A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease
Abstract
:1. Introduction
2. The Importance of Brain Insulin
2.1. Insulin Receptors
2.2. Glucose Transporters
2.3. Brain Insulin Synthesis De Novo
2.4. Insulin Effects on Specific Brain Cells
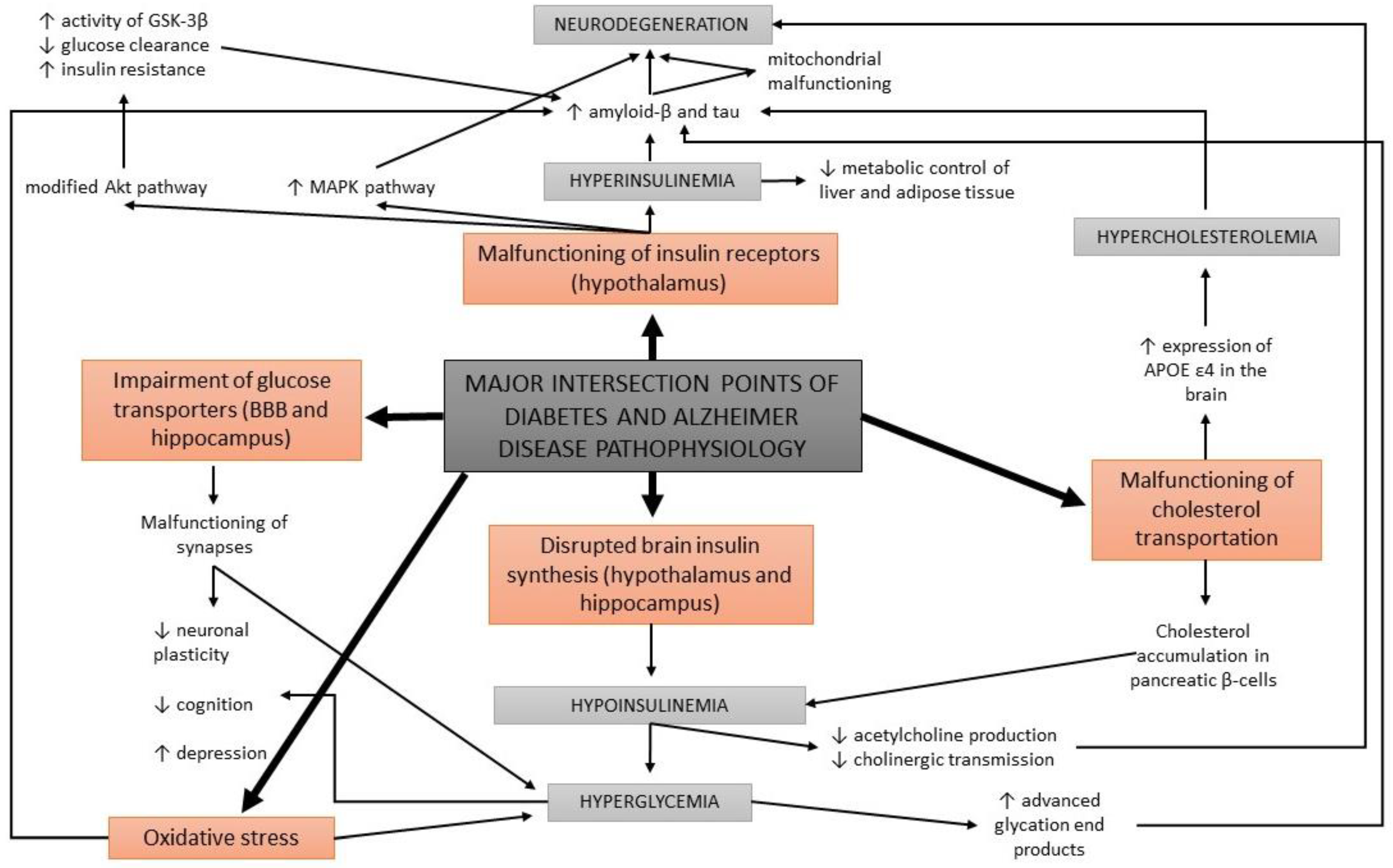
3. Involvement of Diabetes in the Development of Alzheimer’s Disease
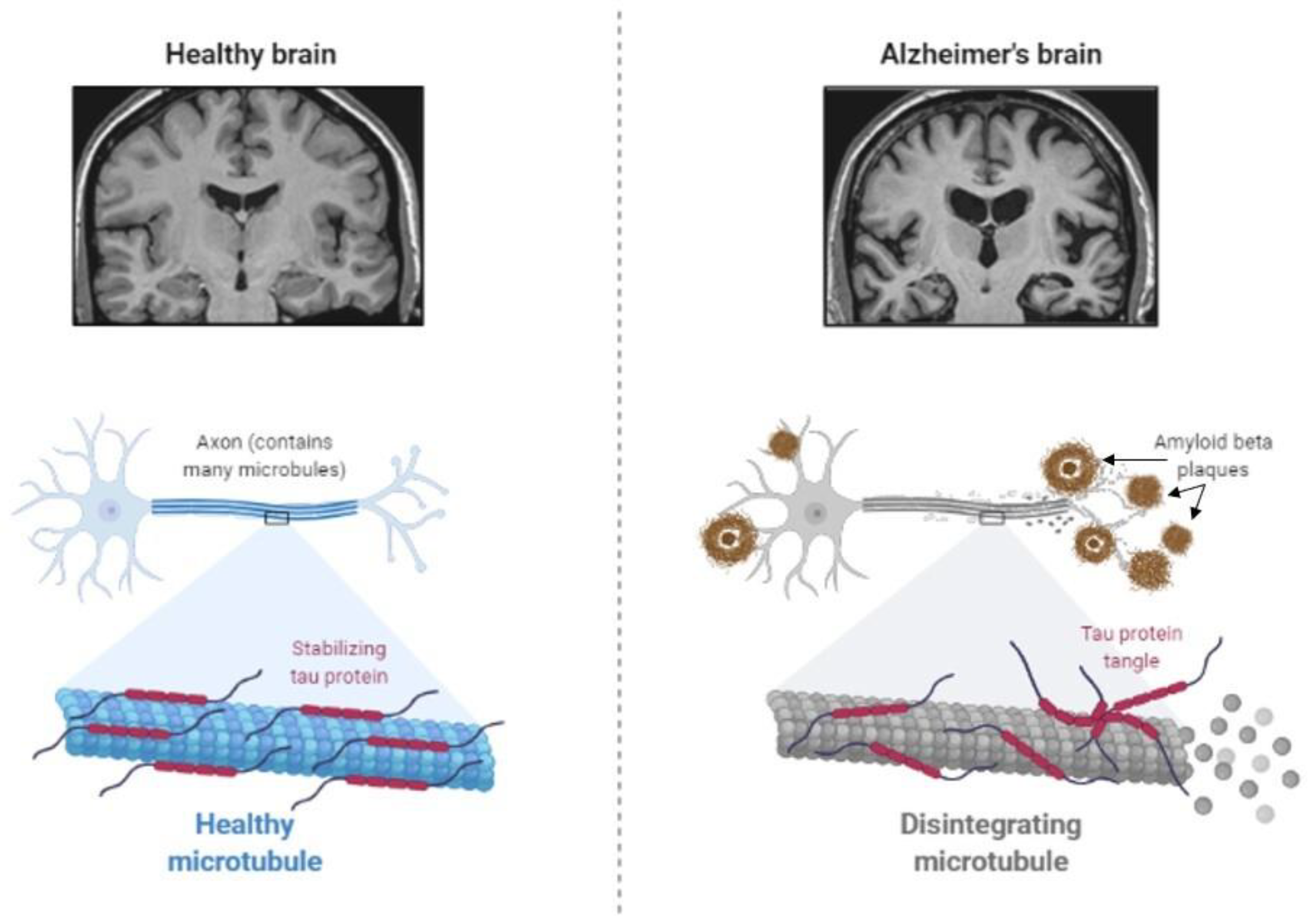
3.1. Abnormal Protein Processing
3.2. Deficient Insulin Signaling
3.3. The Cholinergic Hypothesis
3.4. Glucose Metabolism Disorders
3.5. Oxidative Stress
3.6. Deficits in Mitochondrial Activity
3.7. Cholesterol-Associated Pathologies
4. Insulin Effects on Cognition and Mental Health
4.1. Cognitive Changes
4.2. Mental Changes
5. Combined Effects of Antidepressants and Antidiabetic Drugs on Diabetes, Cognitive Impairment, and Mood Disorders
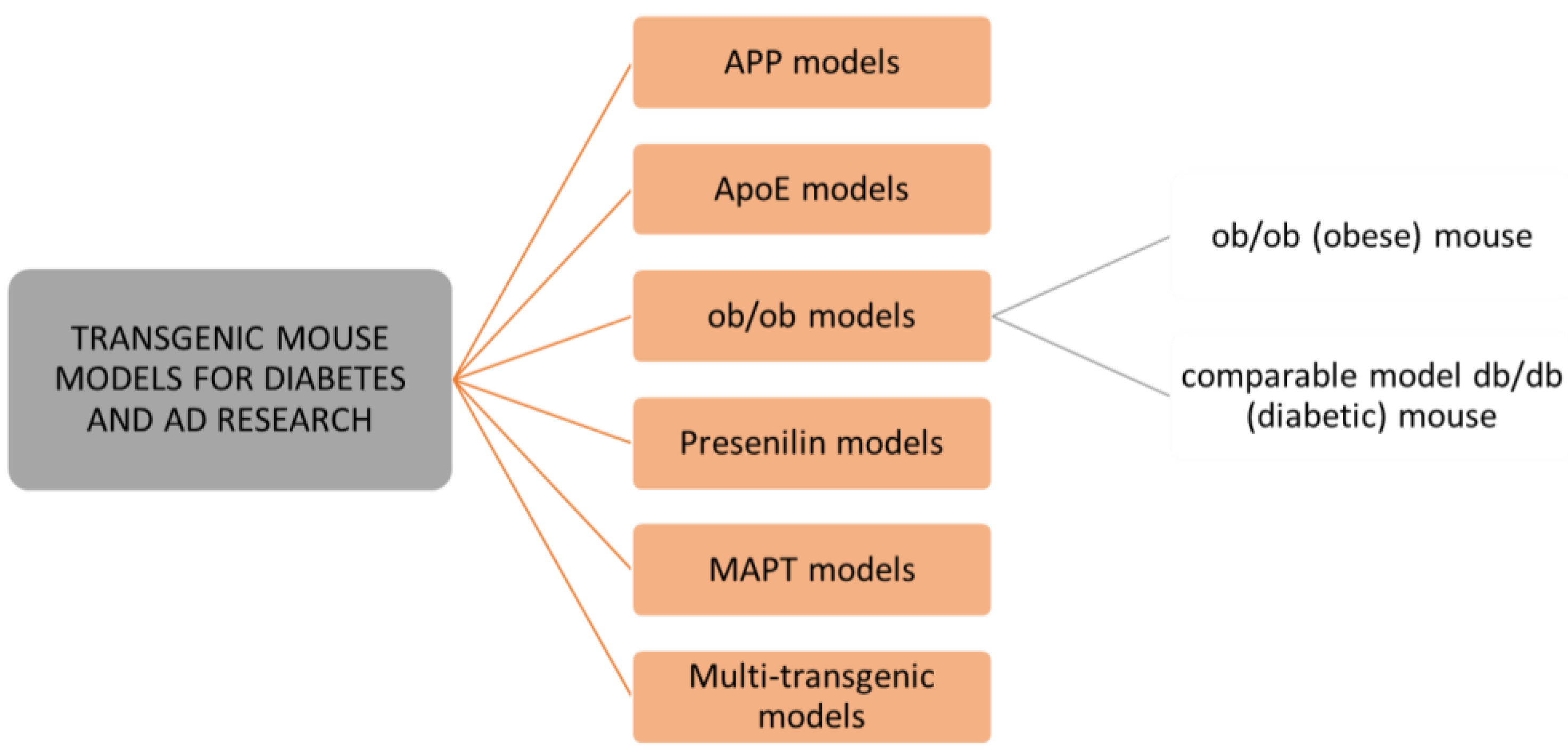
6. Animal Models for Diabetes and Alzheimer’s Disease Research
6.1. Leptin Deficiency Rodent Models
6.2. Monogenic and Polygenic Diabetic Rodent Models
6.3. Transgenic Rodents Models of Alzheimer’s Disease
6.4. Streptozocin-Induced Hyperglycemic and AD Rodent Models
6.5. Acrolein-Induced AD Animal Models
6.6. Diet-Induced Diabetic and AD Rodent Models
6.7. Lesion-Induced Diabetic and AD Rodent Models
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| IGF 1 and 2 | insulin-like growth factor I and II |
| GLUT | glucose transporter |
| BBB | blood–brain barrier |
| NMDA | N-methyl-d-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| IDE | insulin-degrading enzyme |
| MAPK | mitogen-activated protein kinase |
| GSK-3β | glycogen synthase kinase-3β |
References
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Brands, A.M.; Biessels, G.J.; De Haan, E.H.; Kappelle, L.J.; Kessels, R.P. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care 2005, 28, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef]
- Čater, M.; Križančić Bombek, L. Protective Role of Mitochondrial Uncoupling Proteins against Age-Related Oxidative Stress in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef]
- Koutsodendris, N.; Nelson, M.R.; Rao, A.; Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annu. Rev. Pathol. 2022, 17, 73–99. [Google Scholar] [CrossRef]
- Liu, R.-M. Aging, cellular senescence, and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Cipriani, G.; Castelnuovo, G. Technological solutions for diagnosis, management and treatment of Alzheimer0s disease-related symptoms: A structured review of the recent scientific literature. Int. J. Environ. Res. Public Health 2022, 19, 3122. [Google Scholar] [CrossRef]
- Ekblad, L.L.; Rinne, J.O.; Puukka, P.; Laine, H.; Ahtiluoto, S.; Sulkava, R.; Viitanen, M.; Jula, A. Insulin resistance predicts cognitive decline: An 11-year follow-up of a nationally representative adult population sample. Diabetes Care 2017, 40, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Rönnemaa, E.; Zethelius, B.; Sundelöf, J.; Sundström, J.; Degerman-Gunnarsson, M.; Berne, C.; Lannfelt, L.; Kilander, L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology 2008, 71, 1065–1071. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Haan, M.N. Therapy Insight: Type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat. Clin. Pract. Neurol. 2006, 2, 159–166. [Google Scholar] [CrossRef]
- Biessels, G.J.; van der Heide, L.P.; Kamal, A.; Bleys, R.L.; Gispen, W.H. Ageing and diabetes: Implications for brain function. Eur. J. Pharmacol. 2002, 441, 1–14. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.T.; Perluigi, M.; De Marco, C.; Coccia, R.; Keller, J.N.; Markesbery, W.R.; Sultana, R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: Implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007, 1148, 243–248. [Google Scholar] [CrossRef]
- Moreira, P.I.; Santos, M.S.; Seiça, R.; Oliveira, C.R. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J. Neurol. Sci. 2007, 257, 206–214. [Google Scholar] [CrossRef]
- Belinson, H.; Lev, D.; Masliah, E.; Michaelson, D.M. Activation of the amyloid cascade in apolipoprotein E4 transgenic mice induces lysosomal activation and neurodegeneration resulting in marked cognitive deficits. J. Neurosci. 2008, 28, 4690–4701. [Google Scholar] [CrossRef]
- Benarroch, E.E. Brain glucose transporters: Implications for neurologic disease. Neurology 2014, 82, 1374–1379. [Google Scholar] [CrossRef]
- Rebelos, E.; Rinne, J.O.; Nuutila, P.; Ekblad, L.L. Brain Glucose Metabolism in Health, Obesity, and Cognitive Decline—Does Insulin Have Anything to Do with It? A Narrative Review. J. Clin. Med. 2021, 10, 1532. [Google Scholar] [CrossRef]
