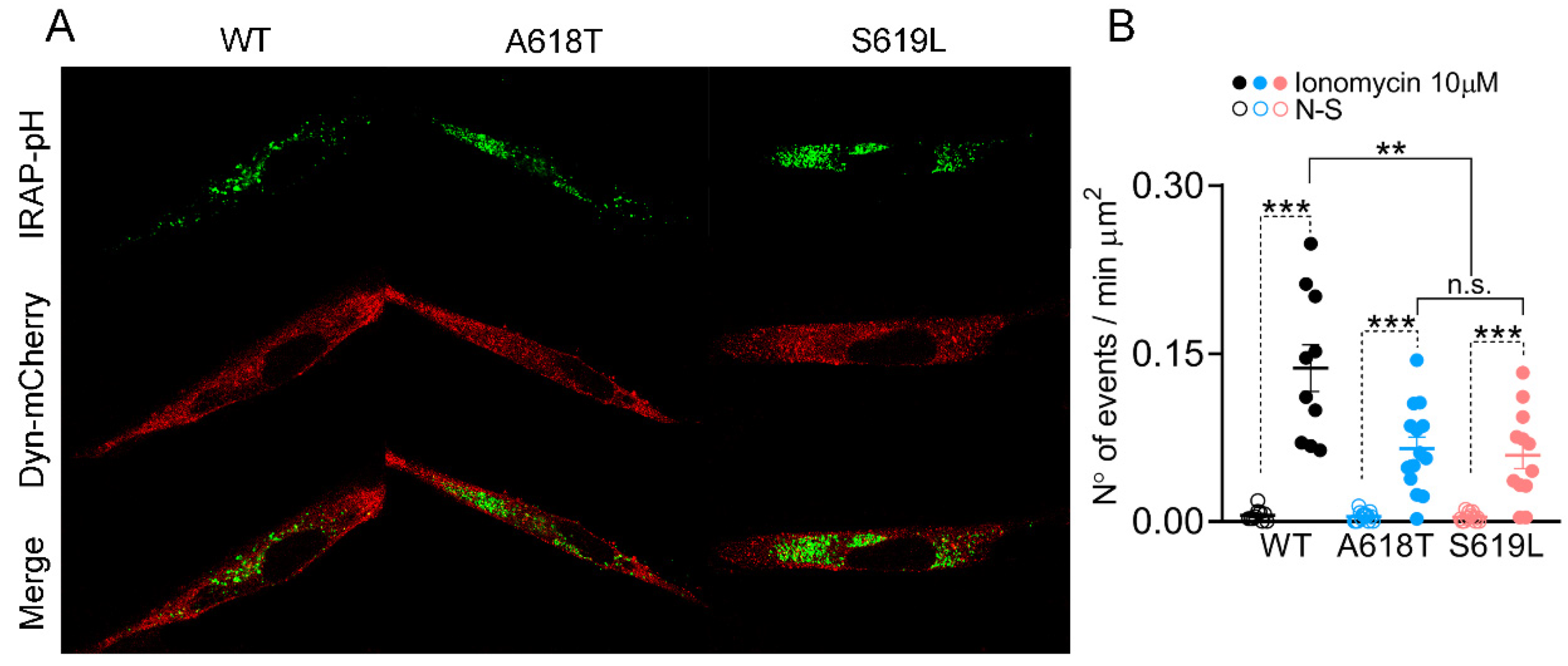
2.3. Dynamin-2 p.A618T and p.S619L Mutations Reduce Exocytosis in C25 Myoblasts
2.4. Dynamin-2 p.A618T and p.S619L Mutations Impairs the Formation of Actin Filaments in C25 Myoblasts
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Culture of Cell Lines and Transfection
4.3. Live-Cell Fluorescence Imaging
4.4. Image Analysis
4.5. Non-Lateral and Lateral Diffusion Event Analysis
4.6. Actin Filaments Formation
4.7. Cytosolic Ca2+ Measurements
4.8. Statistics
4.9. Ethics Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Jamett, A.M.; Bevilacqua, J.A.; Cárdenas Díaz, A.M. Hereditary Myopathies. In Muscle Cell and Tissue—Current Status of Research Field; Sakuma, K., Ed.; IntechOpen: London, U.K, 2018. [Google Scholar] [CrossRef]
- Vandersmissen, I.; Biancalana, V.; Servais, L.; Dowling, J.J.; Vander Stichele, G.; Van Rooijen, S.; Thielemans, L. An integrated modelling methodology for estimating the prevalence of centronuclear myopathy. Neuromuscul. Disord. 2018, 28, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Biancalana, V.; Dechene, E.T.; Bitoun, M.; Pierson, C.R.; Schaefer, E.; Karasoy, H.; Dempsey, M.A.; Klein, F.; Dondaine, N.; et al. Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum. Mutat. 2012, 33, 949–959. [Google Scholar] [CrossRef] [PubMed]
- González-Jamett, A.M.; Haro-Acuña, V.; Momboisse, F.; Caviedes, P.; Bevilacqua, J.A.; Cárdenas, A.M. Dynamin-2 in nervous system disorders. J. Neurochem. 2014, 128, 210–223. [Google Scholar] [CrossRef]
- Arriagada-Diaz, J.; Prado-Vega, L.; Cárdenas, A.M.; Ardiles, A.O.; Gonzalez-Jamett, A.M. Dynamin Superfamily at Pre- and Postsynapses: Master Regulators of Synaptic Transmission and Plasticity in Health and Disease. Neuroscientist 2020, 28, 41–58. [Google Scholar] [CrossRef]
- Gomez, T.S.; Hamann, M.J.; McCarney, S.; Savoy, D.N.; Lubking, C.M.; Heldebrant, M.P.; Labno, C.M.; McKean, D.J.; McNiven, M.A.; Burkhardt, J.K.; et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat. Immunol. 2005, 6, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Abe, T.; Watanabe, M.; Yagisawa, H.; Takei, K.; Yamada, H. Dynamin 2 is required for actin assembly in phagocytosis in Sertoli cells. Biochem. Biophys. Res. Commun. 2009, 378, 478–482. [Google Scholar] [CrossRef] [PubMed]
- González-Jamett, A.M.; Momboisse, F.; Guerra, M.J.; Ory, S.; Báez-Matus, X.; Barraza, N.; Calco, V.; Houy, S.; Couve, E.; Neely, A.; et al. Dynamin-2 regulates fusion pore expansion and quantal release through a mechanism that involves actin dynamics in It neuroendocrine chromaffin cells. PLoS ONE 2013, 8, e70638. [Google Scholar]
