Novel Amoxicillin-Loaded Sericin Biopolymeric Nanoparticles: Synthesis, Optimization, Antibacterial and Wound Healing Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative, Quantitative and SDS-PAGE Analyses of the Extracted Sericin
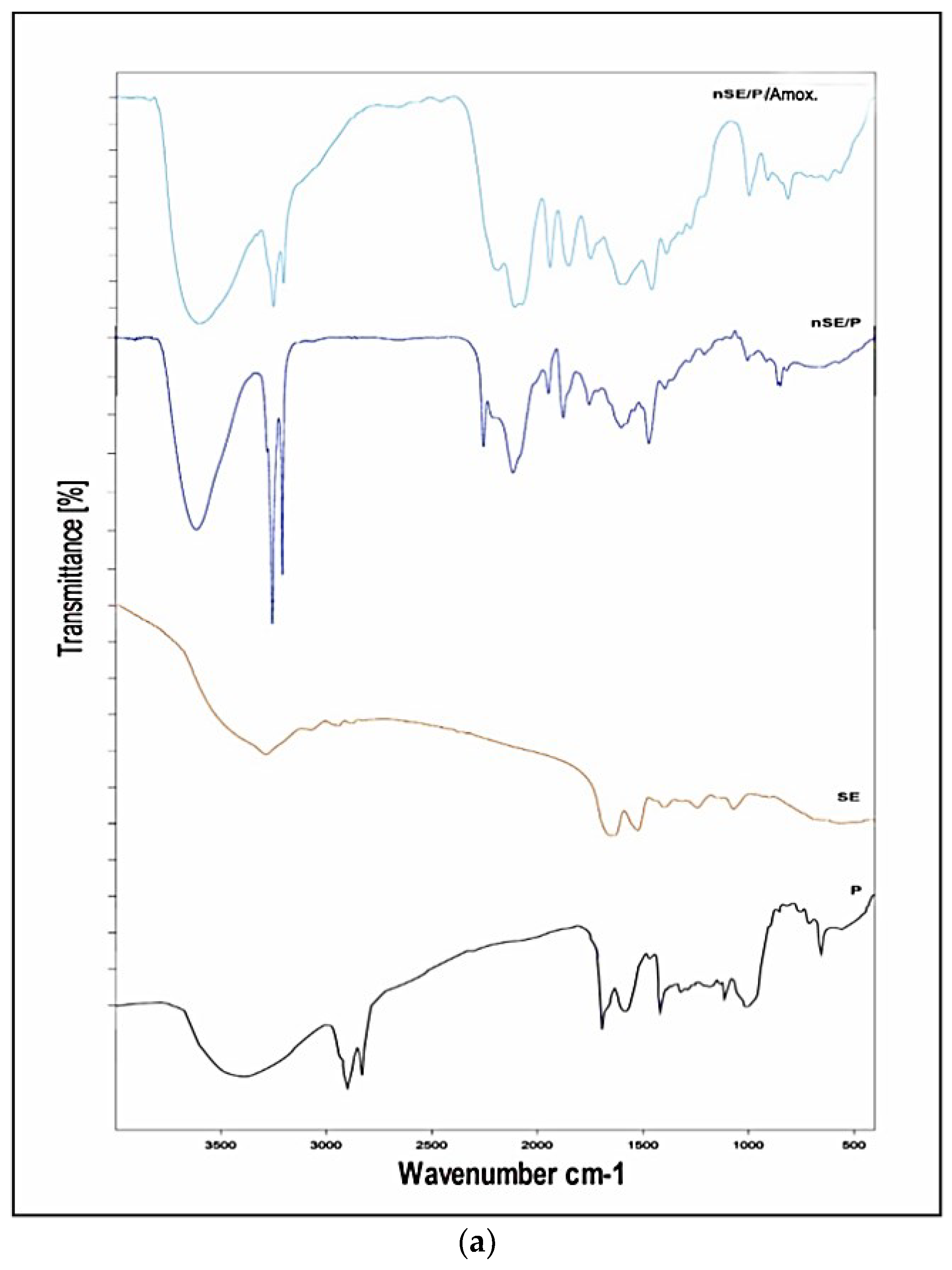
2.2. Amino Acid, X-ray Diffraction (XRD) and Fourier Transform Infrared (FTIR) Analyses
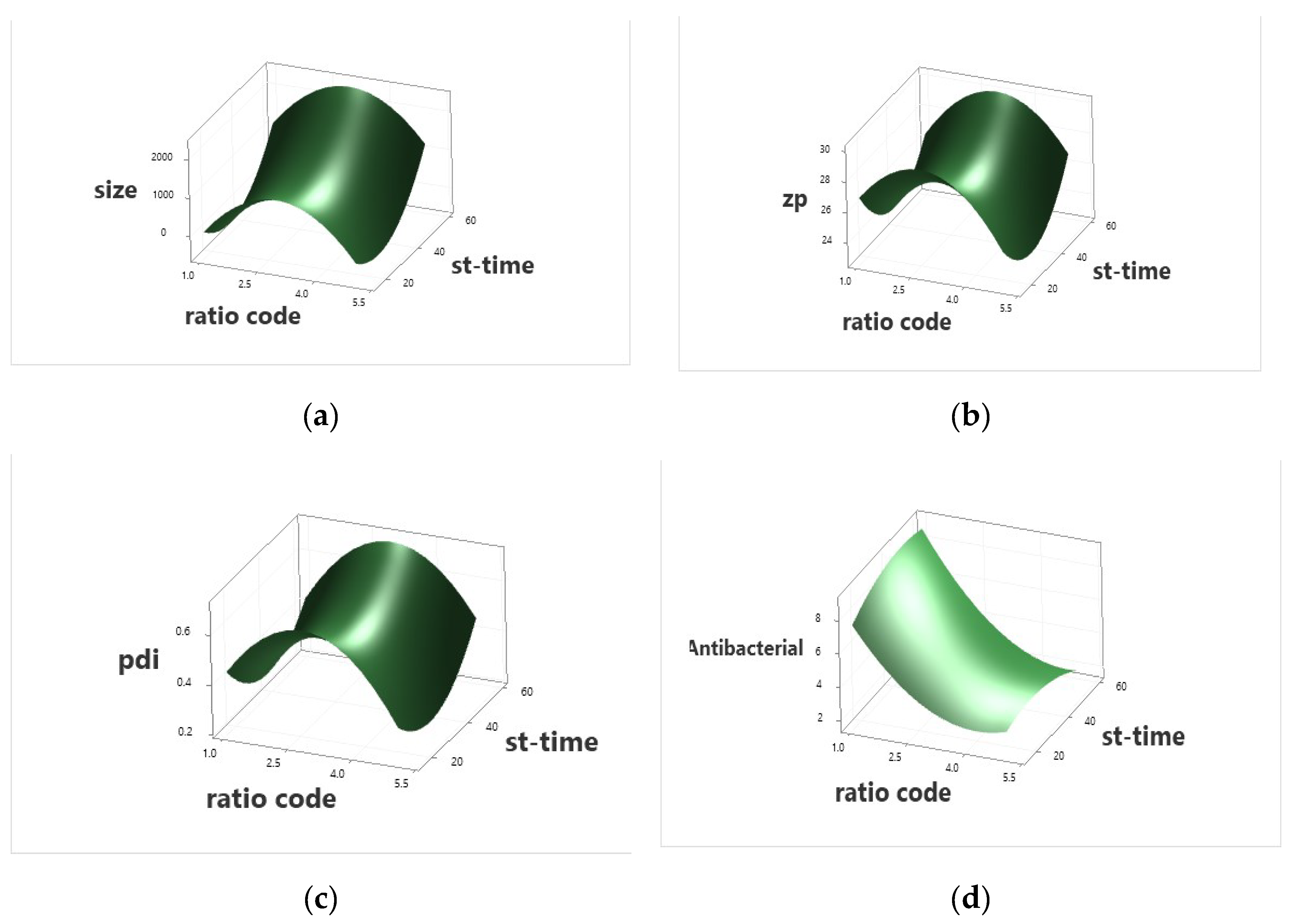
2.3. Sericin/Propolis Nanoparticles (nSE/P) Preparation and Optimization
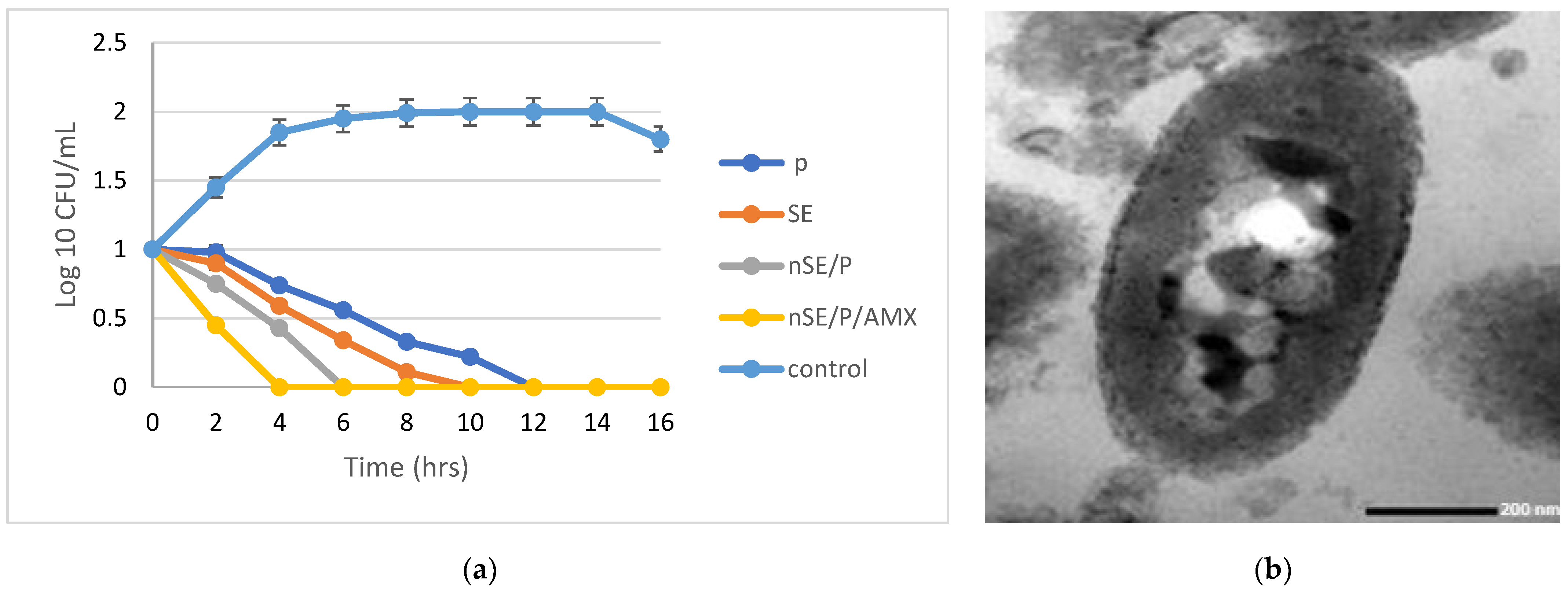