- Reno, C.M.; Puente, E.C.; Sheng, Z.; Daphna-Iken, D.; Bree, A.J.; Routh, V.H.; Kahn, B.B.; Fisher, S.J. Brain GLUT4 knockout mice have impaired glucose tolerance, decreased insulin sensitivity, and impaired hypoglycemic counterregulation. Diabetes 2017, 66, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the brain: Its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—Is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Kuwabara, T.; Kagalwala, M.N.; Onuma, Y.; Ito, Y.; Warashina, M.; Terashima, K.; Sanosaka, T.; Nakashima, K.; Gage, F.H.; Asashima, M. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol. Med. 2011, 3, 742–754. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef]
- Pardini, A.W.; Nguyen, H.T.; Figlewicz, D.P.; Baskin, D.G.; Williams, D.L.; Kim, F.; Schwartz, M.W. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res. 2006, 1112, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pocai, A.; Lam, T.K.; Gutierrez-Juarez, R.; Obici, S.; Schwartz, G.J.; Bryan, J.; Aguilar-Bryan, L.; Rossetti, L. Hypothalamic K ATP channels control hepatic glucose production. Nature 2005, 434, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Scherer, T.; O’Hare, J.; Diggs-Andrews, K.; Schweiger, M.; Cheng, B.; Lindtner, C.; Zielinski, E.; Vempati, P.; Su, K.; Dighe, S. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011, 13, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.H.; Chi, T.; Shin, A.C.; Lindtner, C.; Hsieh, W.; Ehrlich, M.; Gandy, S.; Buettner, C. Increased susceptibility to metabolic dysregulation in a mouse model of Alzheimer’s disease is associated with impaired hypothalamic insulin signaling and elevated BCAA levels. Alzheimer’s Dement. 2016, 12, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Spielman, L.J.; Bahniwal, M.; Little, J.P.; Walker, D.G.; Klegeris, A. Insulin modulates in vitro secretion of cytokines and cytotoxins by human glial cells. Curr. Alzheimer Res. 2015, 12, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.M.; Hernandez-Garzón, E.; Perez-Domper, P.; Perez-Alvarez, A.; Mederos, S.; Matsui, T.; Santi, A.; Trueba-Saiz, A.; García-Guerra, L.; Pose-Utrilla, J.; et al. Insulin Regulates Astrocytic Glucose Handling Through Cooperation With IGF-I. Diabetes 2017, 66, 64–74. [Google Scholar] [CrossRef]
- Banks, W.A.; Owen, J.B.; Erickson, M.A. Insulin in the brain: There and back again. Pharmacol. Ther. 2012, 136, 82–93. [Google Scholar] [CrossRef]
- Simpson, I.A.; Dwyer, D.; Malide, D.; Moley, K.H.; Travis, A.; Vannucci, S.J. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E242–E253. [Google Scholar] [CrossRef]
- Apelt, J.; Mehlhorn, G.; Schliebs, R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J. Neurosci. Res. 1999, 57, 693–705. [Google Scholar] [CrossRef]
- Pearson-Leary, J.; McNay, E.C. Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J. Neurosci. 2016, 36, 11851–11864. [Google Scholar] [CrossRef]
- Patel, S.S.; Udayabanu, M. Urtica dioica extract attenuates depressive like behavior and associative memory dysfunction in dexamethasone induced diabetic mice. Metab. Brain Dis. 2014, 29, 121–130. [Google Scholar] [CrossRef]
- Huang, C.C.; Lee, C.C.; Hsu, K.S. The role of insulin receptor signaling in synaptic plasticity and cognitive function. Chang. Gung Med. J. 2010, 33, 115–125. [Google Scholar]
- Devaskar, S.U.; Giddings, S.J.; Rajakumar, P.A.; Carnaghi, L.R.; Menon, R.K.; Zahm, D.S. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J. Biol. Chem. 1994, 269, 8445–8454. [Google Scholar] [CrossRef]
- Schechter, R.; Whitmire, J.; Holtzclaw, L.; George, M.; Harlow, R.; Devaskar, S.U. Developmental regulation of insulin in the mammalian central nervous system. Brain Res. 1992, 582, 27–37. [Google Scholar] [CrossRef]
- Frölich, L.; Blum-Degen, D.; Bernstein, H.-G.; Engelsberger, S.; Humrich, J.; Laufer, S.; Muschner, D.; Thalheimer, A.; Türk, A.; Hoyer, S. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J. Neural Transm. 1998, 105, 423–438. [Google Scholar] [CrossRef]
- Crane, P.K.; Walker, R.; Hubbard, R.A.; Li, G.; Nathan, D.M.; Zheng, H.; Haneuse, S.; Craft, S.; Montine, T.J.; Kahn, S.E. Glucose levels and risk of dementia. N. Engl. J. Med. 2013, 369, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.L.; Fragoso, G.; Miron, V.E.; Darlington, P.J.; Mushynski, W.E.; Antel, J.; Almazan, G. Response of human oligodendrocyte progenitors to growth factors and axon signals. J. Neuropathol. Exp. Neurol. 2010, 69, 930–944. [Google Scholar] [CrossRef]
