Insights into the Metabolic Response of Lactiplantibacillus plantarum CCFM1287 upon Patulin Exposure
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Concentrations of PAT on L. plantarum CCFM1287 Cell Growth
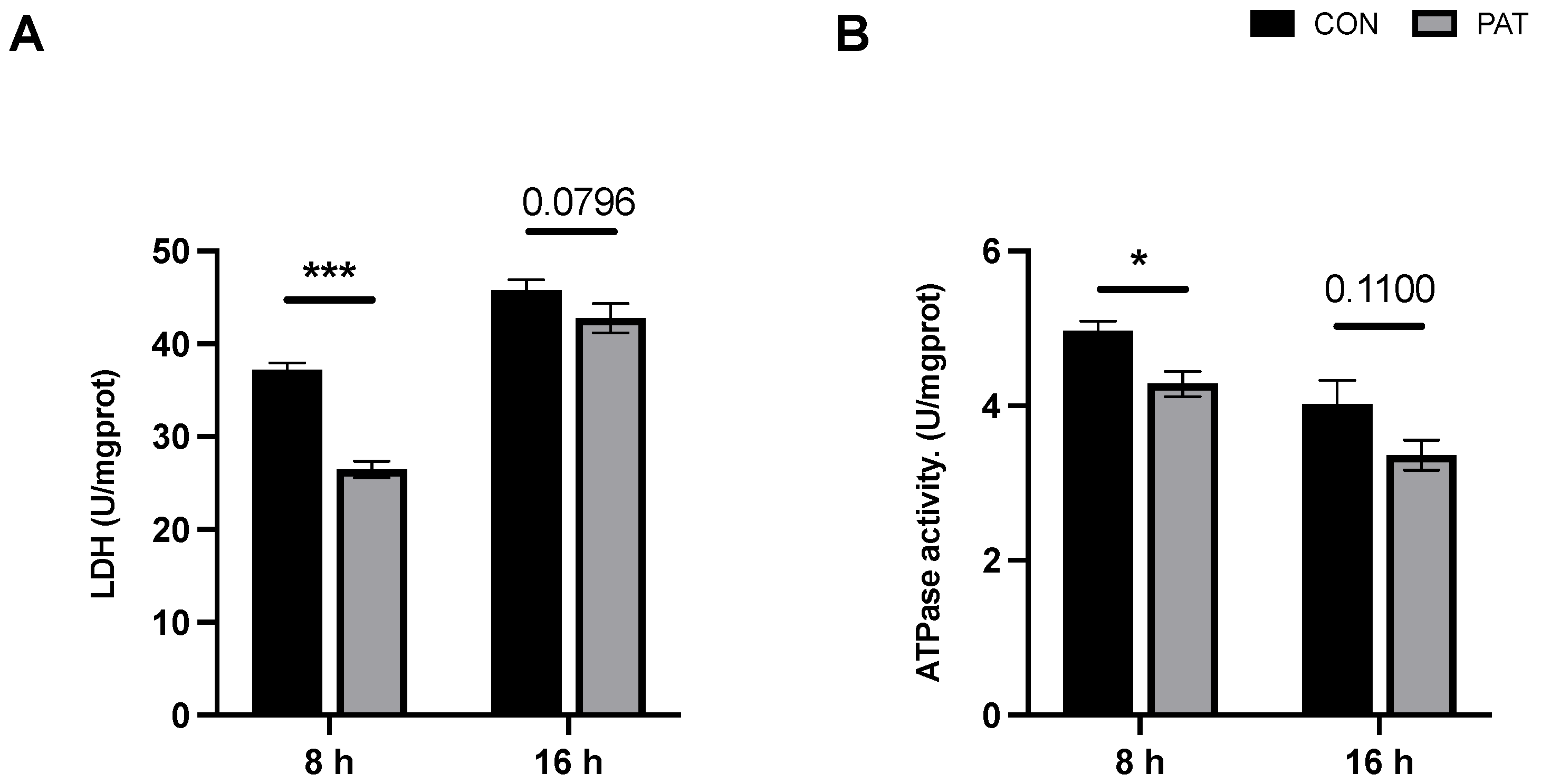
2.2. Changes in Total Intracellular Antioxidant Capacity and Superoxide Dismutase Activity Triggered by PAT
2.3. Changes in Metabolic Capacity Triggered by PAT
2.4. Changes in Metabolic Capacity Triggered by PAT
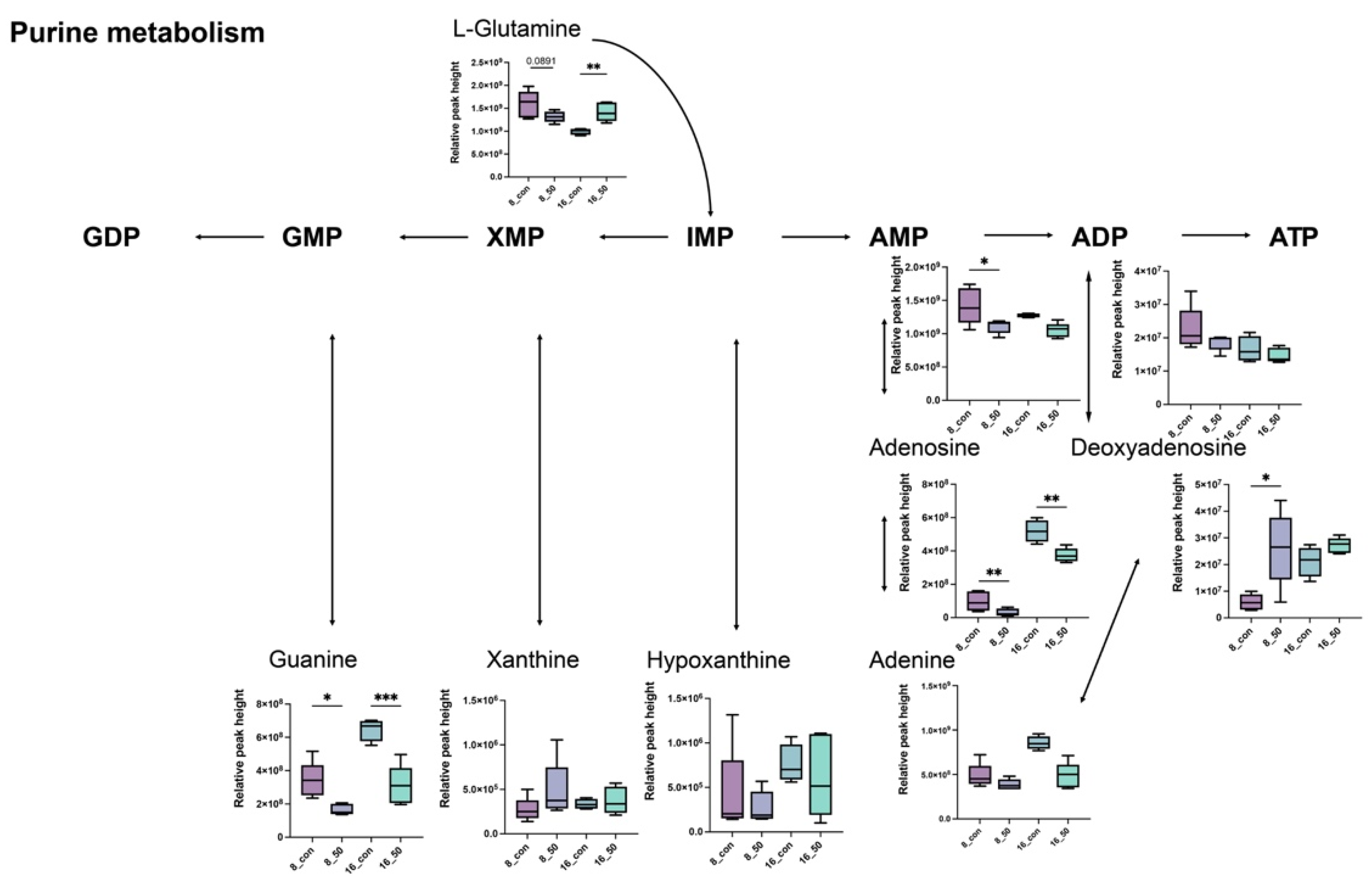
2.5. PAT Exposure Triggers Changes in Intracellular Metabolites of L. plantarum
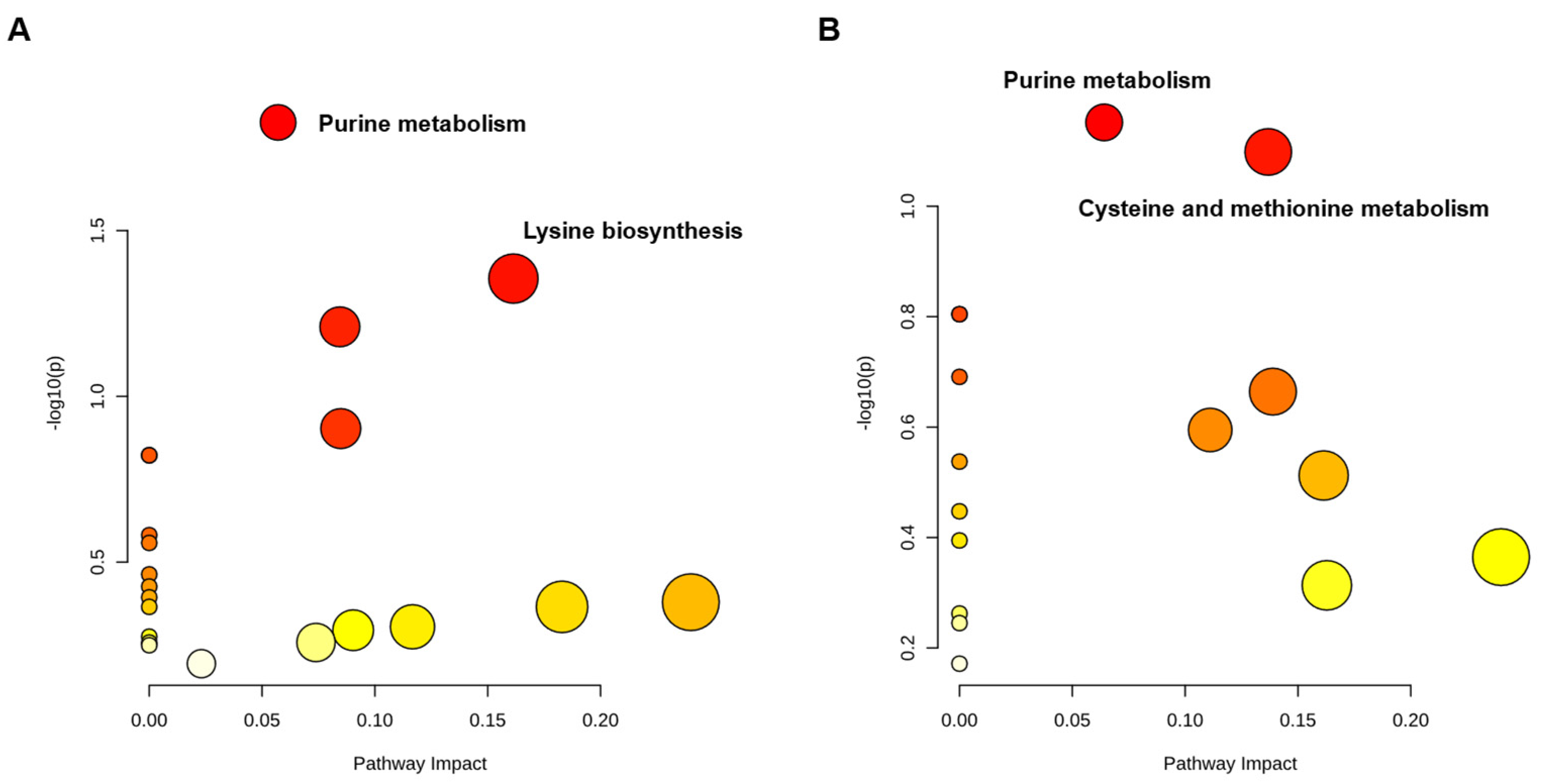
2.6. PAT Exposure Affects Intracellular Metabolic Pathways in L. plantarum
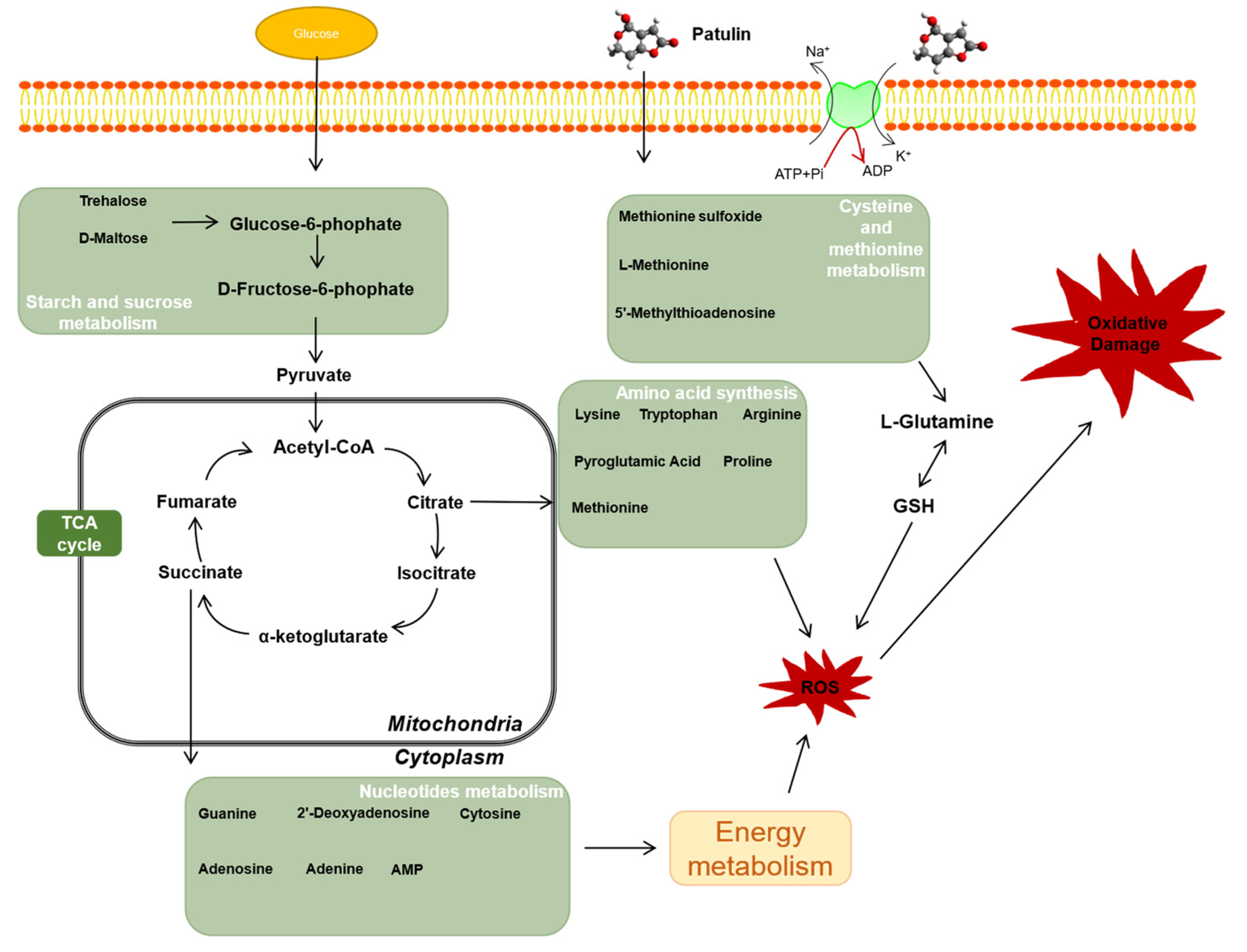
3. Discussion
4. Materials and Methods
4.1. Chemicals and Bacterial Strain
4.2. Culture of L. plantarum CCFM1287 and Detection of PAT
4.3. Determination of Important Intracellular Antioxidant and Physiological Indicators of Bacteria
4.4. Sample Preparation for Metabolic Analysis
4.5. Metabolite Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Wei, W.; Zhou, W.; Li, H.; Rao, S.; Gao, L.; Yang, Z. Prevention and detoxification of patulin in apple and its products: A review. Food Res. Int. 2021, 140, 110034. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Zhai, Q.; Tian, F.; Chen, W. Progress in the distribution, toxicity, control, and detoxification of patulin: A review. Toxicon 2020, 184, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Ioi, J.D.; Zhou, T.; Tsao, R.; Marcone, M.F. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Mehmood, S.; Yuan, Y.; Yue, T. Mycotoxin patulin in food matrices: Occurrence and its biological degradation strategies. Drug Metab. Rev. 2019, 51, 105–120. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, L.; Yuan, L.; Li, J.; Wang, X. The characteristics of patulin degradation by probiotic yeast-Pichia guilliermondii S15-8. Food Control 2022, 133, 108627. [Google Scholar] [CrossRef]
- Ji, J.; Yu, J.; Yang, Y.; Yuan, X.; Yang, J.; Zhang, Y.; Sun, J.; Sun, X. Exploration on the Enhancement of Detoxification Ability of Zearalenone and Its Degradation Products of Aspergillus niger FS10 under Directional Stress of Zearalenone. Toxins 2021, 13, 720. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, X.; Yang, Q.; Zhang, H.; Apaliya, M.T.; Dhanasekaran, S.; Zhang, X.; Zhao, L.; Li, J.; Jiang, Z. S-Adenosylmethionine-Dependent Methyltransferase Helps Pichia caribbica Degrade Patulin. J. Agric. Food Chem. 2019, 67, 11758–11768. [Google Scholar] [CrossRef]
- Xing, M.; Chen, Y.; Li, B.; Tian, S. Highly efficient removal of patulin using immobilized enzymes of Pseudomonas aeruginosa TF-06 entrapped in calcium alginate beads. Food Chem. 2022, 377, 131973. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, W.; Rao, S.; Gao, L.; Li, H.; Yang, Z. Degradation of patulin in fruit juice by a lactic acid bacteria strain Lactobacillus casei YZU01. Food Control 2020, 112, 107147. [Google Scholar] [CrossRef]
- Lai, W.; Cai, R.; Yang, K.; Yue, T.; Gao, Z.; Yuan, Y.; Wang, Z. Detoxification of patulin by Lactobacillus pentosus DSM 20314 during apple juice fermentation. Food Control 2022, 131, 108446. [Google Scholar] [CrossRef]