- Yamada, H.; Takeda, T.; Michiue, H.; Abe, T.; Takei, K. Actin bundling by dynamin 2 and cortactin is implicated in cell migration by stabilizing filopodia in human non-small cell lung carcinoma cells. Int. J. Oncol. 2016, 49, 877–886. [Google Scholar] [CrossRef]
- Chuang, M.C.; Lin, S.S.; Ohniwa, R.L.; Lee, G.H.; Su, Y.A.; Chang, Y.C.; Tang, M.J.; Liu, Y.W. Tks5 and Dynamin-2 enhance actin bundle rigidity in invadosomes to promote myoblast fusion. J. Cell. Biol. 2019, 218, 1670–1685. [Google Scholar] [CrossRef]
- Arneson, L.N.; Segovis, C.M.; Gomez, T.S.; Schoon, R.A.; Dick, C.J.; Lou, Z.; Billadeau, D.D.; Leibson, P.J. Dynamin 2 regulates granule exocytosis during NK cell-mediated cytotoxicity. J. Immunol. 2008, 181, 6995–7001. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Ji, C.; Wu, Y.; Ferguson, S.M.; Tamarina, N.; Philipson, L.H.; Lou, X. Dynamin 2 regulates biphasic insulin secretion and plasma glucose homeostasis. J. Clin. Investig. 2015, 125, 4026–4041. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Ishii, T.; Kohda, T.; Ishizuka, M.; Yamazaki, K.; Nishimura, Y.; Tanaka, T.; Dan, S.; Nakajima, M. Mechanistic study of PpIX accumulation using the JFCR39 cell panel revealed a role for dynamin 2-mediated exocytosis. Sci. Rep. 2019, 9, 8666. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Q.; Liu, B.; Li, Y.; Wu, X.; Kuo, S.; Zheng, L.; Wang, C.; Zhu, F.; Zhou, Z. Dynamin 1 Restrains Vesicular Release to a Subquantal Mode in Mammalian Adrenal Chromaffin Cells. J. Neurosci. 2019, 39, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.W.; Matusica, D.; Wattchow, D.A.; McCluskey, A.; Robinson, P.J.; Keating, D.J. Dynamin regulates L cell secretion in human gut. Mol. Cell. Endocrinol. 2021, 535, 111398. [Google Scholar] [CrossRef]
- González-Jamett, A.M.; Báez-Matus, X.; Hevia, M.A.; Guerra, M.J.; Olivares, M.J.; Martínez, A.D.; Neely, A.; Cárdenas, A.M. The association of dynamin with synaptophysin regulates quantal size and duration of exocytotic events in chromaffin cells. J. Neurosci. 2010, 30, 10683–10691. [Google Scholar] [CrossRef] [PubMed]
- Lasič, E.; Stenovec, M.; Kreft, M.; Robinson, P.J.; Zorec, R. Dynamin regulates the fusion pore of endo- and exocytotic vesicles as revealed by membrane capacitance measurements. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2293–2303. [Google Scholar] [CrossRef]
- Zhou, X.; Shentu, P.; Xu, Y. Spatiotemporal Regulators for Insulin-Stimulated GLUT4 Vesicle Exocytosis. J. Diabetes Res. 2017, 2017, 1683678. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2021, 236, 2393–2412. [Google Scholar] [CrossRef]
- Chin, Y.H.; Lee, A.; Kan, H.W.; Laiman, J.; Chuang, M.C.; Hsieh, S.T.; Liu, Y.W. Dynamin-2 mutations associated with centronuclear myopathy are hypermorphic and lead to T-tubule fragmentation. Hum. Mol. Genet. 2015, 24, 5542–5554. [Google Scholar] [CrossRef]
