Resveratrol Affects Sphingolipid Metabolism in A549 Lung Adenocarcinoma Cells
Abstract
:1. Introduction
2. Results
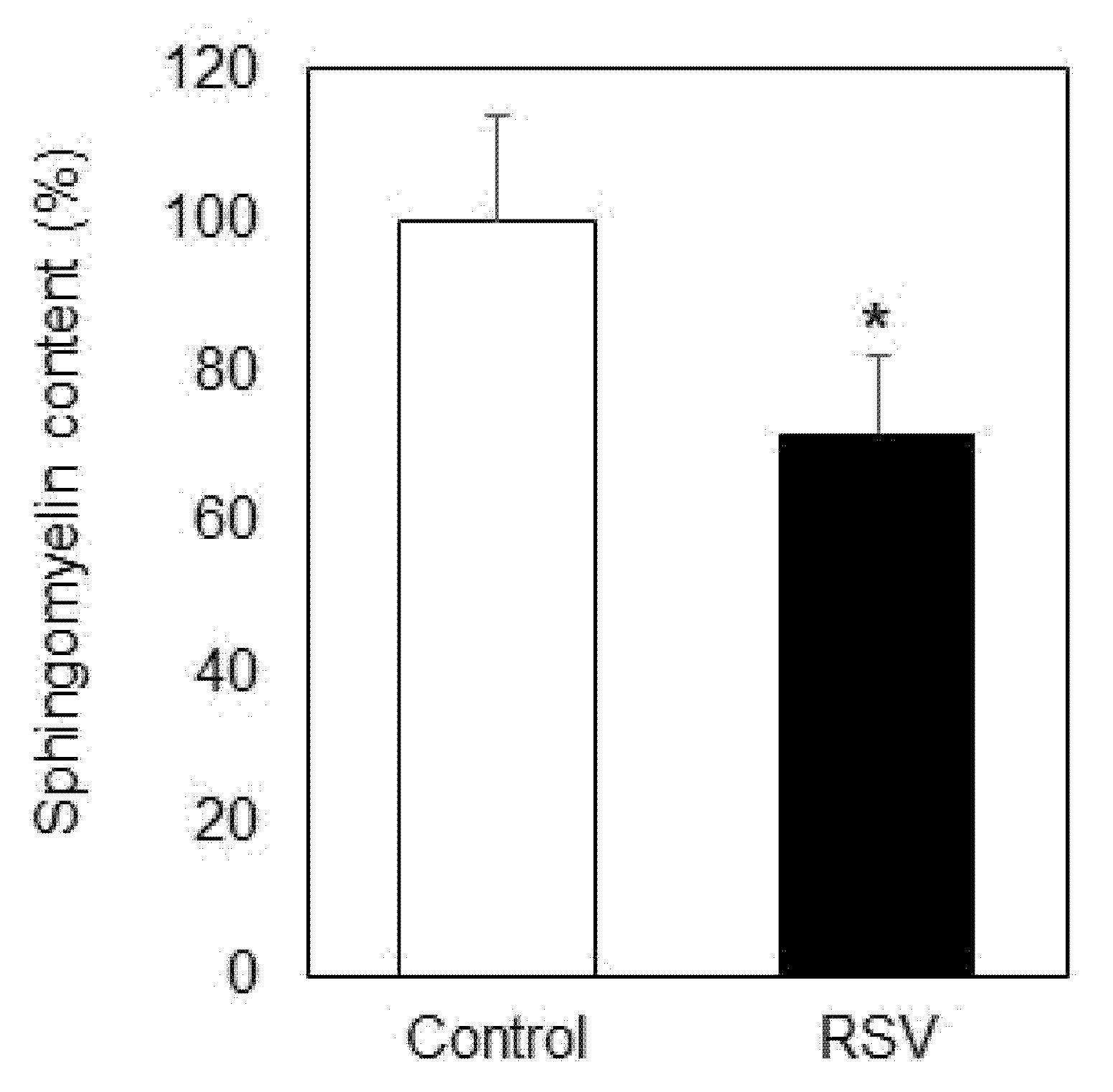
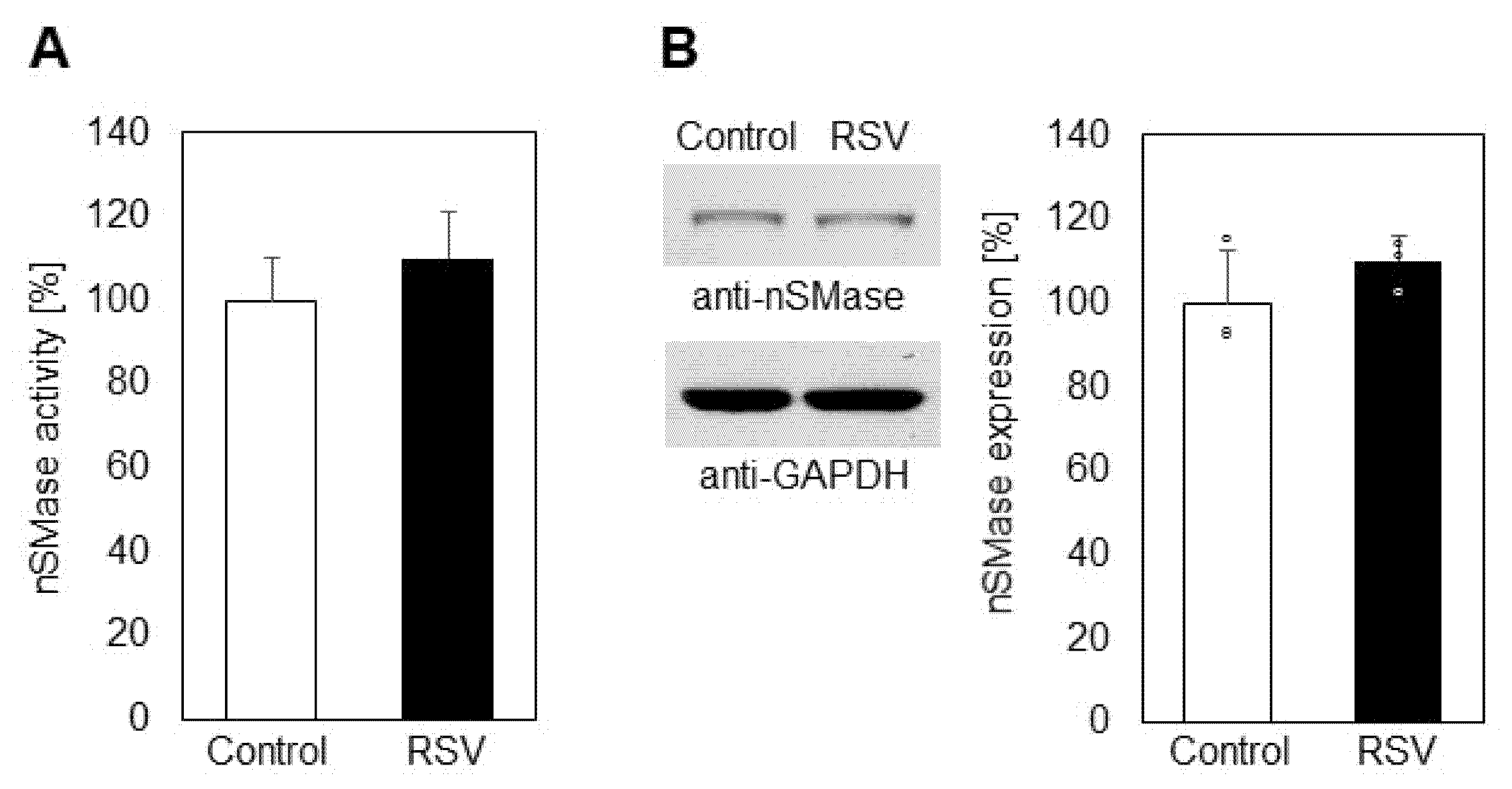
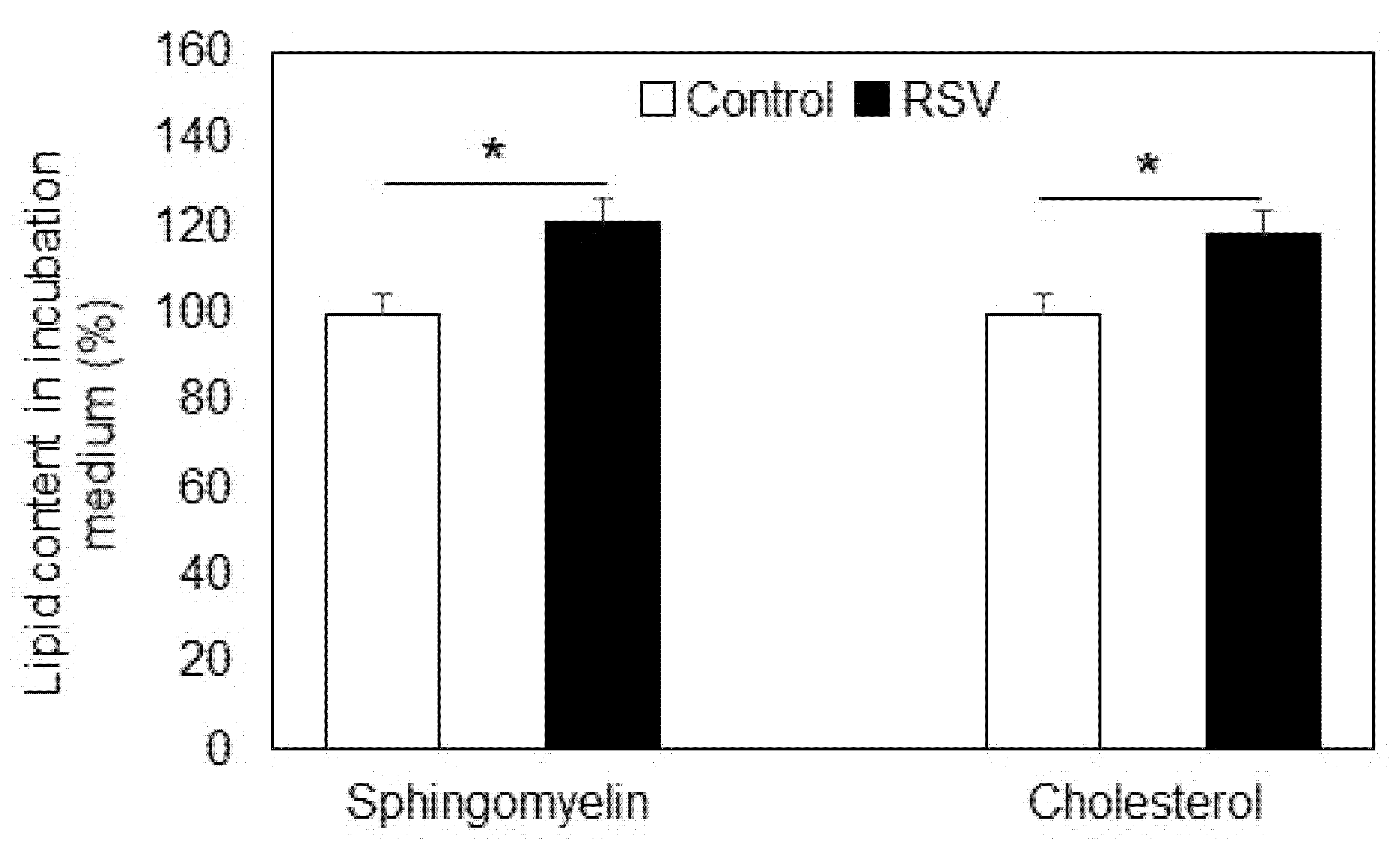
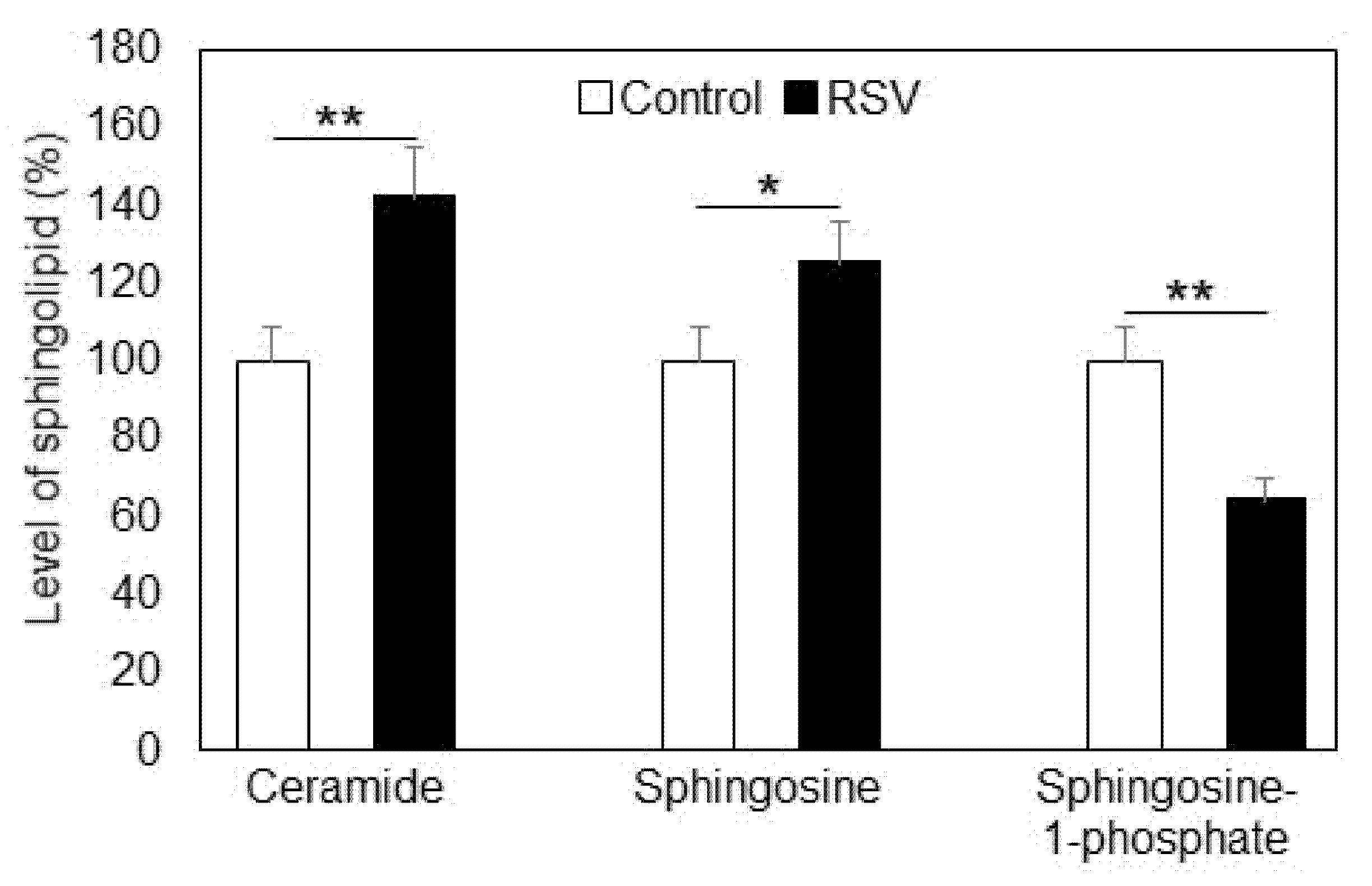