- Wei, C.; Yu, L.; Qiao, N.; Wang, S.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The characteristics of patulin detoxification by Lactobacillus plantarum 13M5. Food Chem. Toxicol. 2020, 146, 111787. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Chen, Y.; Li, B.; Tian, S. Characterization of a short-chain dehydrogenase/reductase and its function in patulin biodegradation in apple juice. Food Chem. 2021, 348, 129046. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, X.; Wang, Y.; Xie, J.; Gao, H.; Li, X.; Huang, Z. Metabolomics analysis based on UHPLC-Q-TOF-MS/MS reveals effects of genistein on reducing mycotoxin citrinin production by Monascus aurantiacus Li AS3.4384. LWT 2020, 130, 109613. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, H.; Yang, Y.; Yu, J.; Ji, J.; Sun, J.; Zhang, S.; Sun, X. Exploration of biodegradation mechanism by AFB1-degrading strain Aspergillus niger FS10 and its metabolic feedback. Food Control 2021, 121, 107609. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, J.; Tang, Y.; Ma, Q.; Zhang, J.; Ji, C.; Zhao, L. Characterization and Genome Analysis of a Zearalenone-Degrading Bacillus velezensis Strain ANSB01E. Curr. Microbiol. 2020, 77, 273–278. [Google Scholar] [CrossRef]
- Horváth, E.; Papp, G.; Belágyi, J.; Gazdag, Z.; Vágvölgyi, C.; Pesti, M. In vivo direct patulin-induced fluidization of the plasma membrane of fission yeast Schizosaccharomyces pombe. Food Chem. Toxicol. 2010, 48, 1898–1904. [Google Scholar] [CrossRef]