- González-Jamett, A.M.; Baez-Matus, X.; Olivares, M.J.; Hinostroza, F.; Guerra-Fernández, M.J.; Vasquez-Navarrete, J.; Bui, M.T.; Guicheney, P.; Romero, N.B.; Bevilacqua, J.A.; et al. Dynamin-2 mutations linked to Centronuclear Myopathy impair actin-dependent trafficking in muscle cells. Sci. Rep. 2017, 7, 4580. [Google Scholar] [CrossRef]
- Cowling, B.S.; Prokic, I.; Tasfaout, H.; Rabai, A.; Humbert, F.; Rinaldi, B.; Nicot, A.S.; Kretz, C.; Friant, S.; Roux, A.; et al. Amphiphysin (BIN1) negatively regulates dynamin 2 for normal muscle maturation. J. Clin. Investig. 2017, 127, 4477–4487. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Hsieh, T.L.; Liou, G.G.; Li, T.N.; Lin, H.C.; Chang, C.W.; Wu, H.Y.; Yao, C.K.; Liu, Y.W. Dynamin-2 Regulates Postsynaptic Cytoskeleton Organization and Neuromuscular Junction Development. Cell Rep. 2020, 33, 108310. [Google Scholar] [CrossRef] [PubMed]
- Fujise, K.; Okubo, M.; Abe, T.; Yamada, H.; Nishino, I.; Noguchi, S.; Takei, K.; Takeda, T. Mutant BIN1-Dynamin 2 complexes dysregulate membrane remodeling in the pathogenesis of centronuclear myopathy. J. Biol. Chem. 2021, 296, 100077. [Google Scholar] [CrossRef] [PubMed]
- Kenniston, J.A.; Lemmon, M.A. Dynamin GTPase regulation is altered by PH domain mutations found in centronuclear myopathy patients. EMBO J. 2010, 29, 3054–3067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lippincott-Schwartz, J. Selective visualization of GLUT4 storage vesicles and associated Rab proteins using IRAP-pHluorin. Methods Mol. Biol. 2015, 1298, 173–179. [Google Scholar] [PubMed]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front. Physiol. 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ferrat, A.; Lavandero, S.; Jaimovich, E.; Klip, A. Calcium signaling in insulin action on striated muscle. Cell Calcium 2014, 56, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Ojuka, E.O.; Goyaram, V.; Smith, J.A. The role of CaMKII in regulating GLUT4 expression in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E322–E331. [Google Scholar] [CrossRef]
- Naro, F.; De Arcangelis, V.; Coletti, D.; Molinaro, M.; Zani, B.; Vassanelli, S.; Reggiani, C.; Teti, A.; Adamo, S. Increase in cytosolic Ca2+ induced by elevation of extracellular Ca2+ in skeletal myogenic cells. Am. J. Physiol. Cell. Physiol. 2003, 284, C969–C976. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Ishikura, S.; Zhang, D.; Gao, J.; Sun, Y.; Contreras-Ferrat, A.; Foley, K.P.; Lavandero, S.; Yao, Z.; et al. Ca2+ signals promote GLUT4 exocytosis and reduce its endocytosis in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E209–E224. [Google Scholar] [CrossRef] [Green Version]