2.1. Effect of Resveratrol on Sphingolipid Level and Neutral Sphingomyelinase
2.2. Effect of Resveratrol on the Enzymes Maintaining Ceramide Level
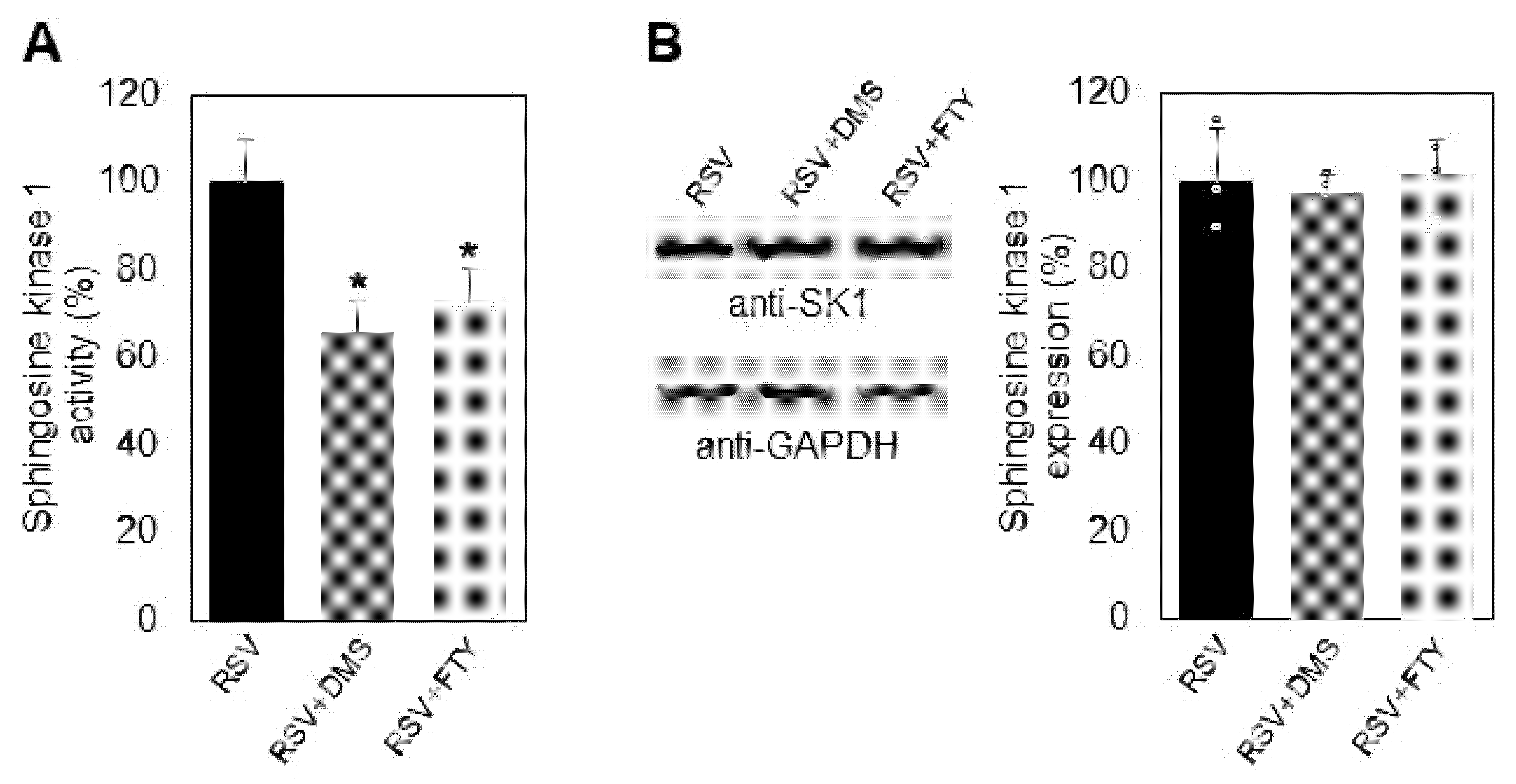
2.3. Influence of Resveratrol on Sphingosine Kinase 1 Activity and Expression