- Iwahashi, Y.; Hosoda, H.; Park, J.-H.; Lee, J.-H.; Suzuki, Y.; Kitagawa, E.; Murata, S.M.; Jwa, N.-S.; Gu, M.-B.; Iwahashi, H. Mechanisms of Patulin Toxicity under Conditions That Inhibit Yeast Growth. J. Agric. Food Chem. 2006, 54, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jin, C.; Zhong, Y.; Zhu, J.; Liu, Q.; Sun, D.; Feng, J.; Xia, X.; Peng, X. Involvement of NADPH oxidase in patulin-induced oxidative damage and cytotoxicity in HEK293 cells. Food Chem. Toxicol. 2021, 150, 112055. [Google Scholar] [CrossRef] [PubMed]
- Boussabbeh, M.; Prola, A.; Ben Salem, I.; Guilbert, A.; Bacha, H.; Lemaire, C.; Abis-Essefi, S. Crocin and quercetin prevent PAT-induced apoptosis in mammalian cells: Involvement of ROS-mediated ER stress pathway. Environ. Toxicol. 2016, 31, 1851–1858. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Wen, R.; Chen, Q.; Kong, B. Metabolomics profiling reveals defense strategies of Pediococcus pentosaceus R1 isolated from Harbin dry sausages under oxidative stress. LWT 2021, 135, 110041. [Google Scholar] [CrossRef]
- Li, X.; Jiang, B.; Pan, B.; Mu, W.; Zhang, T. Purification and Partial Characterization of Lactobacillus Species SK007 Lactate Dehydrogenase (LDH) Catalyzing Phenylpyruvic Acid (PPA) Conversion into Phenyllactic Acid (PLA). J. Agric. Food Chem. 2008, 56, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jiang, X.; Wang, T.; Zhang, B.; Zhao, H. Lyciumbarbarum Polysaccharide (LBP): A Novel Prebiotics Candidate for Bifidobacterium and Lactobacillus. Front. Microbiol. 