- Jullié, D.; Choquet, D.; Perrais, D. Recycling endosomes undergo rapid closure of a fusion pore on exocytosis in neuronal dendrites. J. Neurosci. 2014, 34, 11106–11118. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Navarrete, J.; Martínez, A.D.; Ory, S.; Baéz-Matus, X.; González-Jamett, A.M.; Brauchi, S.; Caviedes, P.; Cárdenas, A.M. RCAN1 Knockdown Reverts Defects in the Number of Calcium-Induced Exocytotic Events in a Cellular Model of Down Syndrome. Front. Cell. Neurosci. 2018, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; De Angelis, D.; Rothman, J.E.; Ryan, T.A. The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 2000, 79, 2199–2208. [Google Scholar] [CrossRef]
- Leitz, J.; Kavalali, E.T. Ca2+ influx slows single synaptic vesicle endocytosis. J. Neurosci. 2011, 31, 16318–16326. [Google Scholar] [CrossRef] [PubMed]
- Vardjan, N.; Stenovec, M.; Jorgacevski, J.; Kreft, M.; Zorec, R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J. Neurosci. 2007, 27, 4737–4746. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Heinemann, S.F. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron 2009, 61, 397–411. [Google Scholar] [CrossRef]
- González-Jamett, A.M.; Momboisse, F.; Haro-Acuña, V.; Bevilacqua, J.A.; Caviedes, P.; Cárdenas, A.M. Dynamin-2 function and dysfunction along the secretory pathway. Front. Endocrinol. 2013, 4, 126. [Google Scholar] [CrossRef]
- Wang, L.; Barylko, B.; Byers, C.; Ross, J.A.; Jameson, D.M.; Albanesi, J.P. Dynamin 2 mutants linked to centronuclear myopathies form abnormally stable polymers. J. Biol. Chem. 2010, 285, 22753–22757. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Witczak, C.A.; Jessen, N.; Warro, D.M.; Toyoda, T.; Fujii, N.; Anderson, M.E.; Hirshman, M.F.; Goodyear, L.J. CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1150–E1160. [Google Scholar] [CrossRef] [PubMed]
- Park, D.R.; Park, K.H.; Kim, B.J.; Yoon, C.S.; Kim, U.H. Exercise ameliorates insulin resistance via Ca2+ signals distinct from those of insulin for GLUT4 translocation in skeletal muscles. Diabetes 2015, 64, 1224–1234. [Google Scholar] [CrossRef]
- Buvinic, S.; Almarza, G.; Bustamante, M.; Casas, M.; López, J.; Riquelme, M.; Sáez, J.C.; Huidobro-Toro, J.P.; Jaimovich, E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 2009, 284, 34490–34505. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Fuentealba, C.; Contreras-Ferrat, A.E.; Altamirano, F.; Espinosa, A.; Li, Q.; Niu, W.; Lavandero, S.; Klip, A.; Jaimovich, E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes 2013, 62, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ferrat, A.; Llanos, P.; Vásquez, C.; Espinosa, A.; Osorio-Fuentealba, C.; Arias-Calderon, M.; Lavandero, S.; Klip, A.; Hidalgo, C.; Jaimovich, E. Insulin elicits a ROS-activated and an IP3-dependent Ca2+ release, which both impinge on GLUT4 translocation. J. Cell Sci. 2014, 127, 1911–1923. [Google Scholar] [PubMed]