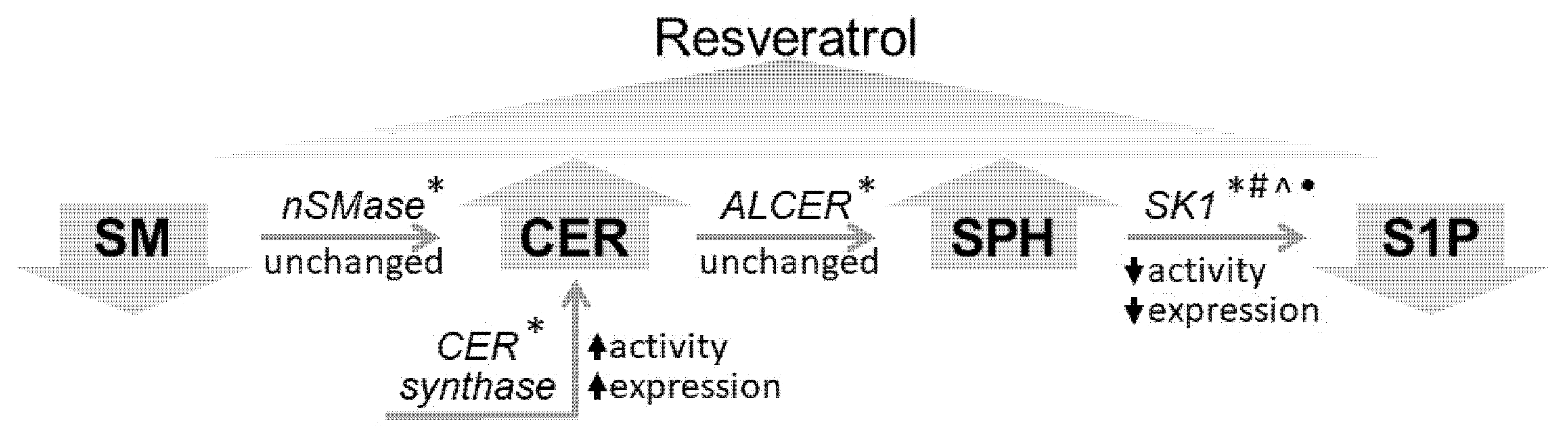
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Incubation with Resveratrol
4.3. Cell Viability Assay after Incubation with Resveratrol
4.4. Isolation of Plasma Membrane from A549 Cells
4.5. Lipid Analysis
4.6. Determination of Sphingomyelin
4.7. Determination of Ceramide
4.8. Determination of Sphingosine
4.9. Determination of Sphingosine-1-Phosphate (S1P)
4.10. Neutral Sphingomyelinase (nSM) Activity Assay
4.11. Ceramide Synthase 6 Activity Assay
4.12. Alkaline Ceramidase (ALCER) Activity Assay
4.13. Sphingosine Kinase 1 Activity Assay
4.14. Western Blotting and Antibodies
4.15. Statistical Analysis
5. Conclusions
- Resveratrol treatment of A549 cells induced an increase in ceramide and sphingosine and decrease in sphingomyelin and sphingosine-1-phosphate.