2018, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Zhen, N.; Zeng, X.; Wang, H.; Yu, J.; Pan, D.; Wu, Z.; Guo, Y. Effects of heat shock treatment on the survival rate of Lactobacillus acidophilus after freeze-drying. Food Res. Int. 2020, 136, 109507. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shen, W.; Gurunathan, S. Biologically Synthesized Gold Nanoparticles Ameliorate Cold and Heat Stress-Induced Oxidative Stress in Escherichia coli. Molecules 2016, 21, 731. [Google Scholar] [CrossRef] [Green Version]
- Mráček, T.; Pecina, P.; Vojtíšková, A.; Kalous, M.; Šebesta, O.; Houštěk, J. Two components in pathogenic mechanism of mitochondrial ATPase deficiency: Energy deprivation and ROS production. Exp. Gerontol. 2006, 41, 683–687. [Google Scholar] [CrossRef]
- Mázala-de-Oliveira, T.; de Figueiredo, C.S.; de Rezende Corrêa, G.; da Silva, M.S.; Miranda, R.L.; de Azevedo, M.A.; Cossenza, M.; dos Santos, A.A.; Giestal-de-Araujo, E. Ouabain-Na+/K+-ATPase Signaling Regulates Retinal Neuroinflammation and ROS Production Preventing Neuronal Death by an Autophagy-Dependent Mechanism Following Optic Nerve Axotomy In Vitro. Neurochem. Res. 2022, 47, 723–738. [Google Scholar] [CrossRef]
- Yu, X.; Jin, X.; Tang, J.; Wang, N.; Yu, Y.; Sun, R.; Deng, F.; Huang, C.; Sun, J.; Zhu, L. Metabolomic analysis and oxidative stress response reveals the toxicity in Escherichia coli induced by organophosphate flame retardants tris(2-chloroethyl) phosphate and triphenyl phosphate. Chemosphere 2022, 291, 133125. [Google Scholar] [CrossRef] [PubMed]