- Clarke, D.; Boyd, F., Jr.; Kappy, M.; Raizada, M. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain. J. Biol. Chem. 1984, 259, 11672–11675. [Google Scholar] [CrossRef]
- Albrecht, J.; Wróblewska, B.; Mossakowski, M.J. The binding of insulin to cerebral capillaries and astrocytes of the rat. Neurochem. Res. 1982, 7, 489–494. [Google Scholar] [CrossRef]
- Wender, R.; Brown, A.M.; Fern, R.; Swanson, R.A.; Farrell, K.; Ransom, B.R. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 2000, 20, 6804–6810. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.-L.; Stella, N.; Magistretti, P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Li, L.; Hölscher, C. Common pathological processes in Alzheimer disease and type 2 diabetes: A review. Brain Res. Rev. 2007, 56, 384–402. [Google Scholar] [CrossRef]
- Johnson, G.V.; Stoothoff, W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 2004, 117, 5721–5729. [Google Scholar] [CrossRef]
- Chung, C.-W.; Song, Y.-H.; Kim, I.-K.; Yoon, W.-J.; Ryu, B.-R.; Jo, D.-G.; Woo, H.-N.; Kwon, Y.-K.; Kim, H.-H.; Gwag, B.-J. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol. Dis. 2001, 8, 162–172. [Google Scholar] [CrossRef]
- Kim, B.; Backus, C.; Oh, S.; Hayes, J.M.; Feldman, E.L. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology 2009, 150, 5294–5301. [Google Scholar] [CrossRef]
- Li, Z.-G.; Zhang, W.; Sima, A.A. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes 2007, 56, 1817–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clodfelder-Miller, B.J.; Zmijewska, A.A.; Johnson, G.V.; Jope, R.S. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes 2006, 55, 3320–3325. [Google Scholar] [CrossRef] [PubMed]
- Planel, E.; Tatebayashi, Y.; Miyasaka, T.; Liu, L.; Wang, L.; Herman, M.; Yu, W.H.; Luchsinger, J.A.; Wadzinski, B.; Duff, K.E. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J. Neurosci. 2007, 27, 13635–13648. [Google Scholar] [CrossRef] [PubMed]
- Jolivalt, C.; Lee, C.; Beiswenger, K.; Smith, J.; Orlov, M.; Torrance, M.; Masliah, E. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: Parallels with Alzheimer’s disease and correction by insulin. J. Neurosci. Res. 2008, 86, 3265–3274. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer’s disease. J. Neurochem. 2009, 111, 242–249. [Google Scholar] [CrossRef]
- Zhao, W.-Q.; Lacor, P.N.; Chen, H.; Lambert, M.P.; Quon, M.J.; Krafft, G.A.; Klein, W.L. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric Aβ. J. Biol. Chem. 2009, 284, 18742–18753. [Google Scholar] [CrossRef]
- Zhao, W.-Q.; Alkon, D.L. Role of insulin and insulin receptor in learning and memory. Mol. Cell. Endocrinol. 2001, 177, 125–134. [Google Scholar] [CrossRef]
- Gasparini, L.; Xu, H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends Neurosci. 2003, 26, 404–406. [Google Scholar] [CrossRef]
- Pedersen, W.A.; McMillan, P.J.; Kulstad, J.J.; Leverenz, J.B.; Craft, S.; Haynatzki, G.R. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol. 2006, 199, 265–273. [Google Scholar] [CrossRef]
- Watson, G.S.; Cholerton, B.A.; Reger, M.A.; Baker, L.D.; Plymate, S.R.; Asthana, S.; Fishel, M.A.; Kulstad, J.J.; Green, P.S.; Cook, D.G. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. Am. J. Geriatr. Psychiatry 2005, 13, 950–958. [Google Scholar] [CrossRef]
- Lesort, M.; Johnson, G. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience 2000, 99, 305–316. [Google Scholar] [CrossRef]
- Freude, S.; Plum, L.; Schnitker, J.; Leeser, U.; Udelhoven, M.; Krone, W.; Bruning, J.C.; Schubert, M. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes 2005, 54, 3343–3348. [Google Scholar] [CrossRef]
- Schubert, M.; Brazil, D.P.; Burks, D.J.; Kushner, J.A.; Ye, J.; Flint, C.L.; Farhang-Fallah, J.; Dikkes, P.; Warot, X.M.; Rio, C. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J. Neurosci. 2003, 23, 7084–7092. [Google Scholar] [CrossRef]
- Pearson, L.L.; Castle, B.E.; Kehry, M.R. CD40-mediated signaling in monocytic cells: Up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int. Immunol. 2001, 13, 273–283. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A.; Zheng, N.Y.; Nael, R.; Robinson, K.A.; Nguyen, X.; Pye, Q.N.; Stewart, C.A.; Geddes, J.; Markesbery, W.R. p38 kinase is activated in the Alzheimer’s disease brain. J. Neurochem. 1999, 72, 2053–2058. [Google Scholar] [CrossRef]
- Munoz, L.; Ammit, A.J. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef]
- Kelleher, I.; Garwood, C.; Hanger, D.P.; Anderton, B.H.; Noble, W. Kinase activities increase during the development of tauopathy in htau mice. J. Neurochem. 2007, 103, 2256–2267. [Google Scholar] [CrossRef]