- Sphingomyelinase was not responsible for sphingomyelin reduction, but an elevation in sphingomyelin was observed in the incubation medium after resveratrol treatment.
- Resveratrol induced upregulation and elevated expression of ceramide synthase in A549 cells.
- Sphingosine kinase 1 was downregulated, and its expression was reduced in resveratrol treated A549 cells.
- Incubation of A549 with the SK1 inhibitors DMS and fingolimod induced additional downregulation of SK1 in resveratrol-treated lung adenocarcinoma cells, implying that the combined use of anti-proliferative effectors with naturally occurring anti-tumor agents could turn out to be a useful tool for reducing the pro-survival potential of cancer cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilello, K.; Murin, S.; Matthay, R. Epidemiology, etiology, and prevention of lung cancer. Clin. Chest Med. 2002, 23, 1–25. [Google Scholar] [CrossRef]
- Walker, A.J.; Baldwin, D.R.; Card, T.R.; Powell, H.A.; Hubbard, R.B.; Grainge, M.J. Risk of venous thromboembolism in people with lung cancer: A cohort study using linked UK healthcare data. Br. J. Cancer 2016, 115, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R.J. A new class of phytoalexins from grapevines. Experientia 1977, 33, 51–152. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Orallo, F. Trans-resveratrol: A magical elixir of eternal youth? Curr. Med. Chem. 2008, 5, 1887–1898. [Google Scholar] [CrossRef]
- Cowart, L.A. Sphingolipids: Players in the pathology of metabolic disease. Trends Endocrinol. Metab. 2009, 20, 34–42. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Meyers-Needham, M.; Senkal, C.E.; Saddoughi, S.A.; Sentelle, D.; Selvam, S.P.; Salas, A.; Ogretmen, B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010, 6, 1603–1624. [Google Scholar] [CrossRef]
- Gomez-Larrauri, A.; Presa, N.; Dominguez-Herrera, A.; Ouro, A.; Trueba, M.; Gomez-Muñoz, A. Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. 2020, 64, 579–589. [Google Scholar] [CrossRef]
- Maeng, H.J.; Song, J.H.; Kim, G.T.; Song, Y.J.; Lee, K.; Kim, J.Y.; Park, T.S. Celecoxib-mediated activation of endoplasmic reticulum stress induces de novo ceramide biosynthesis and apoptosis in hepatoma HepG2 cells mobilization. BMB Rep. 2017, 50, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.W.; Hojabrpour, P.; Zhang, P.; Kolesnick, R.N.; Steinbrecher, U.P.; Gómez-Muñoz, A.; Duronio, V. Regulation of ceramide generation during macrophage apoptosis by ASMase and de novo synthesis. Biochim Biophys Acta 2015, 1851, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Smith, C.D.; Foroozesh, M.; Miele, L.; Qin, Z. The sphingosine kinase 2 inhibitor ABC294640 displays anti-non-small cell lung cancer activities in vitro and in vivo. Int J Cancer 2018, 142, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, C.Y.; Lin, Y.C.; Wu, M.T.; Su, K.L.; Yuan, S.S.; Wang, H.D.; Fong, Y.; Lin, Y.H.; Chiu, C.C. C(2)-ceramide-induced Rb-dominant senescence-like phenotype leads to human breast cancer MCF-7 escape from p53-dependent cell death. Int. J. Mol. Sci. 2019, 20, 4292. [Google Scholar] [CrossRef]
- Rahman, A.; Pallichankandy, S.; Thayyullathil, F.; Galadari, S. Critical role of H2O2 in mediating sanguinarine-induced apoptosis in prostate cancer cells via facilitating ceramide generation, ERK1/2 phosphorylation, and Par-4 cleavage. Free Radic. Biol. Med. 2019, 134, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. The ceramide-centric universe of lipid-mediated cell regulation: Stress encounters of the lipid kind. J. Biol. Chem. 2002, 277, 25847–25850. [Google Scholar] [CrossRef]