- Cone, M.C.; Marchitto, K.; Zehfus, B.; Ferro, A.J. Utilization by Saccharomyces cerevisiae of 5′-methylthioadenosine as a source of both purine and methionine. J. Bacteriol. 1982, 151, 510–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peifer, S.; Barduhn, T.; Zimmet, S.; Volmer, D.A.; Heinzle, E.; Schneider, K. Metabolic engineering of the purine biosynthetic pathway in Corynebacterium glutamicum results in increased intracellular pool sizes of IMP and hypoxanthine. Microb. Cell Factories 2012, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Scribani Rossi, C.; Barrientos-Moreno, L.; Paone, A.; Cutruzzolà, F.; Paiardini, A.; Espinosa-Urgel, M.; Rinaldo, S. Nutrient Sensing and Biofilm Modulation: The Example of L-arginine in Pseudomonas. Int. J. Mol. Sci. 2022, 23, 4386. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Fang, Z.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W.; Li, H.; Lu, W. Integration of Transcriptome and Metabolome Reveals the Genes and Metabolites Involved in Bifidobacterium bifidum Biofilm Formation. Int. J. Mol. Sci. 2021, 22, 7596. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Zhao, J.; Zhang, H.; Chen, W. Untargeted metabolomics reveals metabolic state of Bifidobacterium bifidum in the biofilm and planktonic states. LWT 2020, 118, 108772. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Seo, K.S.; Park, N.; Rutter, J.K.; Park, Y.; Baker, C.L.; Thornton, J.A.; Park, J.Y. Role of Glucose-6-Phosphate in Metabolic Adaptation of Staphylococcus aureus in Diabetes. Microbiol. Spectr. 2021, 9, e00857-00821. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactiplantibacillusand Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Yang, Q.; Tao, R.; Yang, B.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Optimization of the quenching and extraction procedures for a metabolomic analysis of Lactobacillus plantarum. Anal. Biochem. 2018, 557, 62–68. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Wu, X.; Kuang, H.; Xu, C. Sex-Dependent Environmental Health Risk Analysis of Flupyradifurone. Environ. Sci. Technol. 2022, 56, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, L.; Shen, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. Int. J. Mol. Sci. 2021, 22, 11045. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Zhang, C.; Gao, Y.; Yu, L.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Insights into the Metabolic Response of Lactiplantibacillus plantarum CCFM1287 upon Patulin Exposure. Int. J. Mol. Sci. 2022, 23, 11652. https://doi.org/10.3390/ijms231911652
Wei C, Zhang C, Gao Y, Yu L, Zhao J, Zhang H, Chen W, Tian F. Insights into the Metabolic Response of Lactiplantibacillus plantarum CCFM1287 upon Patulin Exposure. International Journal of Molecular Sciences. 2022; 23(19):11652. https://doi.org/10.3390/ijms231911652
Chicago/Turabian StyleWei, Chaozhi, Chuan Zhang, Yuhang Gao, Leilei Yu, Jianxin Zhao, Hao Zhang, Wei Chen, and Fengwei Tian. 2022. "Insights into the Metabolic Response of Lactiplantibacillus plantarum CCFM1287 upon Patulin Exposure" International Journal of Molecular Sciences 23, no. 19: 11652. https://doi.org/10.3390/ijms231911652