- Baumann, P.; Schriever, S.; Kullmann, S.; Zimprich, A.; Feuchtinger, A.; Amarie, O.; Peter, A.; Walch, A.; Gailus-Durner, V.; Fuchs, H.; et al. Dusp8 affects hippocampal size and behavior in mice and humans. Sci. Rep. 2019, 9, 19483. [Google Scholar] [CrossRef]
- Baumann, P.; Schriever, S.; Kullmann, S.; Zimprich, A.; Peter, A.; Gailus-Durner, V.; Fuchs, H.; Hrabe de Angelis, M.; Wurst, W.; Tschöp, M.; et al. Diabetes type 2 risk gene Dusp8 is associated with altered sucrose reward behavior in mice and humans. Brain Behav. 2021, 11, e01928. [Google Scholar] [CrossRef]
- Schriever, S.; Kabra, D.; Pfuhlmann, K.; Baumann, P.; Baumgart, E.; Nagler, J.; Seebacher, F.; Harrison, L.; Irmler, M.; Kullmann, S.; et al. Type 2 diabetes risk gene Dusp8 regulates hypothalamic Jnk signaling and insulin sensitivity. J. Clin. Investig. 2020, 130, 6093–6108. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Wands, J.R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. J. Alzheimer’s Dis. 2005, 7, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Brazil, D.P.; Hemmings, B.A. Ten years of protein kinase B signalling: A hard Akt to follow. Trends Biochem. Sci. 2001, 26, 657–664. [Google Scholar] [CrossRef]
- Tremblay, M.L.; Giguère, V. Phosphatases at the heart of FoxO metabolic control. Cell Metab. 2008, 7, 101–103. [Google Scholar] [CrossRef]
- Balaraman, Y.; Limaye, A.; Levey, A.; Srinivasan, S. Glycogen synthase kinase 3β and Alzheimer’s disease: Pathophysiological and therapeutic significance. Cell. Mol. Life Sci. CMLS 2006, 63, 1226–1235. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.-S. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res. Clin. Pract. 2007, 77, S49–S57. [Google Scholar] [CrossRef]
- Sims-Robinson, C.; Kim, B.; Rosko, A.; Feldman, E.L. How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 2010, 6, 551–559. [Google Scholar] [CrossRef]
- Phiel, C.J.; Wilson, C.A.; Lee, V.M.-Y.; Klein, P.S. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 2003, 423, 435–439. [Google Scholar] [CrossRef]
- Noble, W.; Planel, E.; Zehr, C.; Olm, V.; Meyerson, J.; Suleman, F.; Gaynor, K.; Wang, L.; LaFrancois, J.; Feinstein, B. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 6990–6995. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Kroner, Z. The Relationship between Alzheimer’s Disease and Diabetes: Type 3 Diabetes. Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar]
- Abeysekera, W.K.S.M.; Ratnasooriya, W.D.; Abeysekera, W.P.K.M.; Premakumara, G.A.S. Anti-Acetylcholinesterase Activity of Commercially Important Ceylon Black Tea (Camellia sinensis L.) Grades Belonging to Different Elevations: Potential Natural Product for Type 3 Diabetes Management? Asian Food Sci. J. 2021, 20, 36–46. [Google Scholar] [CrossRef]
- Leszek, J.; Trypka, E.; Tarasov, V.V.; Ashraf, G.M.; Aliev, G. Type 3 diabetes mellitus: A novel implication of Alzheimers disease. Curr. Top. Med. Chem. 2017, 17, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Wands, J.R. Alzheimer’s Disease Is Type 3 Diabetes—Evidence Reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Brownlee, M.M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef]
- Ledesma, M.D.; Bonay, P.; Colaço, C.; Avila, J. Analysis of microtubule-associated protein tau glycation in paired helical filaments. J. Biol. Chem. 1994, 269, 21614–21619. [Google Scholar] [CrossRef]
- Toth, C.; Schmidt, A.M.; Tuor, U.I.; Francis, G.; Foniok, T.; Brussee, V.; Kaur, J.; Yan, S.F.; Martinez, J.A.; Barber, P.A. Diabetes, leukoencephalopathy and rage. Neurobiol. Dis. 2006, 23, 445–461. [Google Scholar] [CrossRef]
- Gironès, X.; Guimerà, A.; Cruz-Sánchez, C.-Z.; Ortega, A.; Sasaki, N.; Makita, Z.; Lafuente, J.V.; Kalaria, R.; Cruz-Sánchez, F.F. Nϵ-Carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer’s disease. Free Radic. Biol. Med. 2004, 36, 1241–1247. [Google Scholar] [CrossRef]
- Russell, J.W.; Berent-Spillson, A.; Vincent, A.M.; Freimann, C.L.; Sullivan, K.A.; Feldman, E.L. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol. Dis. 2008, 30, 420–429. [Google Scholar] [CrossRef]
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Picciano, M.; La Francois, J.; Duff, K. Fibrillar β-amyloid evokes oxidative damage in a transgenic mouse model of Alzheimer’s disease. Neuroscience 2001, 104, 609–613. [Google Scholar] [CrossRef]