2.4. The Combined Action of the Tested Antibiotics and the Optimized Nanoformula
2.5. Characterization of the Potent Sericin/Propolis/Amoxicillin Polymeric Nanoparticles
In Vitro Drug Release
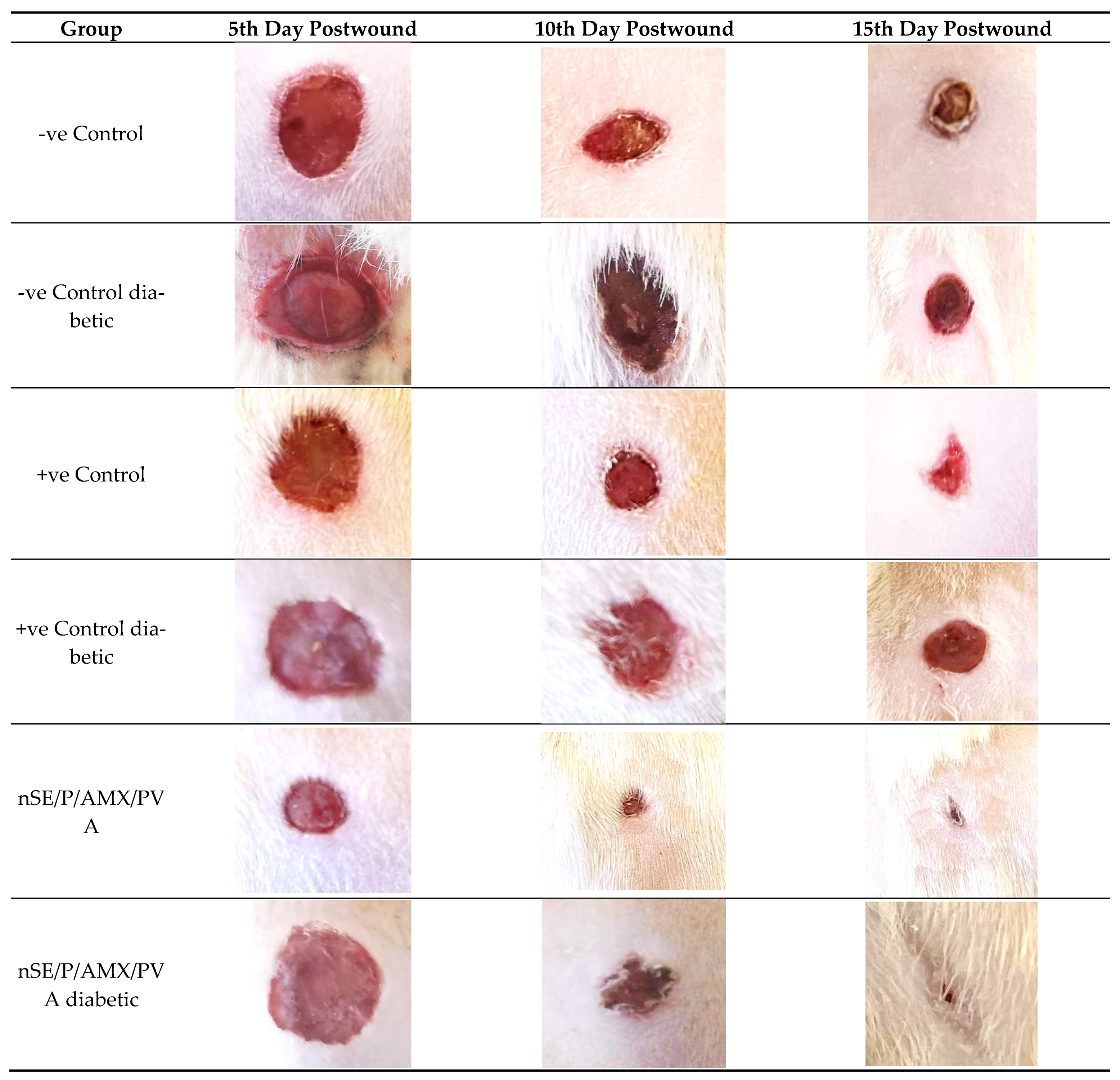
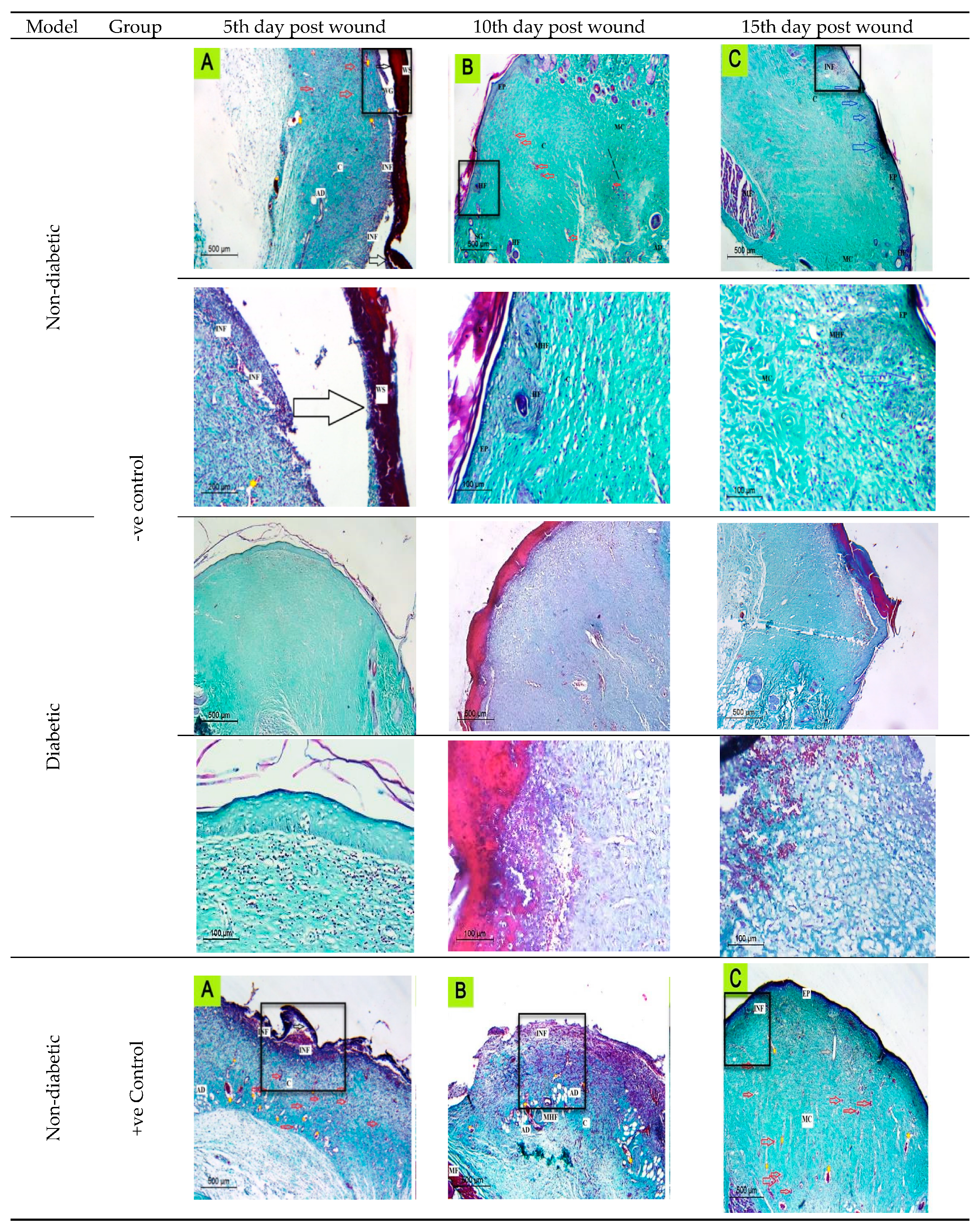
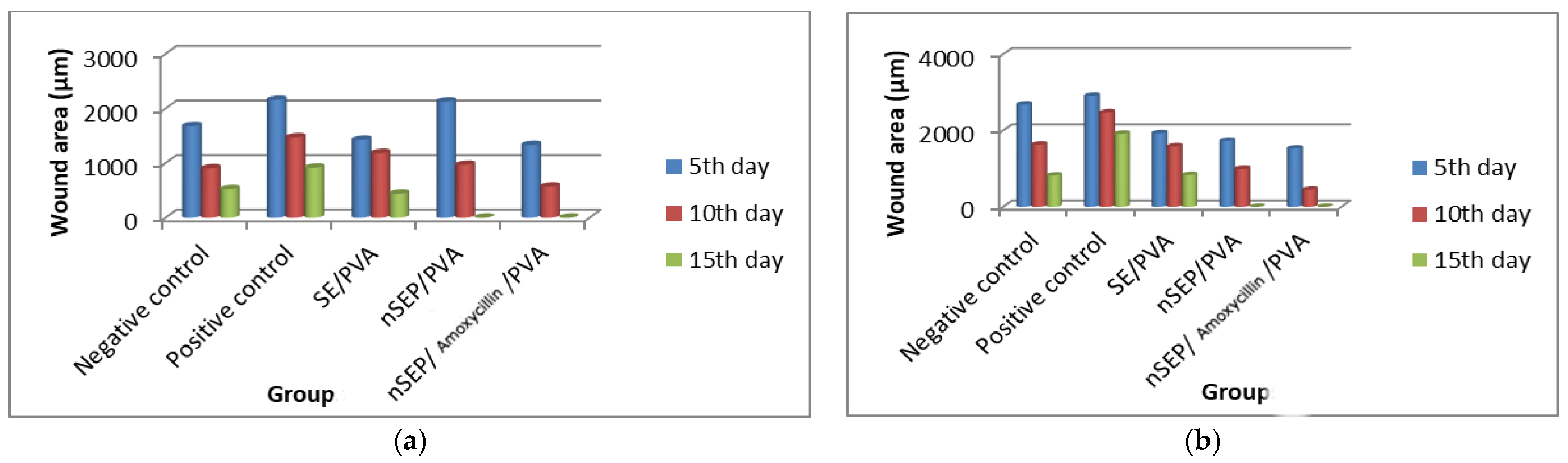
2.6. In Vivo Studies Using Nondiabetic and Diabetic Rats
2.6.1. Histological Observation of the Negative Control (Wounded Noninfected Nontreated) Rats
2.6.2. Histological Observation of the Bacterial Infected (Nontreated) Wounded Rat Skin
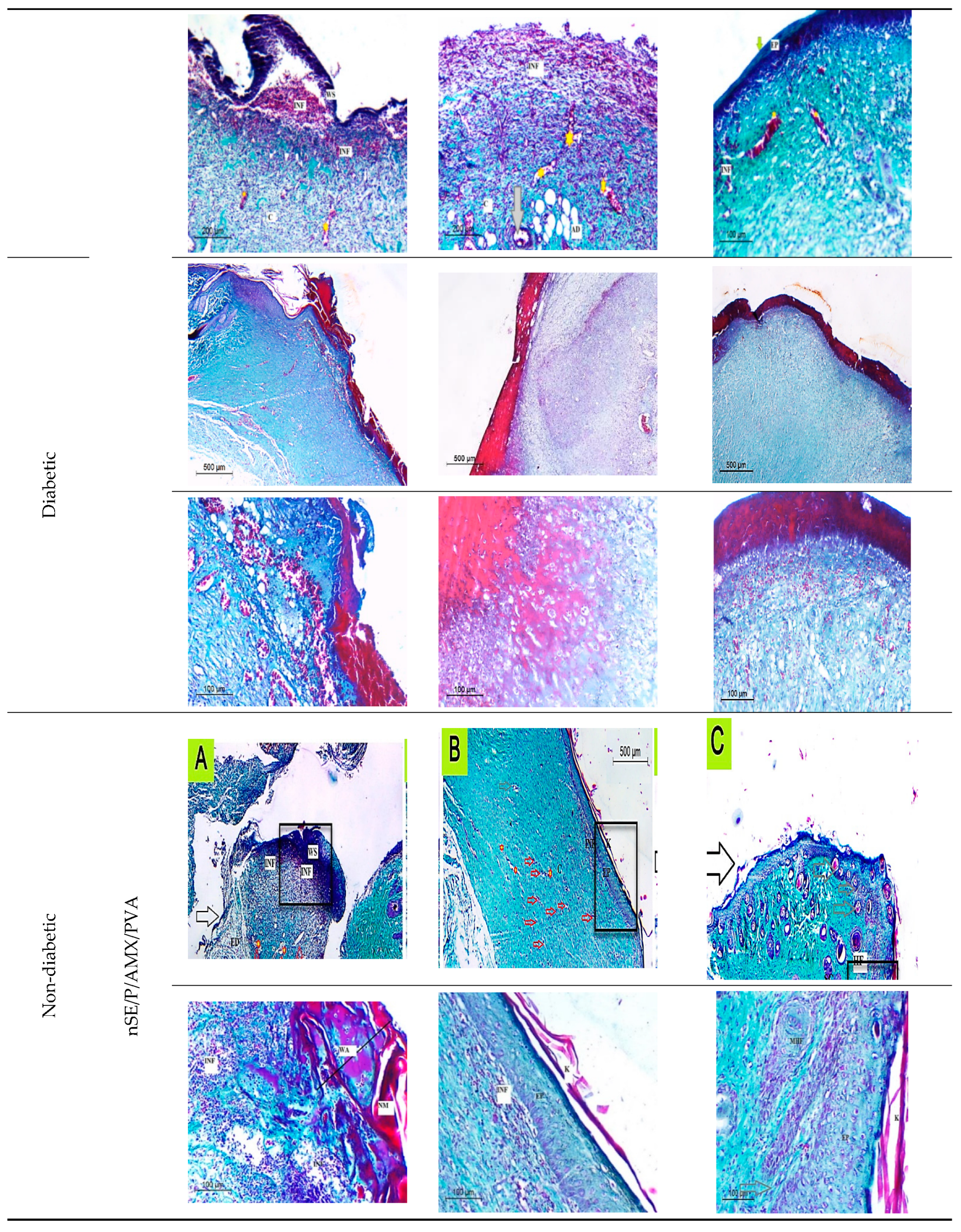
