Atomic Scale Optimization Strategy of Al-Based Layered Double Hydroxide for Alkali Stability and Supercapacitors
Abstract
:1. Introduction
2. Results
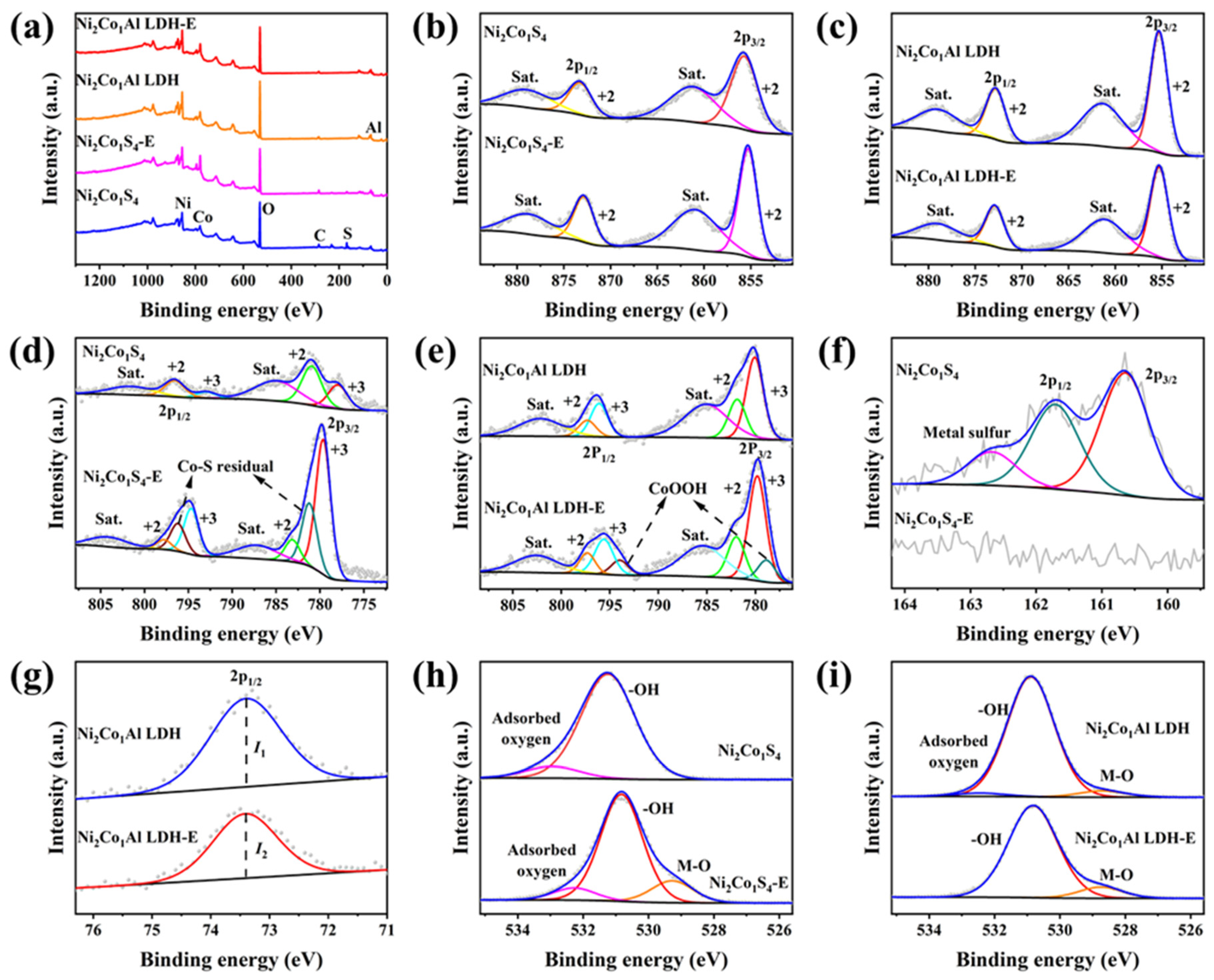
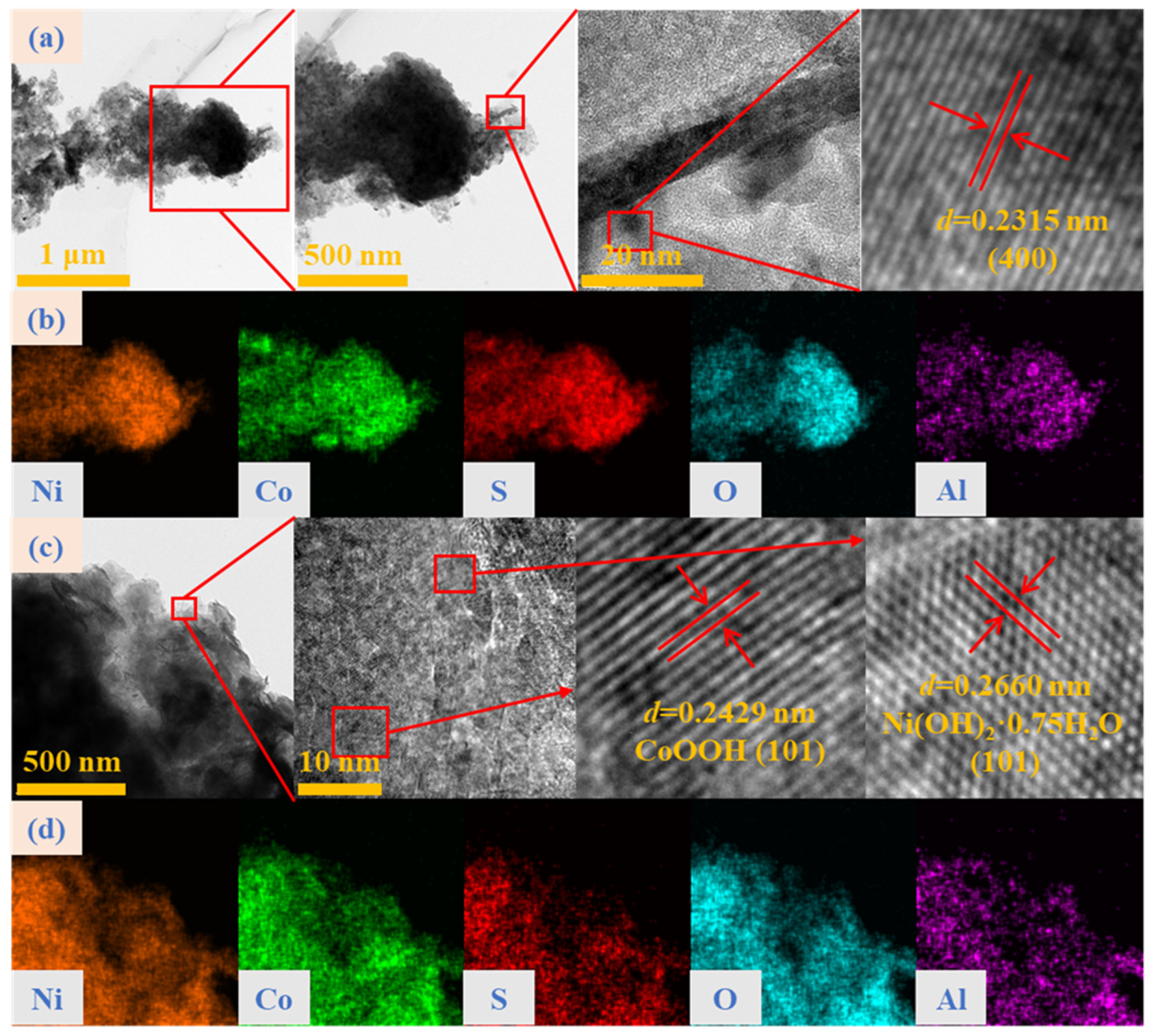
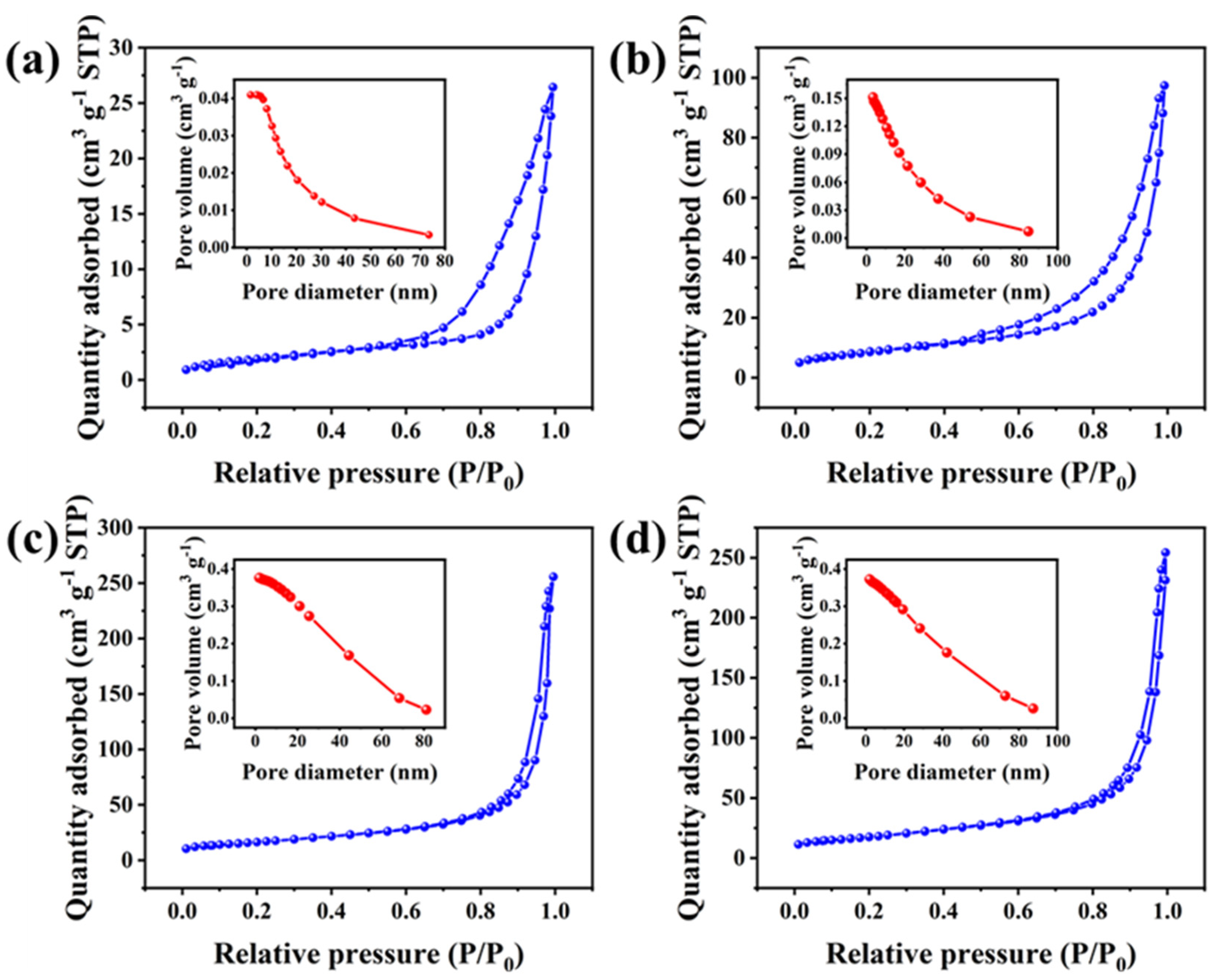
2.1. Morphology and Structural Characteristics
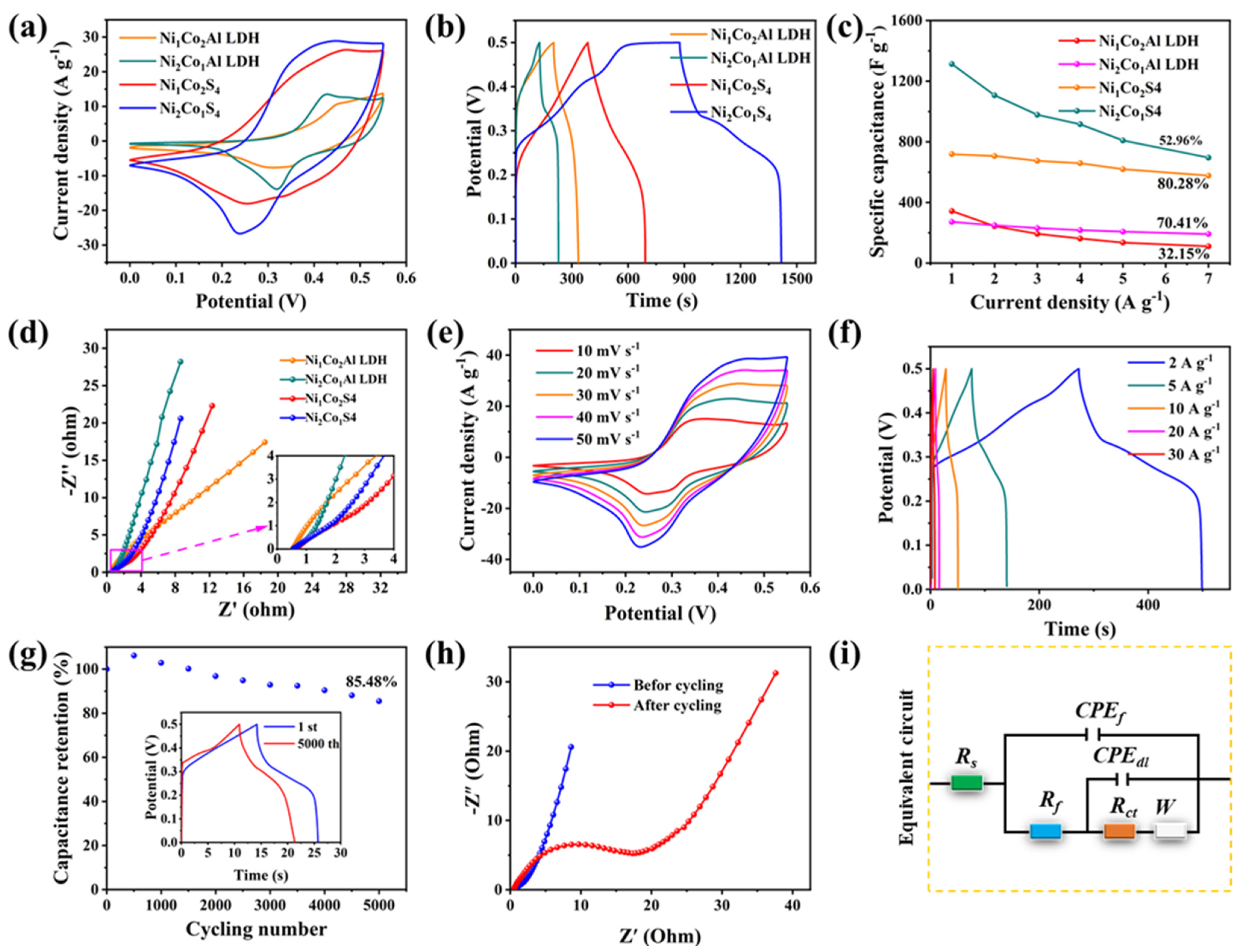
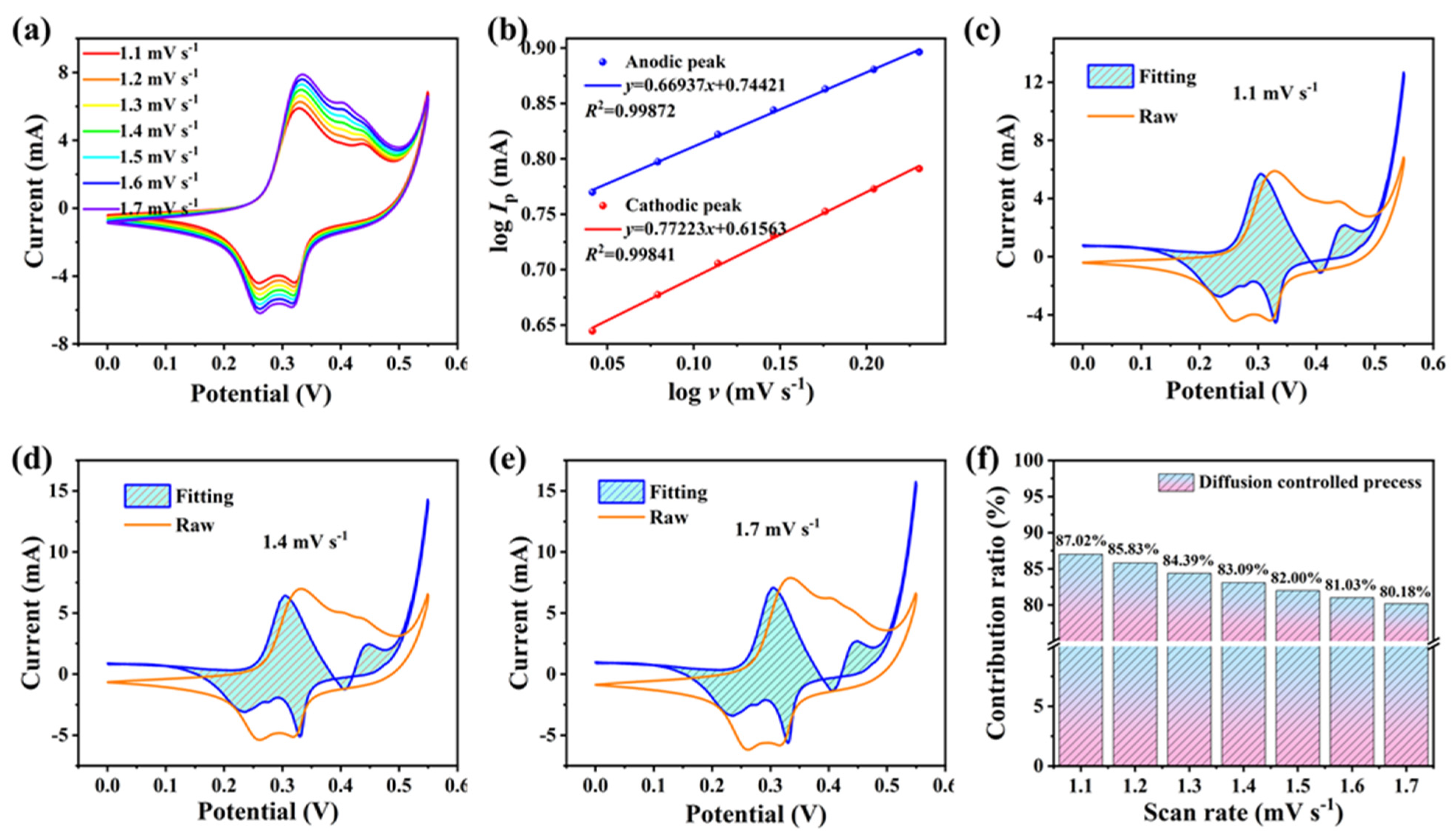
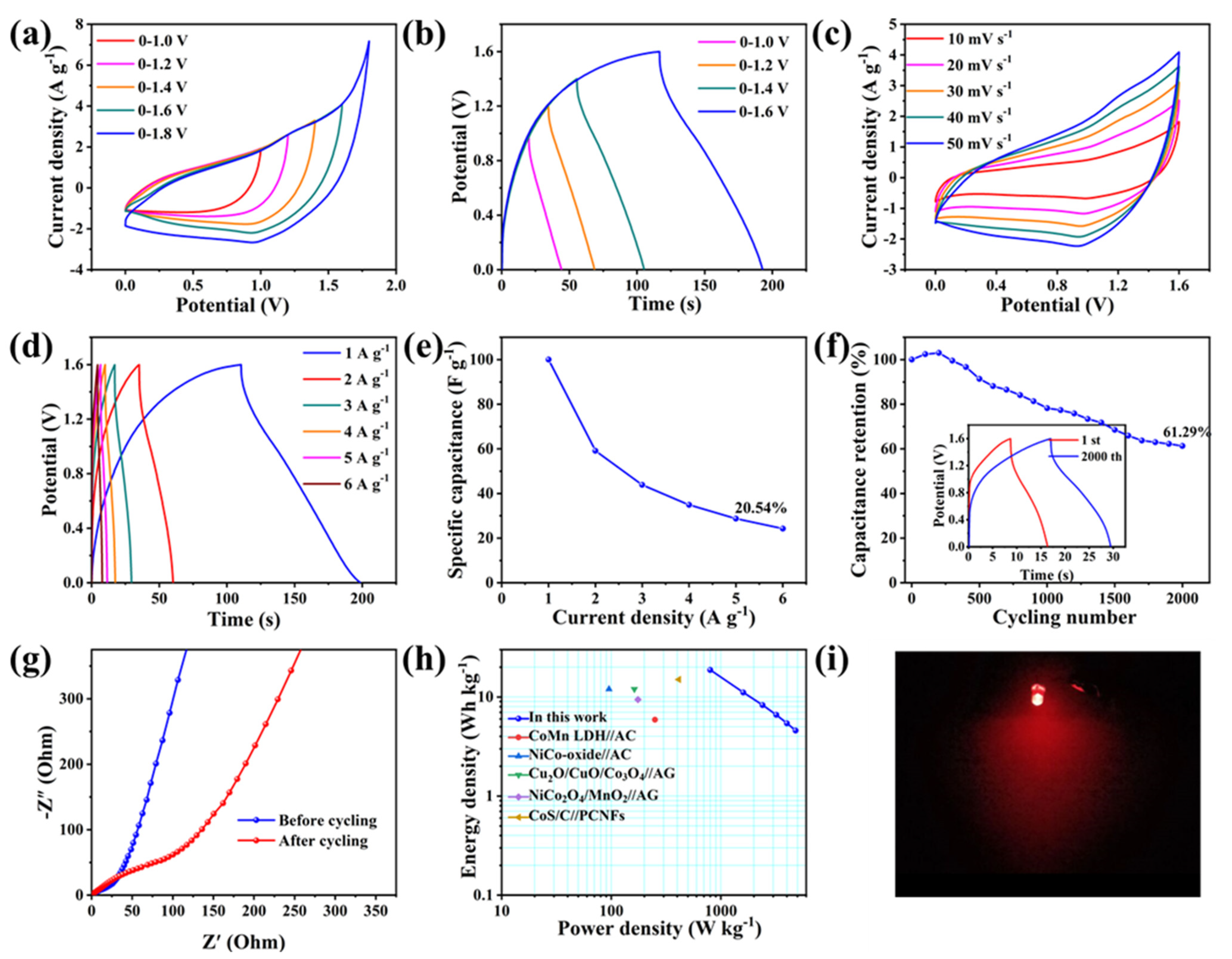
2.2. Electrochemical Properties
3. Discussion
4. Materials and Methods
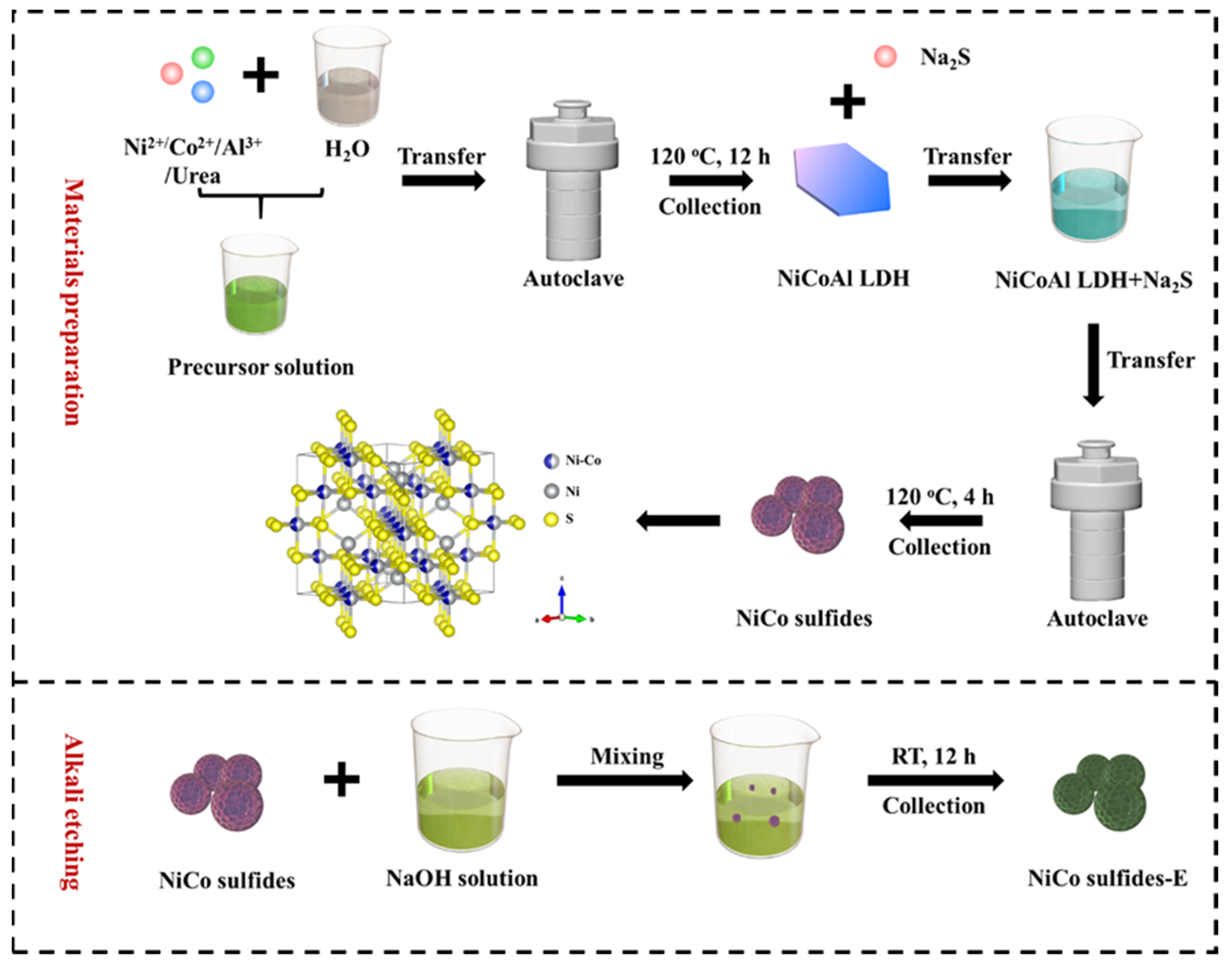
4.1. Electrode Materials Preparation
4.2. Materials Characterization
4.3. Electrochemical Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayaramulu, K.; Horn, M.; Schneemann, A.; Saini, H.; Bakandritsos, A.; Ranc, V.; Petr, M.; Stavila, V.; Narayana, C.; Scheibe, B.; et al. Covalent Graphene-MOF Hybrids for High-Performance Asymmetric Supercapacitors. Adv. Mater. 2021, 33, 2004560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Lu, H.Y.; Zhang, Y.; Yu, S.X.; Malyi, O.I.; Zhao, X.X.; Wang, L.T.; Wang, H.B.; Peng, J.H.; Li, X.F.; et al. Direct coherent multi-ink printing of fabric supercapacitors. Sci. Adv. 2021, 7, eabd6978. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.C.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Jiang, S.H.; Ding, J.; Wang, R.H.; Deng, Y.X.; Chen, F.Y.; Zhou, M.Q.; Gui, H.; Li, X.L.; Xu, C.H. High performance NiCo-LDH//Fe2O3 asymmetric supercapacitors based on binder-free electrodes with dual conductive networks. Chem. Eng. J. 2022, 431, 133936. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Liang, Z.B.; Jin, Y.K.; Gao, S.; Zou, R.Q. Understanding and tackling lattice manganese exfoliation and deactivation of battery-type NiMn-LDH in fast electrochemical energy storage. J. Mater. Chem. A 2021, 9, 23286–23295. [Google Scholar] [CrossRef]
- Cheng, S.T.; Zhang, Y.X.; Liu, Y.P.; Sun, Z.H.; Cui, P.; Zhang, J.L.; Hua, X.H.; Su, Q.; Fu, J.C.; Xie, E.Q. Energizing Fe2O3-based supercapacitors with tunable surface pseudocapacitance via physical spatial-confining strategy. Chem. Eng. J. 2021, 406, 126875. [Google Scholar] [CrossRef]
- Huang, X.; Sun, R.; Li, Y.; Jiang, J.; Li, M.; Xu, W.; Wang, Y.; Cong, H.; Tang, J.; Han, S. Two-step electrodeposition synthesis of heterogeneous NiCo-layered double hydroxides@MoO3 nanocomposites on nickel foam with high performance for hybrid supercapacitors. Electrochim. Acta 2022, 403, 139680. [Google Scholar] [CrossRef]