- Resende, R.; Moreira, P.I.; Proença, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Szekely, C.A.; Thorne, J.E.; Zandi, P.P.; Ek, M.; Messias, E.; Breitner, J.C.; Goodman, S.N. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: A systematic review. Neuroepidemiology 2004, 23, 159–169. [Google Scholar] [CrossRef]
- Aisen, P.S.; Schafer, K.A.; Grundman, M.; Pfeiffer, E.; Sano, M.; Davis, K.L.; Farlow, M.R.; Jin, S.; Thomas, R.G.; Thal, L.J.; et al. Effects of Rofecoxib or Naproxen vs. Placebo on Alzheimer Disease Progression. JAMA 2003, 289, 2819. [Google Scholar] [CrossRef]
- Reines, S.; Block, G.; Morris, J.; Liu, G.; Nessly, M.; Lines, C.; Norman, B.; Baranak, C. Rofecoxib: No effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 2004, 62, 66–71. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Brini, M.; Chiesa, A.; Filippin, L.; Pozzan, T. Mitochondria as biosensors of calcium microdomains. Cell Calcium 1999, 26, 193–200. [Google Scholar] [CrossRef]
- Blass, J.; Gibson, G. The role of oxidative abnormalities in the pathophysiology of Alzheimer’s disease. Rev. Neurol. 1991, 147, 513–525. [Google Scholar]
- Nixon, R.A.; Saito, K.I.; Grynspan, F.; Griffin, W.R.; Katayama, S.; Honda, T.; Mohan, P.S.; Shea, T.B.; Beermann, M. Calcium-Activated Neutral Proteinase (Calpain) System in Aging and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1994, 747, 77–91. [Google Scholar] [CrossRef]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001, 21, 3017–3023. [Google Scholar] [CrossRef]
- Crouch, P.J.; Blake, R.; Duce, J.A.; Ciccotosto, G.D.; Li, Q.-X.; Barnham, K.J.; Curtain, C.C.; Cherny, R.A.; Cappai, R.; Dyrks, T. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-β1-42. J. Neurosci. 2005, 25, 672–679. [Google Scholar] [CrossRef]
- Johnson, G.V.; Cox, T.M.; Lockhart, J.P.; Zinnerman, M.D.; Miller, M.L.; Powers, R.E. Transglutaminase activity is increased in Alzheimer’s disease brain. Brain Res. 1997, 751, 323–329. [Google Scholar] [CrossRef]
- Querfurth, H.W.; Selkoe, D.J. Calcium ionophore increases amyloid. beta. Peptide production by cultured cells. Biochemistry 1994, 33, 4550–4561. [Google Scholar] [CrossRef]
- Levy, J.; Gavin, J.R., III; Sowers, J.R. Diabetes mellitus: A disease of abnormal cellular calcium metabolism? Am. J. Med. 1994, 96, 260–273. [Google Scholar] [CrossRef]
- Levy, J.; Zemel, M.B.; Sowers, J.R. Role of cellular calcium metabolism in abnormal glucose metabolism and diabetic hypertension. Am. J. Med. 1989, 87, S7–S16. [Google Scholar] [CrossRef]
- Moreira, P.I.; Santos, M.S.; Sena, C.; Seiça, R.; Oliveira, C.R. Insulin protects against amyloid β-peptide toxicity in brain mitochondria of diabetic rats. Neurobiol. Dis. 2005, 18, 628–637. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Ishikawa, M.; Iwasaki, Y.; Yatoh, S.; Kato, T.; Kumadaki, S.; Inoue, N.; Yamamoto, T.; Matsuzaka, T.; Nakagawa, Y.; Yahagi, N. Cholesterol accumulation and diabetes in pancreatic β-cell-specific SREBP-2 transgenic mice: A new model for lipotoxicity. J. Lipid Res. 2008, 49, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.P.; Noble, W.J.; Olm, V.; Gaynor, K.; Casey, E.; LaFrancois, J.; Wang, L.; Duff, K. Co-localization of cholesterol, apolipoprotein E and fibrillar Aβ in amyloid plaques. Mol. Brain Res. 2003, 110, 119–125. [Google Scholar] [CrossRef]
- Peila, R.; Rodriguez, B.L.; Launer, L.J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 2002, 51, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Brecht, W.J.; Harris, F.M.; Chang, S.; Tesseur, I.; Yu, G.-Q.; Xu, Q.; Fish, J.D.; Wyss-Coray, T.; Buttini, M.; Mucke, L. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 2004, 24, 2527–2534. [Google Scholar] [CrossRef]
- Miles, W.; Root, H. Psychologic tests applied to diabetic patients. Arch. Intern. Med. 1922, 30, 767–777. [Google Scholar] [CrossRef]
- Awad, N.; Gagnon, M.; Messier, C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J. Clin. Exp. Neuropsychol. 2004, 26, 1044–1080. [Google Scholar] [CrossRef]
- Strachan, M.W.; Deary, I.J.; Ewing, F.M.; Frier, B.M. Is type II diabetes associated with an increased risk of cognitive dysfunction?: A critical review of published studies. Diabetes Care 1997, 20, 438–445. [Google Scholar] [CrossRef]
- Ferretti, L.; McCurry, S.M.; Logsdon, R.; Gibbons, L.; Teri, L. Anxiety and Alzheimer’s Disease. J. Geriatr. Psychiatry Neurol. 2001, 14, 52–58. [Google Scholar] [CrossRef]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef]
- James, F.E. Insulin treatment in psychiatry. Hist. Psychiatry 1992, 3, 221–235. [Google Scholar] [CrossRef]