2.6.3. Histopathological Evaluation of SE/PVA Gel Treated Group
2.6.4. Histopathological Evaluation of PRO/PVA Gel Treated Group
2.6.5. Histopathological Evaluation of nSE/P/PVA Gel Treated Group
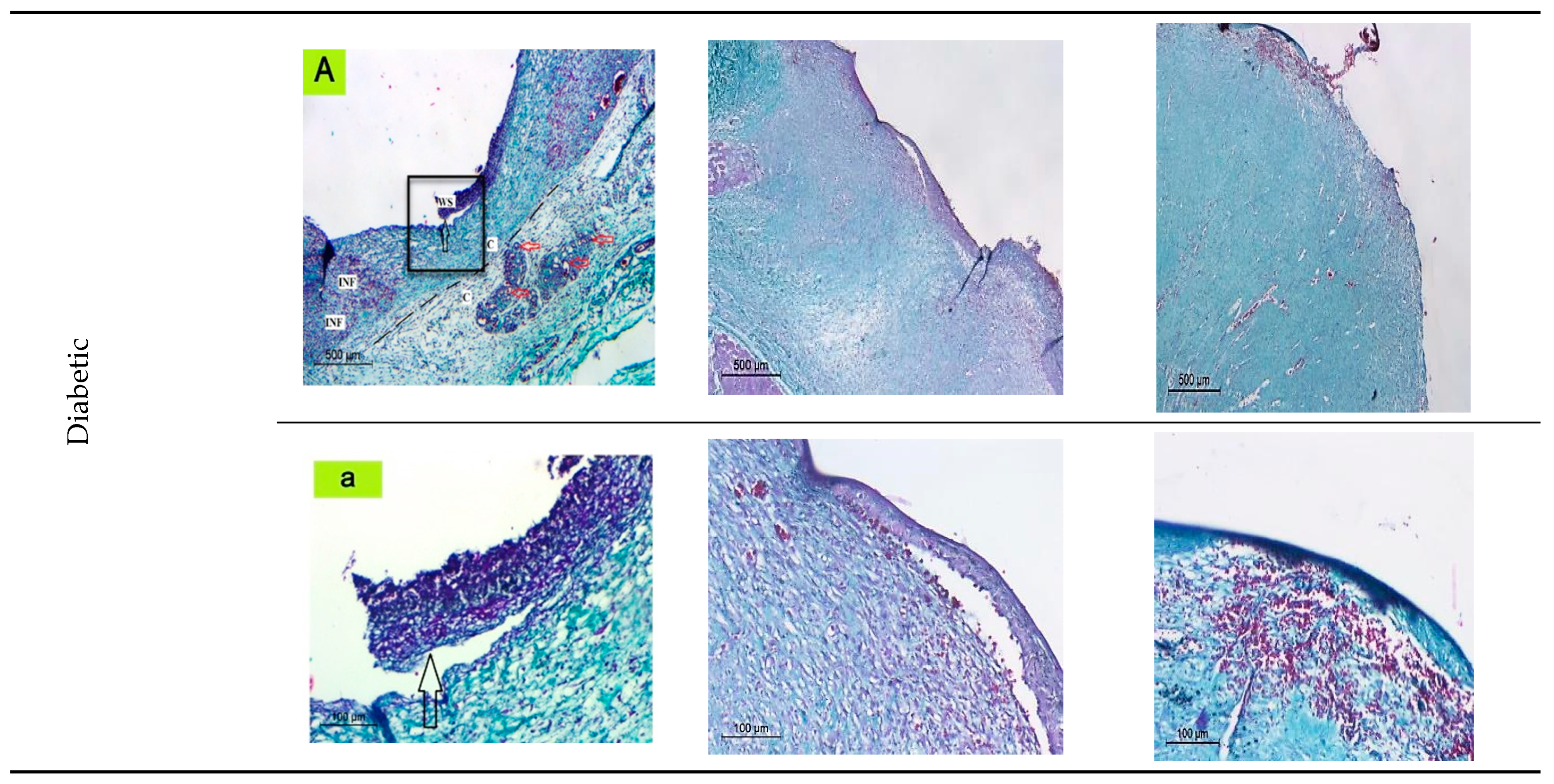
2.6.6. Histopathological Evaluation of nSE/P/Amoxicillin/PVA Gel Treated Group
3. Materials and Methods
3.1. Bombyx mori Cocoons
3.2. Propolis Collection and Extraction
3.3. Microorganisms
3.4. Sericin Extraction
3.4.1. Sericin Characterization
Qualitative and Quantitative Analysis of the Extracted Sericin
3.4.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.4.3. Amino Acid, X-ray Diffraction (XRD) and Fourier Transform Infrared (FTIR) Analyses
3.5. Sericin/Propolis Nanoparticles’ (nSE/P) Preparation and Optimization
Experimental Design
3.6. The Combined Effect of the Tested Antibiotics and the Optimized Nanoformula
3.7. Characterization of the Most Promising Nanoformula
3.8. In Vivo Study on the Wound Healing Efficiency of the Synthesized Nanoparticles in Normal and Diabetic Rats
3.8.1. Fabrication of PVA Gel Combined with the Optimized Nanoformula
3.8.2. Animal Modeling
- -
- Group I: Assigned as a negative control, with neither infection nor treatment regimen.
- -
- Group II: Assigned as a positive control, infected with MDR bacteria with no treatment regimen.
- -
- Group III: MDR bacterial infected rats treated with sericin/PVA.
- -
- Group IV: MDR bacterial infected rats treated with propolis/PVA.
- -
- Group V: MDR bacterial wound infected rats treated with a placebo (nSE/P) nanoparticles/PVA.
- -
- Group VI: MDR bacterial wound infected rats treated with the final nanoformula/PVA.