- Yildiz-Ozer, M.; Oztopcu-Vatan, P.; Kus, G. The investigation of ceranib-2 on apoptosis and drug interaction with carboplatin in human non small cell lung cancer cells in vitro. Cytotechnology 2018, 70, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Cao, K.; Kato, S.; Komizu, Y.; Mizutani, N.; Tanaka, K.; Arima, C.; Tai, M.C.; Yanagisawa, K.; Togawa, N.; et al. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J. Clin. Investig. 2016, 126, 254–265. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Jana, A.; Pahan, K. Sphingolipids in multiple sclerosis. Neuromolecular Med. 2010, 12, 351–361. [Google Scholar] [CrossRef]
- Taha, T.A.; Hannun, Y.A.; Obeid, L.M. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 2006, 39, 113–131. [Google Scholar] [CrossRef]
- Ma, Y.; Xing, X.; Kong, R.; Cheng, C.; Li, S.; Yang, X.; Li, S.; Zhao, F.; Sun, L.; Cao, G. SphK1 promotes development of nonsmall cell lung cancer through activation of STAT3. Int. J. Mol. Med. 2021, 47, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, S.; Tamori, M.; Tanaka, A.; Utsumi, N.; Sato, H.; Hatakeyama, H.; Hisaka, A.; Kohama, T.; Yamagata, K.; Honda, T.; et al. Inhibitory effects of ceramide kinase on Rac1 activation, lamellipodium formation, cell migration, and metastasis of A549 lung cancer cells. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2020, 1865, 158675. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, X.; Zhao, X.; Kong, X.; Meng, Y.-M.; Chen, Y.; Su, L.; Jiang, X.; Qiu, X.; Huang, C.; et al. A circular network of coregulated sphingolipids dictates lung cancer growth and progression. eBioMedicine 2021, 66, 103301. [Google Scholar] [CrossRef] [PubMed]
- Dolfini, E.; Roncoroni, L.; Dogliotti, E.; Sala, G.; Erba, E.; Sacchi, N.; Ghidoni, R. Resveratrol impairs the formation of MDA-MB-231 multicellular tumor spheroids concomitant with ceramide accumulation. Cancer Lett. 2007, 249, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Scarlatti, F.; Sala, G.; Ricci, C.; Maioli, C.; Milani, F.; Minella, M.; Botturi, M.; Ghidoni, R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007, 253, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Huwiler, A.; Loitsch, S.; Schmidt, H.; Stein, J.M. De novo ceramide biosynthesis is associated with resveratrol-induced inhibition of ornithine decarboxylase activity. Biochem. Pharmacol. 2007, 74, 281–289. [Google Scholar] [CrossRef]
- Lim, K.G.; Gray, A.I.; Pyne, S.; Pyne, N.J. Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br. J. Pharmacol. 2012, 166, 1605–1611. [Google Scholar] [CrossRef]
- Lim, K.G.; Tonelli, F.; Berdyshev, E.; Gorshkova, I.; Leclercq, T.; Pitson, S.M.; Bittman, R.; Pyne, S.; Pyne, N.J. Inhibition kinetics and regulation of sphingosine kinase 1 expression in prostate cancer cells: Functional differences between sphingosine kinase 1a and 1b. Int. J. Biochem. Cell. Biol. 2012, 44, 1457–1464. [Google Scholar] [CrossRef]
- Issuree, P.D.; Pushparaj, P.N.; Pervaiz, S.; Melendez, A.J. Resveratrol attenuates C5a-induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009, 23, 2412–2424. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Harasim-Symbor, E.; Polak, A.; Drygalski, K.; Berk, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. Influence of Resveratrol on Sphingolipid Metabolism in Hepatocellular Carcinoma Cells in Lipid Overload State. Anti-Cancer Agents Med. Chem. 2019, 19, 121–129. [Google Scholar] [CrossRef]
- Sanders, T.H.; McMichael, R.W., Jr.; Hendrix, K.W. Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 2000, 48, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Drygalski, K.; Konstantynowicz-Nowicka, K.; Berk, K.; Chabowski, A. Alternative treatment methods attenuate the development of NAFLD: A review of resveratrol molecular mechanisms and clinical trials. Nutrition 2017, 34, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, L.; Dayon, A.; Doumerc, N.; Ader, I.; Golzio, M.; Izard, J.; Hara, Y.; Malavaud, B.; Cuvillier, A.O. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 2010, 24, 3882–3894. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Kamath-Loeb, A.S.; Balakrishna, S.; Whittington, D.; Shen, J.