- Nouwen, A.; Winkley, K.; Twisk, J.; Lloyd, C.E.; Peyrot, M.; Ismail, K.; Pouwer, F. Type 2 diabetes mellitus as a risk factor for the onset of depression: A systematic review and meta-analysis. Diabetologia 2010, 53, 2480–2486. [Google Scholar] [CrossRef]
- Martin, H.; Bullich, S.; Guiard, B.P.; Fioramonti, X. The impact of insulin on the serotonergic system and consequences on diabetes-associated mood disorders. J. Neuroendocrinol. 2021, 33, e12928. [Google Scholar] [CrossRef]
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008, 31, 2383–2390. [Google Scholar] [CrossRef]
- Ehrmann, D.; Schmitt, A.; Reimer, A.; Haak, T.; Kulzer, B.; Hermanns, N. The affective and somatic side of depression: Subtypes of depressive symptoms show diametrically opposed associations with glycemic control in people with type 1 diabetes. Acta Diabetol. 2017, 54, 749–756. [Google Scholar] [CrossRef]
- Carter, J.; Swardfager, W. Mood and metabolism: Anhedonia as a clinical target in Type 2 diabetes. Psychoneuroendocrinology 2016, 69, 123–132. [Google Scholar] [CrossRef]
- Maheux, P.; Ducros, F.; Bourque, J.; Garon, J.; Chiasson, J. Fluoxetine improves insulin sensitivity in obese patients with non-insulin-dependent diabetes mellitus independently of weight loss. Int. J. Obes. 1997, 21, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Erenmemisoglu, A.; Ozdogan, U.; Saraymen, R.; Tutus, A. Effect of some antidepressants on glycaemia and insulin levels of normoglycaemic and alloxan-induced hyperglycaemic mice. J. Pharm. Pharmacol. 1999, 51, 741–743. [Google Scholar] [CrossRef]
- Goodnick, P. Use of Antidepressants in Treatment of Comorbid Diabetes Mellitus and Depression as Well as in Diabetic Neuropathy. Ann. Clin. Psychiatry 2001, 13, 31–41. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Soczynska, J.K.; Konarski, J.Z.; Kennedy, S.H. The effect of antidepressants on glucose homeostasis and insulin sensitivity: Synthesis and mechanisms. Expert Opin. Drug Saf. 2006, 5, 157–168. [Google Scholar] [CrossRef]
- Mago, R.; Tripathi, N.; Andrade, C. Cardiovascular adverse effects of newer antidepressants. Expert Rev. Neurother. 2014, 14, 539–551. [Google Scholar] [CrossRef]
- Grigolon, R.B.; Brietzke, E.; Mansur, R.B.; Idzikowski, M.A.; Gerchman, F.; De Felice, F.G.; McIntyre, R.S. Association between diabetes and mood disorders and the potential use of anti-hyperglycemic agents as antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109720. [Google Scholar] [CrossRef]
- Shivavedi, N.; Kumar, M.; Tej, G.N.V.C.; Nayak, P.K. Metformin and ascorbic acid combination therapy ameliorates type 2 diabetes mellitus and comorbid depression in rats. Brain Res. 2017, 1674, 1–9. [Google Scholar] [CrossRef]
- Bohringer, A.; Schwabe, L.; Richter, S.; Schachinger, H. Intranasal insulin attenuates the hypothalamic–pituitary–adrenal axis response to psychosocial stress. Psychoneuroendocrinology 2008, 33, 1394–1400. [Google Scholar] [CrossRef]
- Beirami, E.; Oryan, S.; Tamijani, S.M.S.; Ahmadiani, A.; Dargahi, L. Intranasal insulin treatment alleviates methamphetamine induced anxiety-like behavior and neuroinflammation. Neurosci. Lett. 2017, 660, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.; Essmat, N.; Mahmoud, M.F.; Mahmoud, A.A. Impact of some oral hypoglycemic agents on type 2 diabetes-associated depression and reserpine-induced depression in rats: The role of brain oxidative stress and inflammation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Essmat, N.; Soliman, E.; Mahmoud, M.F.; Mahmoud, A.A.A. Antidepressant activity of anti-hyperglycemic agents in experimental models: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Poggini, S.; Golia, M.T.; Alboni, S.; Milior, G.; Sciarria, L.P.; Viglione, A.; Matte Bon, G.; Brunello, N.; Puglisi-Allegra, S.; Limatola, C. Combined fluoxetine and metformin treatment potentiates antidepressant efficacy increasing IGF2 expression in the dorsal hippocampus. Neural Plast. 2019, 2019, 4651031. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; De Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Reardon, S. Frustrated Alzheimer’s researchers seek better lab mice. Nature 2018, 563, 611–612. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Mak, M.S.; Lu, J.; Wu, Z.; Chen, Q.; Han, Y.; Li, Y.; Pi, R. Advance of sporadic Alzheimer’s disease animal models. Med. Res. Rev. 2020, 40, 431–458. [Google Scholar] [CrossRef]
- Abraham, K.M. Animal models of obesity and metabolic syndrome: Potential tools for Alzheimer’s disease research. Curr. Alzheimer Res. 2007, 4, 145–146. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse models of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef]