3.8.3. Bacterial Load Assessment
3.8.4. Histological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Boutet-Dubois, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.-P.; Loubet, P. Alternative Approaches for the Management of Diabetic Foot Ulcers. Front. Microbiol. 2021, 2877. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of antibacterial sericin based hydrogel as an injectable and mouldable wound dressing. Mater. Sci. Eng. C 2021, 119, 111597. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65. [Google Scholar] [CrossRef]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Long, M.; Liu, Q.; Wang, D.; Wang, J.; Zhang, Y.; Tang, A.; Liu, N.; Bui, B.; Chen, W.; Yang, H. A new nanoclay-based bifunctional hybrid fiber membrane with hemorrhage control and wound healing for emergency self-rescue. Mater. Today Adv. 2021, 12, 100190. [Google Scholar] [CrossRef]
- Fatima, H.; Goel, N.; Sinha, R.; Khare, S.K. Recent strategies for inhibiting multidrug-resistant and β-lactamase producing bacteria: A review. Colloids Surf. B Biointerfaces 2021, 205, 111901. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Abrahamse, H. Sericin-based nanomaterials and their applications in drug delivery. In Bio-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 211–229. [Google Scholar]
- Lamboni, L.; Li, Y.; Liu, J.; Yang, G. Silk sericin-functionalized bacterial cellulose as a potential wound-healing biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Abu-Serie, M.M.; Awaad, A.K.; El-Kady, H.; Elwakil, B.H. In vivo bio-distribution and acute toxicity evaluation of greenly synthesized ultra-small gold nanoparticles with different biological activities. Sci. Rep. 2022, 12, 6269. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; Elwakil, B.H.; Elshewemi, S.S.; El-Naggar, M.Y.; Bekhit, A.A.; Olama, Z.A. Novel Siwa propolis and colistin-integrated chitosan nanoparticles: Elaboration; in vitro and in vivo appraisal. Nanomedicine 2020, 15, 1269–1284. [Google Scholar] [CrossRef]
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In vitro and in vivo performance of a propolis-coated polyurethane wound dressing with high porosity and antibacterial efficacy. Colloids Surf. B Biointerfaces 2019, 178, 177–184. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein polymer-based nanoparticles: Fabrication and medical applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [Green Version]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Atlas, M.D.; Kaur, J.; Wang, X. The impact of degumming conditions on the properties of silk films for biomedical applications. Text. Res. J. 2016, 86, 275–287. [Google Scholar] [CrossRef]
- Gupta, D.; Agrawal, A.; Rangi, A. Extraction and characterization of silk sericin. Indian J. Fibre Text. Res. 2014, 39, 364–372. [Google Scholar]
- Jo, Y.Y.; Kweon, H.; Oh, J.H. Sericin for tissue engineering. Appl. Sci. 2020, 10, 8457. [Google Scholar] [CrossRef]
- Chanu, S.B.; Devi, S.K.; Singh, L.R. Silk Protein Sericin: Structure, Secretion, Composition and Antimicrobial Potential. Curr. Perspect. Anti-Infect. Agents 2019, 1, 183. [Google Scholar]
- Ahsan, F.; Ansari, T.M.; Usmani, S.; Bagga, P. An insight on silk protein sericin: From processing to biomedical application. Drug Res. 2018, 68, 317–327. [Google Scholar] [CrossRef]
- Kanoujia, J.; Faizan, M.; Parashar, P.; Singh, N.; Saraf, S.A. Curcumin loaded sericin nanoparticles: Assessment for biomedical application. Food Hydrocoll. Health 2021, 1, 100029. [Google Scholar] [CrossRef]
- Radu, I.C.; Zaharia, C.; Hudiță, A.; Tanasă, E.; Ginghină, O.; Marin, M.; Gălățeanu, B.; Costache, M. In vitro interaction of doxorubicin-loaded silk sericin nanocarriers with mcf-7 breast cancer cells leads to DNA damage. Polymers 2021, 13, 2047. [Google Scholar] [CrossRef]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of Silk Sericin from cocoons of three Southern African Wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef]
- Deryabin, D.; Galadzhieva, A.; Kosyan, D.; Duskaev, G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: Modes of action. Int. J. Mol. Sci. 2019, 20, 5588. [Google Scholar] [CrossRef] [Green Version]
- Rangi, A.; Jajpura, L. The biopolymer sericin: Extraction and applications. J. Text. Sci. Eng. 2015, 5, 1–5. [Google Scholar]