- Kang, L.; Huang, C.; Zhang, J.; Zhang, M.; Zhang, N.; Liu, S.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C.; et al. Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 2020, 390, 124643. [Google Scholar] [CrossRef]
- Chang, J.; Zang, S.; Liang, W.; Wu, D.; Lian, Z.; Xu, F.; Jiang, K.; Gao, Z. Enhanced faradic activity by construction of p-n junction within reduced graphene oxide@cobalt nickel sulfide@nickle cobalt layered double hydroxide composite electrode for charge storage in hybrid supercapacitor. J. Colloid Interf. Sci. 2021, 590, 114–124. [Google Scholar] [CrossRef]
- Wei, X.J.; Song, Y.Z.; Song, L.X.; Liu, X.D.; Li, Y.H.; Yao, S.R.; Xiao, P.; Zhang, Y.H. Phosphorization Engineering on Metal-Organic Frameworks for Quasi-Solid-State Asymmetry Supercapacitors. Small 2021, 17, 2007062. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Du, C.C.; Yang, H.K.; Huang, J.L.; Zhang, X.H.; Chen, J.H. NiCoP@CoS tree-like core-shell nanoarrays on nickel foam as battery-type electrodes for supercapacitors. Chem. Eng. J. 2021, 421, 127871. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Zhu, W.; Lei, X.D.; Williams, G.R.; O’Hare, D.; Chang, Z.; Sun, X.M.; Duan, X. High pseudocapacitive cobalt carbonate hydroxide films derived from CoAl layered double hydroxides. Nanoscale 2012, 4, 3640–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.H.; Wu, X.C.; Li, Y.J.; Xu, W.W.; Lu, Z.Y.; Li, Y.P.; Lei, X.D.; Sun, X.M. Morphology and Phase Evolution of CoAl Layered Double Hydroxides in an Alkaline Environment with Enhanced Pseudocapacitive Performance. Chemelectrochem 2015, 2, 679–683. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zheng, L.R.; Shi, R.; Zhang, S.; Bian, X.N.; Wu, F.; Cao, X.Z.; Waterhouse, G.I.N.; Zhang, T.R. Alkali Etching of Layered Double Hydroxide Nanosheets for Enhanced Photocatalytic N-2 Reduction to NH3. Adv. Energy Mater. 2020, 10, 2002199. [Google Scholar] [CrossRef]
- He, W.J.; Cao, D.; Ma, D.Q.; Li, Y.; Chen, C.; Liang, L.M.; Liu, H. Engineering nickel vacancies in NiCo LDH nanoarrays accelerates hydrogen evolution and oxygen evolution reactions. Chem. Commun. 2022, 58, 7757–7760. [Google Scholar] [CrossRef]
- Li, K.L.; Teng, H.; Dai, X.J.; Wang, Y.; Wang, D.S.; Zhang, X.F.; Yao, Y.J.; Liu, X.Y.; Feng, L.; Rao, J.S.; et al. Atomic scale modulation strategies and crystal phase transition of flower-like CoAl layered double hydroxides for supercapacitors. Crystengcomm 2022, 24, 2081–2088. [Google Scholar] [CrossRef]