- Myers, A.; McGonigle, P. Overview of Transgenic Mouse Models for Alzheimer’s Disease. Curr. Protoc. Neurosci. 2019, 89, e81. [Google Scholar] [CrossRef]
- Hollander, C.F.; Mos, J. The old animal as a model in research on brain aging and Alzheimer’s disease/senile dementia of the Alzheimer type. Prog. Brain Res. 1986, 70, 337–343. [Google Scholar]
- Canudas, A.M.; Gutierrez-Cuesta, J.; Rodríguez, M.I.; Acuña-Castroviejo, D.; Sureda, F.X.; Camins, A.; Pallàs, M. Hyperphosphorylation of microtubule-associated protein tau in senescence-accelerated mouse (SAM). Mech. Ageing Dev. 2005, 126, 1300–1304. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Moult, P.R.; Milojkovic, B.; Harvey, J. Leptin reverses long-term potentiation at hippocampal CA1 synapses. J. Neurochem. 2009, 108, 685–696. [Google Scholar] [CrossRef]
- Morrison, C.D. Leptin signaling in brain: A link between nutrition and cognition? Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2009, 1792, 401–408. [Google Scholar] [CrossRef]
- King, A.; Brain, A.; Hanson, K.; Dittmann, J.; Vickers, J.; Fernandez-Martos, C. Disruption of leptin signalling in a mouse model of Alzheimer’s disease. Metab. Brain Dis. 2018, 33, 1097–1110. [Google Scholar] [CrossRef]
- Bonda, D.J.; Stone, J.G.; Torres, S.L.; Siedlak, S.L.; Perry, G.; Kryscio, R.; Jicha, G.; Casadesus, G.; Smith, M.A.; Zhu, X. Dysregulation of leptin signaling in Alzheimer disease: Evidence for neuronal leptin resistance. J. Neurochem. 2014, 128, 162–172. [Google Scholar] [CrossRef]
- Lieb, W.; Beiser, A.S.; Vasan, R.S.; Tan, Z.S.; Au, R.; Harris, T.B.; Roubenoff, R.; Auerbach, S.; DeCarli, C.; Wolf, P.A. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 2009, 302, 2565–2572. [Google Scholar] [CrossRef]
- Searcy, J.L.; Phelps, J.T.; Pancani, T.; Kadish, I.; Popovic, J.; Anderson, K.L.; Beckett, T.L.; Murphy, M.P.; Chen, K.-C.; Blalock, E.M. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 30, 943–961. [Google Scholar] [CrossRef]
- Sato, T.; Hanyu, H.; Hirao, K.; Kanetaka, H.; Sakurai, H.; Iwamoto, T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging 2011, 32, 1626–1633. [Google Scholar] [CrossRef]
- Fewlass, D.C.; Noboa, K.; Pi-Sunyer, F.X.; Johnston, J.M.; Yan, S.D.; Tezapsidis, N. Obesity-related leptin regulates Alzheimer’s Aβ. FASEB J. 2004, 18, 1870–1878. [Google Scholar] [CrossRef]
- Tezapsidis, N.; Johnston, J.M.; Smith, M.A.; Ashford, J.W.; Casadesus, G.; Robakis, N.K.; Wolozin, B.; Perry, G.; Zhu, X.; Greco, S.J. Leptin: A novel therapeutic strategy for Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 16, 731–740. [Google Scholar] [CrossRef]
- Johnston, J.M.; Greco, S.J.; Hamzelou, A.; Ashford, J.W.; Tezapsidis, N. Repositioning leptin as a therapy for Alzheimer’s disease. Therapy 2011, 8, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Greco, S.J.; Bryan, K.J.; Sarkar, S.; Zhu, X.; Smith, M.A.; Ashford, J.W.; Johnston, J.M.; Tezapsidis, N.; Casadesus, G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 19, 1155–1167. [Google Scholar] [CrossRef]
- Greco, S.J.; Sarkar, S.; Johnston, J.M.; Zhu, X.; Su, B.; Casadesus, G.; Ashford, J.W.; Smith, M.A.; Tezapsidis, N. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochem. Biophys. Res. Commun. 2008, 376, 536–541. [Google Scholar] [CrossRef]
- Harvey, J.; Solovyova, N.; Irving, A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Yoshitomi, T.; Inoue, M.; Iigo, Y.; Matsumoto, K.; Kubota, K.; Shinagawa, A. Pathological and gene expression analysis of a polygenic diabetes model, NONcNZO10/LtJ mice. Gene 2017, 629, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Thamarai, K.; Kandimalla, R.; Manczak, M.; Yin, X.; Kumar, S.; Vijayan, M.; Reddy, P.H. Mitochondria-Targeted Small Peptide, SS31 Ameliorates Diabetes Induced Mitochondrial Dynamics in Male TallyHO/JngJ Mice. Mol. Neurobiol. 2021, 58, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, B.; Reddy, P.H. Are TallyHo Mice A True Mouse Model for Type 2 Diabetes and Alzheimer’s Disease? J. Alzheimer’s Dis. 2019, 72, S81–S93. [Google Scholar] [CrossRef]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Borthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef]
- Laurijssens, B.; Aujard, F.; Rahman, A. Animal models of Alzheimer0s disease and drug development. Transl. Pharmacol. 2013, 10, e319–e327. [Google Scholar]
- LaFerla, F.M.; Green, K.N. Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006320. [Google Scholar] [CrossRef]