- Saha, J.H.; Mondal, M.I.; Karim Sheikh, M.R.; Habib, M.A. Extraction, structural and functional properties of silk sericin biopolymer from Bombyx mori silk cocoon waste. J. Text. Sci. Eng. 2019, 9, 2. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of resveratrol-loaded sericin nanoparticles: Promising bionanocarriers for drug delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Ray, P.; Singh, S.; Gupta, S. Topical antimicrobial therapy: Current status and challenges. Indian J. Med. Microbiol. 2019, 37, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Z.; Zhu, X.; Kang, E.T.; Neoh, K.G. Silk-functionalized titanium surfaces for enhancing osteoblast functions and reducing bacterial adhesion. Biomaterials 2008, 29, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Perteghella, S.; Bari, E.; Sorrenti, M.; Tripodo, G.; Chlapanidas, T.; Torre, M.L. Silk nanoparticles: From inert supports to bioactive natural carriers for drug delivery. Soft Matter 2018, 14, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.Y.; Kim, M.K.; Um, I.C.; Lee, K.H. Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol. 2011, 48, 32–37. [Google Scholar] [CrossRef]
- Zhang, X.; Tsukada, M.; Morikawa, H.; Aojima, K.; Zhang, G.; Miura, M. Production of silk sericin/silk fibroin blend nanofibers. Nanoscale Res. Lett. 2011, 6, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- H Elwakil, B.; Shaaban, M.M.; Bekhit, A.A.; El-Naggar, M.Y.; Olama, Z.A. Potential anti-COVID-19 activity of Egyptian propolis using computational modeling. Future Virol. 2021, 16, 107–116. [Google Scholar] [CrossRef]
- Liu, F.J.; Zhang, X.J.; Li, X. Silkworm (Bombyx mori) cocoon vs. wild cocoon multi-layer structure and performance characterization. Therm. Sci. 2019, 23, 2135–2142. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Jena, K.; Pandey, J.P.; Kumari, R.; Sinha, A.K.; Gupta, V.P.; Singh, G.P. Tasar silk fiber waste sericin: New source for anti-elastase, anti-tyrosinase and anti-oxidant compounds. Int. J. Biol. Macromol. 2018, 114, 1102–1108. [Google Scholar] [CrossRef]
- Shehata, T.M.; Elnahas, H.M.; Elsewedy, H.S. Development, Characterization and Optimization of the Anti-Inflammatory Influence of Meloxicam Loaded into a Eucalyptus Oil-Based Nanoemulgel. Gels 2022, 8, 262. [Google Scholar] [CrossRef]
- Siritienthong, T.; Ratanavaraporn, J.; Aramwit, P. Development of ethyl alcohol-precipitated silk sericin/polyvinyl alcohol scaffolds for accelerated healing of full-thickness wounds. Int. J. Pharm. 2012, 439, 175–186. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Elnahas, R.A.; Elwakil, B.H.; Elshewemi, S.S.; Olama, Z.A. Egyptian Olea europaea leaves bioactive extract: Antibacterial and wound healing activity in normal and diabetic rats. J. Tradit. Complement. Med. 2021, 11, 427–434. [Google Scholar] [CrossRef] [PubMed]





| Extraction Method | Protein Content (g/dL) | A-Ratio |
|---|---|---|
| High temperature and high pressure (HTHP) | 4.40 ± 0.1 | 1.34 |
| High temperature | 4.60 ± 0.07 | 1.70 |
| Alkali and high-temperature degumming | 4.90 ± 0.09 | 1.77 |
| Alkali/high-temperature/high-pressure degumming | 4.58 ± 0.5 | 1.65 |
| Acid-degumming | 3.70 ± 0.7 | 1.21 |
| Urea buffer | 3.70 ± 0.2 | 1.20 |
| Type | Amino Acids | Molar % |
|---|---|---|
| Polar | Serine | 30.40 |
| Cysteine | 3.05 | |
| Proline | 0.80 | |
| Threonine | 6.00 | |
| Tyrosine | 3.80 | |
| Nonpolar | Isoleusine | 1.40 |
| Leucine | 0.60 | |
| Proline | 0.80 | |
| Glycine | 12.20 | |
| Phenylalanine | 0.40 | |
| Methionine | 0.05 | |
| Alanine | 4.60 | |
| Basic | Histadine | 0.90 |
| Arginine | 2.80 | |
| Lysine | 10.20 | |
| Acidic | Aspartic acid | 19.10 |
| Glutamic acid | 4.10 |
| Trial Number | Sericin Percentage (%) | Stirring Time (min) | Physical Characteristics | ||
|---|---|---|---|---|---|
| Zeta Potential (mV) | Size (nm) | PDI | |||
| 1 | 30.0 | 15.0 | 32.7 | 268.0 | 0.30 |
| 2 | 30.0 | 30.0 | 23.7 | 234.0 | 0.36 |
| 3 | 30.0 | 45.0 | 39.3 | 116.0 | 0.24 |
| 4 | 30.0 | 60.0 | 24.4 | 222.6 | 0.32 |
| 5 | 40.0 | 15.0 | 20.5 | 762.8 | 0.76 |
| 6 | 40.0 | 30.0 | 13.4 | 1438.0 | 0.97 |
| 7 | 40.0 | 45.0 | 33.3 | 143.2 | 0.27 |
| 8 | 40.0 | 60.0 | 20.4 | 642.3 | 0.75 |
| 9 | 50.0 | 15.0 | 35.5 | 629.6 | 0.65 |
| 10 | 50.0 | 30.0 | 27.5 | 166.1 | 0.28 |
| 11 | 50.0 | 45.0 | 35.6 | 169.3 | 0.27 |
| 12 | 50.0 | 60.0 | 28.2 | 7540.0 | 1.00 |
| 13 | 60.0 | 15.0 | 28.6 | 389.6 | 0.45 |
| 14 | 60.0 | 30.0 | 23.0 | 362.9 | 0.39 |
| 15 | 60.0 | 45.0 | 23.6 | 617.3 | 0.71 |
| 16 | 60.0 | 60.0 | 24.8 | 375.0 | 0.36 |
| 17 | 70.0 | 15.0 | 25.7 | 319.1 | 0.39 |
| 18 | 70.0 | 30.0 | 23.0 | 270.3 | 0.21 |
| 19 | 70.0 | 45.0 | 22.7 | 269.9 | 0.37 |
| 20 | 70.0 | 60.0 | 27.2 | 394.4 | 0.46 |
| Trial Number | Sericin Percentage (%) | Stirring Time (min) | Inhibition Zone Diameter (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus 1 | S. aureus 2 | K. pneumoniae 1 | K. pneumoniae 2 | E. coli 1 | E. coli 2 | P. aeruginosa 1 | P. aeruginosa 2 | A. baumannii 1 | A. baumannii 2 | |||
| 1 | 30.0 | 15.0 | 18.0 | 11.0 | 8.0 | 6.0 | 6.0 | 13.0 | 6.0 | 6.0 | 15.0 | 11.0 |
| 2 | 30.0 | 30.0 | 24.0 | 12.0 | 8.0 | 7.0 | 6.0 | 8.0 | 6.0 | 6.0 | 15.0 | 12.0 |
| 3 | 30.0 | 45.0 | 32.0 | 22.0 | 12.0 | 9.0 | 9.0 | 14.0 | 8.0 | 12.0 | 15.0 | 12.0 |
| 4 | 30.0 | 60.0 | 26.0 | 21.0 | 12.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 14.0 | 12.0 |