- Yong, J.X.; Luan, X.B.; Dai, X.P.; Zhang, X.; Yang, Y.; Zhao, H.H.; Cui, M.L.; Ren, Z.T.; Nie, F.; Huang, X.L. Alkaline-Etched NiMgAl Trimetallic Oxide-Supported KMoS-Based Catalysts for Boosting Higher Alcohol Selectivity in CO Hydrogenation. Acs. Appl. Mater. Interfaces 2019, 11, 19066–19076. [Google Scholar] [CrossRef]
- Li, Z.H.; Li, X.; Xiang, L.; Xie, X.; Li, X.; Xiao, D.R.; Shen, J.; Lu, W.Q.; Lu, L.; Liu, S.Y. Three-dimensional hierarchical nickel-cobalt-sulfide nanostructures for high performance electrochemical energy storage electrodes. J. Mater. Chem. A 2016, 4, 18335–18341. [Google Scholar] [CrossRef]
- Hao, Z.Q.; Xu, L.L.; Wei, B.; Fan, L.L.; Liu, Y.; Zhang, M.Y.; Gao, H. Nanosize alpha-Bi2O3 decorated Bi2MoO6 via an alkali etching process for enhanced photocatalytic performance. Rsc. Adv. 2015, 5, 12346–12353. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zheng, L.C.; Tang, R.R.; Zhou, W.H.; Gao, J.; Wu, H.W. Porous Fe-Co-P nanowire arrays through alkaline etching as self-supported electrodes for efficient hydrogen production. J. Solid State Electrochem. 2021, 25, 1623–1631. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, C.; Liu, Y.; Wang, Z.; Wang, P.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H.; Huang, B. Sulfuration of NiV-layered double hydroxide towards novel supercapacitor electrode with enhanced performance. Chem. Eng. J. 2018, 351, 119–126. [Google Scholar] [CrossRef]
- Qin, K.Q.; Wang, L.P.; Wen, S.W.; Diao, L.C.; Liu, P.; Li, J.J.; Ma, L.Y.; Shi, C.S.; Zhong, C.; Hu, W.B.; et al. Designed synthesis of NiCo-LDH and derived sulfide on heteroatom-doped edge-enriched 3D rivet graphene films for high-performance asymmetric supercapacitor and efficient OER. J. Mater. Chem. A 2018, 6, 8109–8119. [Google Scholar] [CrossRef]
- Jing, C.; Liu, X.Y.; Yao, H.C.; Yan, P.; Zhao, G.; Bai, X.L.; Dong, B.Q.; Dong, F.; Li, S.C.; Zhang, Y. Phase and morphology evolution of CoAl LDH nanosheets towards advanced supercapacitor applications. Crystengcomm 2019, 21, 4934–4942. [Google Scholar] [CrossRef]
- Wang, L.; You, J.H.; Zhao, Y.; Bao, W.T. Core-shell CuO@NiCoMn-LDH supported by copper foam for high-performance supercapacitors. Dalton Trans. 2022, 51, 3314–3322. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.A.; Wang, J.X.; Tang, D.J.; Tong, Z.F.; Ji, H.B.; Qu, H.Y. A forest geotexture-inspired ZnO@Ni/Co layered double hydroxide-based device with superior electrochromic and energy storage performance. J. Mater. Chem. A 2022, 10, 12643–12655. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.Q.; Li, X.M.; Du, X.; Ma, X.L.; Hao, X.G.; Abudula, A. Ultrathin nanoflakes of cobalt-manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Tang, C.H.; Tang, Z.; Gong, H. Hierarchically Porous Ni-Co Oxide for High Reversibility Asymmetric Full-Cell Supercapacitors. J. Electrochem. Soc. 2012, 159, A651–A656. [Google Scholar] [CrossRef]
- Kuang, M.; Li, T.T.; Chen, H.; Zhang, S.M.; Zhang, L.L.; Zhang, Y.X. Hierarchical Cu2O/CuO/Co3O4 core-shell nanowires: Synthesis and electrochemical properties. Nanotechnology 2015, 26, 304002. [Google Scholar] [CrossRef]
- Kuang, M.; Wen, Z.Q.; Guo, X.L.; Zhang, S.M.; Zhang, Y.X. Engineering firecracker-like beta-manganese dioxides@ spinel nickel cobaltates nanostructures for high-performance supercapacitors. J. Power Sources 2014, 270, 426–433. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Fu, W.; Zhang, P.; Pan, X.; Xie, E. In situ synthesis of CoSx@ carbon core-shell nanospheres decorated in carbon nanofibers for capacitor electrodes with superior rate and cycling performances. Carbon 2017, 114, 187–197. [Google Scholar] [CrossRef]