- Marengo, F.D.; Cárdenas, A.M. How does the stimulus define exocytosis in adrenal chromaffin cells? Pflugers Arch. 2018, 470, 155–167. [Google Scholar] [CrossRef]
- Elhamdani, A.; Palfrey, H.C.; Artalejo, C.R. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron 2001, 31, 819–830. [Google Scholar] [CrossRef]
- Álvarez de Toledo, G.; Montes, M.Á.; Montenegro, P.; Borges, R. Phases of the exocytotic fusion pore. FEBS Lett. 2018, 592, 3532–3541. [Google Scholar] [CrossRef]
- Tsuboi, T.; Rutter, G.A. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr. Biol. 2003, 13, 563–567. [Google Scholar] [CrossRef]
- Stenkula, K.G.; Lizunov, V.A.; Cushman, S.W.; Zimmerberg, J. Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal. Cell. Metab. 2010, 12, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Wu, Y.; Hara, M.; Rizk, A.; Ji, C.; Nerad, D.; Tamarina, N.; Lou, X. Dynamin deficiency causes insulin secretion failure and hyperglycemia. Proc. Natl. Acad. Sci. USA 2021, 118, e2021764118. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; van Nifterick, A.; Toonen, R.F.; Verhage, M. Dynamin controls neuropeptide secretion by organizing dense-core vesicle fusion sites. Sci. Adv. 2021, 7, eabf0659. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.S.; Obel, L.F.; Waagepetersen, H.S.; Schousboe, A.; Bak, L.K. Complex actions of ionomycin in cultured cerebellar astrocytes affecting both calcium-induced calcium release and store-operated calcium entry. Neurochem. Res. 2013, 38, 1260–1265. [Google Scholar] [CrossRef]
- Di Fiore, P.P.; De Camilli, P. Endocytosis and signaling: An inseparable partnership. Cell 2001, 106, 1–4. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Gallo, L.I.; Marengo, F.D. Exocytosis, endocytosis and recycling of secretory vesicles in neuroendocrine cells, and its regulation by cortical actin. BioCell 2022, 46, 1867–1873. [Google Scholar]
- Knudsen, J.R.; Madsen, A.B.; Li, Z.; Andersen, N.R.; Schjerling, P.; Jensen, T.E. Gene deletion of γ-actin impairs insu-lin-stimulated skeletal muscle glucose uptake in growing mice but not in mature adult mice. Physiol. Rep. 2022, 10, e15183. [Google Scholar] [CrossRef]
- Madsen, A.B.; Knudsen, J.R.; Henriquez-Olguin, C.; Angin, Y.; Zaal, K.J.; Sylow, L.; Schjerling, P.; Ralston, E.; Jensen, T.E. β-actin shows limited mobility and is required only for supraphysiological insulin-stimulated glucose transport in young adult soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E110–E125. [Google Scholar] [CrossRef]