-C.; Emond, M.J.; Okabe, T.; Masutani, C.; Hanaoka, F.; Nishimura, S.; Loeb, L.A. Sphingosine: A Modulator of Human Translesion DNA Polymerase Activity. J. Biol. Chem. 2014, 289, 21663–21672. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Lee, K.T.; Kim, H.J.; Lee, H.-J.; Choi, Y.H.; Lee, C.-M.; Kim, L.K.; Kim, G.-Y. Anti-inflammatory mechanism of alpha-viniferin regulates lipopolysaccharide-induced release of pro-inflammatory mediators in BV2 microglial cells. Cell Immunol. 2014, 290, 21–29. [Google Scholar] [CrossRef]
- Kunkel, G.T.; Maceyka, M.; Milstien, S.; Spiegel, S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 688–702. [Google Scholar] [CrossRef]
- Momchilova, A.; Petkova, D.; Staneva, G.; Markovska, T.; Pankov, R.; Skrobanska, R.; Nikolova-Karakashian, M.; Koumanov, K. Resveratrol alters the lipid composition, metabolism and peroxide level in senescent rat hepatocytes. Chem. Biol. Interact. 2014, 207, 74–80. [Google Scholar] [CrossRef]
- Simons, K.; Vaz, W. Model systems, lipid rafts and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Lightle, A.; Oakley, J.; Nikolova-Karakashian, M. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech. Ageing Dev. 2000, 120, 111–125. [Google Scholar] [CrossRef]
- Subbaiah, P.; Sircar, D.; Lankalpalli, R.S.; Bittman, R. Effect of double bond geometry in sphingosine base on the antioxidant function of sphingomyelin. Arch. Biochem. Biophys. 2009, 481, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, V.; Tzoneva, R.; Stoyanova, T.; Pankov, R.; Skrobanska, R.; Georgiev, G.; Maslenkova, L.; Tsonchev, Z.; Momchilova, A. Dimethylsphingosine and miltefosine induce apoptosis in lung adenocarcinoma A549 cells in a synergistic manner. Chem.-Biol. Interact. 2019, 310, 108731. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Takabe, K.; Kapitonov, D.; Milstien, S.; Spiegel, S. Targeting SphK1 as a new strategy against cancer. Curr. Drug Targets 2008, 9, 662–673. [Google Scholar] [CrossRef]
- Herr, D.R.; Chun, J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr. Drug Targets 2007, 8, 155–167. [Google Scholar] [CrossRef]
- Long, J.S.; Edwards, J.; Watson, C.; Tovey, S.; Mair, K.M.; Schiff, R.; Natarajan, V.; Pyne, N.J.; Pyne, S. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol. Cell. Biol. 2010, 30, 3827–3841. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.G.; Tonelli, F.; Li, Z.; Lu, X.; Bittman, R.; Pyne, S.; Pyne, N.J. FTY720 analogues as sphingosine kinase 1 inhibitors: Enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J. Biol. Chem. 2011, 286, 18633–18640. [Google Scholar] [CrossRef]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef]
- Evans, H. (Ed.) Biological Membranes. A Practical Approach; IRL Press: Washington, DC, USA, 1987; pp. 1–35. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momchilova, A.; Pankov, R.; Staneva, G.; Pankov, S.; Krastev, P.; Vassileva, E.; Hazarosova, R.; Krastev, N.; Robev, B.; Nikolova, B.; et al. Resveratrol Affects Sphingolipid Metabolism in A549 Lung Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 10870. https://doi.org/10.3390/ijms231810870
Momchilova A, Pankov R, Staneva G, Pankov S, Krastev P, Vassileva E, Hazarosova R, Krastev N, Robev B, Nikolova B, et al. Resveratrol Affects Sphingolipid Metabolism in A549 Lung Adenocarcinoma Cells. International Journal of Molecular Sciences. 2022; 23(18):10870. https://doi.org/10.3390/ijms231810870
Chicago/Turabian StyleMomchilova, Albena, Roumen Pankov, Galya Staneva, Stefan Pankov, Plamen Krastev, Evgenia Vassileva, Rusina Hazarosova, Nikolai Krastev, Bozhil Robev, Biliana Nikolova, and et al. 2022. "Resveratrol Affects Sphingolipid Metabolism in A549 Lung Adenocarcinoma Cells" International Journal of Molecular Sciences 23, no. 18: 10870. https://doi.org/10.3390/ijms231810870