| 5 | 40.0 | 15.0 | 12.0 | 6.0 | 6.0 | 6.0 | 7.0 | 8.0 | 6.0 | 6.0 | 10.0 | 6.0 |
| 6 | 40.0 | 30.0 | 8.0 | 6.0 | 8.0 | 6.0 | 9.0 | 10.0 | 6.0 | 6.0 | 8.0 | 6.0 |
| 7 | 40.0 | 45.0 | 12.0 | 6.0 | 8.0 | 6.0 | 6.0 | 11.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| 8 | 40.0 | 60.0 | 18.0 | 10.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 13.0 | 11.0 |
| 9 | 50.0 | 15.0 | 14.0 | 14.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 11.0 | 11.0 |
| 10 | 50.0 | 30.0 | 14.0 | 12.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 9.0 | 6.0 |
| 11 | 50.0 | 45.0 | 18.0 | 17.0 | 8.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 12.0 | 6.0 |
| 12 | 50.0 | 60.0 | 12.0 | 10.0 | 8.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 8.0 | 8.0 |
| 13 | 60.0 | 15.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| 14 | 60.0 | 30.0 | 10.0 | 6.0 | 8.0 | 8.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| 15 | 60.0 | 45.0 | 10.0 | 6.0 | 10.0 | 6.0 | 6.0 | 6.0 | 8.0 | 8.0 | 7.0 | 7.0 |
| 16 | 60.0 | 60.0 | 6.0 | 6.0 | 8.0 | 6.0 | 6.0 | 6.0 | 6.0 | 7.0 | 6.0 | 6.0 |
| 17 | 70.0 | 15.0 | 10.0 | 6.0 | 7.0 | 6.0 | 6.0 | 6.0 | 8.0 | 6.0 | 6.0 | 6.0 |
| 18 | 70.0 | 30.0 | 12.0 | 10.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 11.0 | 6.0 | 6.0 |
| 19 | 70.0 | 45.0 | 10.0 | 6.0 | 6.0 | 6.0 | 6.0 | 8.0 | 6.0 | 8.0 | 6.0 | 6.0 |
| 20 | 70.0 | 60.0 | 6.0 | 6.0 | 6.0 | 6.0 | 7.0 | 7.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Treatment/M.OS | Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Klebsiella pneumoniae | Escherichia coli | Pseudomonas aeruginosa | Acinetobacter baumannii | |
| Sericin | 8.0 | 8.0 | 7.5 | 7.0 | 7.5 |
| Propolis | 7.5 | 8.0 | 7.0 | 7.0 | 7.0 |
| nSE/P | 22.0 | 9.0 | 9.0 | 8.0 | 12.0 |
| Cephalexin | 11.7 | 12.5 | 10.5 | 9.5 | 10.0 |
| nSE/P/Cephalexin | 45.0 | 30.0 | 25.5 | 24.5 | 25.3 |
| Colistin | 10.0 | 11.0 | 9.0 | 8.6 | 9.0 |
| nSE/P/Colistin | 22.0 | 27.5 | 19.0 | 18.0 | 19.0 |
| Amoxicillin | 11.0 | 12.0 | 9.7 | 9.0 | 9.5 |
| nSE/P/Amoxicillin | 34.0 | 26.4 | 22.0 | 20.0 | 21.0 |
| Tetracycline | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| nSE/P/Tetracycline | 15.0 | 8.0 | 6.0.0 | 6.0 | 6.0 |
| Tegycicline | 22.0 | 17.0 | 25.0 | 17.0 | 14.0 |
| nSE/P/Tegycicline | 15.0 | 6.0 | 6.0 | 6.0 | 12.0 |
| Chloramphenicol | 25.0 | 20.0 | 6.0 | 6.0 | 7.0 |
| nSE/P/Chloramphenicol | 25.0 | 20.0 | 6.0 | 6.0 | 7.0 |
| Ampicillin-sulbactam | 16.0 | 6.0 | 13.0 | 6.0 | 10.0 |
| nSE/P/Ampicillin-sulbactam | 19.0 | 6.0 | 13.0 | 6.0 | 12.0 |
| Cefotaxime | 12.0 | 13.0 | 15.0 | 15.0 | 14.0 |
| nSE/P/Cefotaxime | 14.0 | 11.0 | 14.0 | 10.0 | 12.0 |
| Cefuroxime | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| nSE/P/Cefuroxime | 17.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Gentamicin | 23.0 | 22.0 | 10.0 | 10.0 | 8.0 |
| nSE/P/Gentamicin | 23.0 | 24.0 | 10.0 | 12.0 | 8.0 |
| Cefoperazone | 22.0 | 25.0 | 20.0 | 19.0 | 14.0 |
| nSE/P/Cefoperazone | 25.0 | 25.0 | 23.0 | 22.0 | 16.0 |
| Azithromycin | 6.0 | 15.0 | 13.0 | 6.0 | 12.0 |
| nSE/P/Azithromycin | 15.0 | 14.0 | 10.0 | 6.0 | 10.0 |
| Ceftazidim | 6.0 | 13.0 | 13.0 | 6.0 | 10.0 |
| nSE/P/Ceftazidim | 15.0 | 6.0 | 6.0 | 6.0 | 10.0 |
| Amikacin | 25.0 | 23.0 | 24.0 | 25.0 | 7.0 |
| nSE/P/Amikacin | 30.0 | 24.0 | 24.0 | 28.0 | 9.0 |
| M.OS | Pseudomonas aeruginosa | ||
|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC Index | |
| Sericin | 750.0 | 3000.0 | 4.0 |
| Propolis | 1250.0 | 5000.0 | 4.0 |
| nSE/P | 250.0 | 1000.0 | 4.0 |
| nSE/P/Amoxicillin | 1.0 | 4.0 | 4.0 |
| Independent Variables | Levels of Variation | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | |
| Sericin concentration (%) | 30.0 | 40 | 50 | 60 | 70 |
| Stirring time (min) | 0.0 | 15 | 30 | 45 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, S.E.; Tayea, N.A.; Elwakil, B.H.; Gad, A.A.E.M.; Ghareeb, D.A.; Olama, Z.A. Novel Amoxicillin-Loaded Sericin Biopolymeric Nanoparticles: Synthesis, Optimization, Antibacterial and Wound Healing Activities. Int. J. Mol. Sci. 2022, 23, 11654. https://doi.org/10.3390/ijms231911654
Diab SE, Tayea NA, Elwakil BH, Gad AAEM, Ghareeb DA, Olama ZA. Novel Amoxicillin-Loaded Sericin Biopolymeric Nanoparticles: Synthesis, Optimization, Antibacterial and Wound Healing Activities. International Journal of Molecular Sciences. 2022; 23(19):11654. https://doi.org/10.3390/ijms231911654
Chicago/Turabian StyleDiab, Shaimaa E., Nourhan A. Tayea, Bassma H. Elwakil, Abir Abd El Mageid Gad, Doaa A. Ghareeb, and Zakia A. Olama. 2022. "Novel Amoxicillin-Loaded Sericin Biopolymeric Nanoparticles: Synthesis, Optimization, Antibacterial and Wound Healing Activities" International Journal of Molecular Sciences 23, no. 19: 11654. https://doi.org/10.3390/ijms231911654