- Kalback, W.; Watson, M.D.; Kokjohn, T.A.; Kuo, Y.-M.; Weiss, N.; Luehrs, D.C.; Lopez, J.; Brune, D.; Sisodia, S.S.; Staufenbiel, M.; et al. APP transgenic mice Tg2576 accumulate Abeta peptides that are distinct from the chemically modified and insuluble peptides deposited in Alzheimer’s disease senile plaques. Biochemistry 2002, 41, 922–928. [Google Scholar] [CrossRef]
- Murtishaw, A.S.; Heaney, C.F.; Bolton, M.M.; Belmonte, K.C.D.; Langhardt, M.A.; Kinney, J.W. Intermittent streptozotocin administration induces behavioral and pathological features relevant to Alzheimer’s disease and vascular dementia. Neuropharmacology 2018, 137, 164–177. [Google Scholar] [CrossRef]
- Braganza, D.C.; Flores, E.; Crew, L.A.; Wirt, R.A.; Ortiz, A.A.; McNeela, A.M.; Kinney, J.W.; Hyman, J.M. Diabetes Mellitus Affects Working Memory. Spectra Undergrad. Res. J. 2021, 1, 3. [Google Scholar] [CrossRef]
- Ke, Y.D.; Delerue, F.; Gladbach, A.; Götz, J.; Ittner, L.M. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 2009, 4, e7917. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Hurford, R.; Lee, C.A.; Dumaop, W.; Rockenstein, E.; Masliah, E. Type 1 diabetes exaggerates features of Alzheimer’s disease in APP transgenic mice. Exp. Neurol. 2010, 223, 422–431. [Google Scholar] [CrossRef]
- Takeda, S.; Sato, N.; Uchio-Yamada, K.; Sawada, K.; Kunieda, T.; Takeuchi, D.; Kurinami, H.; Shinohara, M.; Rakugi, H.; Morishita, R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. USA 2010, 107, 7036–7041. [Google Scholar] [CrossRef]
- Shinohara, M.; Sato, N. Bidirectional interactions between diabetes and Alzheimer’s disease. Neurochem. Int. 2017, 108, 296–302. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Tota, S.K.; Kumar, A.; Ahmad, A.S. Streptozotocin Intracerebroventricular-Induced Neurotoxicity and Brain Insulin Resistance: A Therapeutic Intervention for Treatment of Sporadic Alzheimer’s Disease (sAD)-Like Pathology. Mol. Neurobiol. 2016, 53, 4548–4562. [Google Scholar] [CrossRef]
- Lauretti, E.; Li, J.G.; Di Meco, A.; Praticò, D. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl. Psychiatry 2017, 7, e1020. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, R.; Wang, S.; Ren, Z.; Xu, L.; Zhang, X.; Zhao, J.; Ding, Y.; Wu, Y.; Gong, Y. Effect of intraperitoneal or intracerebroventricular injection of streptozotocin on learning and memory in mice. Exp. Ther. Med. 2018, 16, 2375–2380. [Google Scholar] [CrossRef]
- Huang, Y.J.; Jin, M.H.; Pi, R.B.; Zhang, J.J.; Ouyang, Y.; Chao, X.Y.; Chen, M.H.; Liu, P.Q.; Yu, J.C.; Ramassamy, C.; et al. Acrolein induces Alzheimer’s disease-like pathologies in vitro and in vivo. Toxicol. Lett. 2013, 217, 184–191. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The high-fat diet–fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53, S215–S219. [Google Scholar] [CrossRef]
- Schemmel, R.; Mickelsen, O.; Gill, J. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. J. Nutr. 1970, 100, 1041–1048. [Google Scholar] [CrossRef]
- Popp, J.; Meichsner, S.; Kölsch, H.; Lewczuk, P.; Maier, W.; Kornhuber, J.; Jessen, F.; Lütjohann, D. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem. Pharmacol. 2013, 86, 37–42. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.-W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, X.; Yang, J.; Suo, J.; Chen, J.; Liu, X.; Zhao, X. Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neurosci. Lett. 2016, 610, 200–206. [Google Scholar] [CrossRef]
- Thenmozhi, J.A.; William Raja, T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Julien, J.; Joubert, S.; Ferland, M.C.; Frenette, L.C.; Boudreau-Duhaime, M.M.; Malo-Véronneau, L.; de Guise, E. Association of traumatic brain injury and Alzheimer disease onset: A systematic review. Ann. Phys. Rehabil. Med. 2017, 60, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Song, H.; Cui, J.; Johnson, C.E.; Hubler, G.K.; DePalma, R.G.; Gu, Z.; Xia, W. Proteomic Profiling of Mouse Brains Exposed to Blast-Induced Mild Traumatic Brain Injury Reveals Changes in Axonal Proteins and Phosphorylated Tau. J. Alzheimer’s Dis. 2018, 66, 751–773. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čater, M.; Hölter, S.M. A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 11562. https://doi.org/10.3390/ijms231911562
Čater M, Hölter SM. A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(19):11562. https://doi.org/10.3390/ijms231911562
Chicago/Turabian StyleČater, Maša, and Sabine M. Hölter. 2022. "A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 19: 11562. https://doi.org/10.3390/ijms231911562