- Malak-Polaczyk, A.; Vix-Guterl, C.; Frackowiak, E. Carbon/layered double hydroxide (LDH) composites for supercapacitor application. Energy Fuels 2010, 24, 3346–3351. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, C.; Shu, K.; Sun, Q.; Zheng, J.; Zhang, S.; Liu, X.; Yao, K.; Zhou, X.; Liu, X. Atomic Scale Optimization Strategy of Al-Based Layered Double Hydroxide for Alkali Stability and Supercapacitors. Int. J. Mol. Sci. 2022, 23, 11645. https://doi.org/10.3390/ijms231911645
Jing C, Shu K, Sun Q, Zheng J, Zhang S, Liu X, Yao K, Zhou X, Liu X. Atomic Scale Optimization Strategy of Al-Based Layered Double Hydroxide for Alkali Stability and Supercapacitors. International Journal of Molecular Sciences. 2022; 23(19):11645. https://doi.org/10.3390/ijms231911645
Chicago/Turabian StyleJing, Chuan, Kai Shu, Qing Sun, Jiayu Zheng, Shuijie Zhang, Xin Liu, Kexin Yao, Xianju Zhou, and Xiaoying Liu. 2022. "Atomic Scale Optimization Strategy of Al-Based Layered Double Hydroxide for Alkali Stability and Supercapacitors" International Journal of Molecular Sciences 23, no. 19: 11645. https://doi.org/10.3390/ijms231911645