- Shin, W.; Ge, L.; Arpino, G.; Villarreal, S.A.; Hamid, E.; Liu, H.; Zhao, W.D.; Wen, P.J.; Chiang, H.C.; Wu, L.G. Visualization of Membrane Pore in Live Cells Reveals a Dynamic-Pore Theory Governing Fusion and Endocytosis. Cell 2018, 173, 934–945.e12. [Google Scholar] [CrossRef]
- Antonescu, C.N.; Díaz, M.; Femia, G.; Planas, J.V.; Klip, A. Clathrin-dependent and independent endocytosis of glucose transporter 4 (GLUT4) in myoblasts: Regulation by mitochondrial uncoupling. Traffic 2008, 9, 1173–1190. [Google Scholar] [CrossRef]
- Hartig, S.M.; Ishikura, S.; Hicklen, R.S.; Feng, Y.; Blanchard, E.G.; Voelker, K.A.; Pichot, C.S.; Grange, R.W.; Raphael, R.M.; Klip, A.; et al. The F-BAR protein CIP4 promotes GLUT4 endocytosis through bidirectional interactions with N-WASp and Dynamin-2. J. Cell. Sci. 2009, 122, 2283–2291. [Google Scholar] [CrossRef]
- Thorley, M.; Duguez, S.; Mazza, E.M.C.; Valsoni, S.; Bigot, A.; Mamchaoui, K.; Harmon, B.; Voit, T.; Mouly, V.; Duddy, W. Skeletal muscle characteristics are preserved in hTERT/cdk4 human myogenic cell lines. Skelet. Muscle 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Báez-Matus, X.; Figueroa-Cares, C.; Gónzalez-Jamett, A.M.; Almarza-Salazar, H.; Arriagada, C.; Maldifassi, M.C.; Guerra, M.J.; Mouly, V.; Bigot, A.; Caviedes, P.; et al. Defects in G-Actin Incorporation into Filaments in Myoblasts Derived from Dysferlinopathy Patients Are Restored by Dysferlin C2 Domains. Int. J. Mol. Sci. 2019, 21, 37. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayonés, L.; Guerra-Fernández, M.J.; Hinostroza, F.; Báez-Matus, X.; Vásquez-Navarrete, J.; Gallo, L.I.; Parra, S.; Martínez, A.D.; González-Jamett, A.; Marengo, F.D.; et al. Gain-of-Function Dynamin-2 Mutations Linked to Centronuclear Myopathy Impair Ca2+-Induced Exocytosis in Human Myoblasts. Int. J. Mol. Sci. 2022, 23, 10363. https://doi.org/10.3390/ijms231810363
Bayonés L, Guerra-Fernández MJ, Hinostroza F, Báez-Matus X, Vásquez-Navarrete J, Gallo LI, Parra S, Martínez AD, González-Jamett A, Marengo FD, et al. Gain-of-Function Dynamin-2 Mutations Linked to Centronuclear Myopathy Impair Ca2+-Induced Exocytosis in Human Myoblasts. International Journal of Molecular Sciences. 2022; 23(18):10363. https://doi.org/10.3390/ijms231810363
Chicago/Turabian StyleBayonés, Lucas, María José Guerra-Fernández, Fernando Hinostroza, Ximena Báez-Matus, Jacqueline Vásquez-Navarrete, Luciana I. Gallo, Sergio Parra, Agustín D. Martínez, Arlek González-Jamett, Fernando D. Marengo, and et al. 2022. "Gain-of-Function Dynamin-2 Mutations Linked to Centronuclear Myopathy Impair Ca2+-Induced Exocytosis in Human Myoblasts" International Journal of Molecular Sciences 23, no. 18: 10363. https://doi.org/10.3390/ijms231810363




