Recombinant Humanized IgG1 Antibody Protects against oxLDL-Induced Oxidative Stress and Apoptosis in Human Monocyte/Macrophage THP-1 Cells by Upregulation of MSRA via Sirt1-FOXO1 Axis
Abstract
:1. Introduction
2. Results
2.1. Recombinant Humanized IgG1 Antibody Alleviates oxLDL-Induced Oxidative Stress and Apoptosis in THP-1 Cells
2.2. OxLDL Reduces MSRA Protein Expression in THP-1 Cells, While Recombinant Humanized IgG1 Antibody Upregulates MSRA Expression
2.3. Recombinant Humanized IgG1 Antibody Alleviates oxLDL-Induced Oxidative Stress and Apoptosis in THP-1 Cells Is MSRA Dependent
2.4. The Upregulation of MSRA Is Regulated by Transcription Factor FOXO1
2.5. Recombinant Humanized IgG1 Antibody Alleviates oxLDL-Induced Apoptosis in THP-1 Cells via Modulation of SIRT1-Dependent FOXO1 Deacetylation
3. Discussion
4. Materials and Methods
4.1. Preparation of CD14+ Human Monocytes
4.2. Cell Culture
4.3. Protein Extraction, Digestion and Analysis
4.4. Bioinformatics Analysis and Functional Enrichment
4.5. Western Blot Analysis
4.6. Cell Transfection
4.7. Luciferase Reporter Gene Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.O.; Regine, M.A.; Subrata, C.; Long, S. Molecular mechanisms and genetic regulation in atherosclerosis. IJC Heart Vasc. 2018, 21, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Mushenkova, N.V.; Bezsonov, E.E. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage apoptosis in advanced atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. 1), E40–E45. [Google Scholar] [CrossRef] [Green Version]
- Bezsonov, E.E.; Sobenin, I.A. Immunopathology of Atherosclerosis and Related Diseases: Focus on Molecular Biology. Int. J. Mol. Sci. 2021, 22, 4080. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Weissbach, H.; Etienne, F.; Hoshi, T.; Heinemann, S.H.; Lowther, W.T.; Matthews, B.; St John, G.; Nathan, C.; Brot, N. Peptide methionine sulfoxide reductase: Structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 2002, 397, 172–178. [Google Scholar] [CrossRef]
- Yermolaieva, O.; Xu, R.; Schinstock, C.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Hoshi, T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc. Natl. Acad. Sci. USA 2004, 101, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.; Tang, X.D.; Chen, M.-L.; Joiner, M.A.; Sun, G.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Iverson, L.; Wu, C.-F.; et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA 2002, 99, 2748–2753. [Google Scholar] [CrossRef]
- Chung, H.; Kim, A.K.; Jung, S.A.; Kim, S.W.; Yu, K.; Lee, J.H. The Drosophila homolog of methionine sulfoxide reductase A extends lifespan and increases nuclear localization of FOXO. FEBS Lett. 2010, 584, 3609–3614. [Google Scholar] [CrossRef] [Green Version]
- Koc, A.; Gasch, A.P.; Rutherford, J.C.; Kim, H.Y.; Gladyshev, V.N. Methionine sulfoxide reductase regulation of yeast lifespan reveals reactive oxygen species-dependent and -independent components of aging. Proc. Natl. Acad. Sci. USA 2004, 101, 7999–8004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, A.B.; Kim, G.; Liu, C.; Wren, J.D.; Georgescu, C.; Richardson, A.; Levine, R.L. Effects of transgenic methionine sulfoxide reductase A (MsrA) expression on lifespan and age-dependent changes in metabolic function in mice. Redox Biol. 2016, 10, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Wang, F.; Hughes, T.; Yu, J. FOXOs in cancer immunity: Knowns and unknowns. Semin. Cancer Biol. 2018, 50, 53–64. [Google Scholar] [CrossRef]
- Kousteni, S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone 2012, 50, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Hou, H.; Haller, E.M.; Nicosia, S.V.; Bai, W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005, 24, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Lee, Y.M.; Chun, Y.S.; Chen, J.; Kim, J.E.; Park, J.W. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Liu, X.; Su, J.; Zhou, H.; Zeng, Z.; Li, Z.; Xiao, Z.; Zhao, M. Collagen VI antibody reduces atherossclerosis by activating monocyte/macrophage polarization in ApoE−/− mice. Int. Immunopharmacol. 2022, 111, 109100. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Srivastava, S.K. CBP-mediated FOXO-1 acetylation inhibits pancreatic tumor growth by targeting SirT. Mol. Cancer Ther. 2014, 13, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, R.A.; Rijs, K.; Wezel, A.; Hamming, J.F.; Kolodgie, F.D.; Virmani, R.; Schaapherder, A.F.; Lindeman, J.H. Systematic Evaluation of the Cellular Innate Immune Response During the Process of Human Atherosclerosis. J. Am. Heart Assoc. 2016, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yuan, H.Q.; Hao, Y.M.; Ren, Z.; Qu, S.L.; Liu, L.S.; Wei, D.H.; Tang, Z.H.; Zhang, J.F.; Jiang, Z.S. Macrophage polarization in atherosclerosis. Clin. Chim. Acta; Int. J. Clin. Chem. 2020, 501, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H.M.; Moench, I.A.; Rickaway, Z.T.; Dougherty, C.J.; Webster, K.A.; Weissbach, H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem. Biophys. Res. Commun. 2008, 366, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Rahman, M.A.; Strassman, J.; Yancey, S.O.; Kushner, S.R.; Brot, N.; Weissbach, H. Escherichia coli peptide methionine sulfoxide reductase gene: Regulation of expression and role in protecting against oxidative damage. J. Bacteriol. 1995, 177, 502–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oien, D.; Moskovitz, J. Protein-carbonyl accumulation in the non-replicative senescence of the methionine sulfoxide reductase A (msrA) knockout yeast strain. Amino Acids 2007, 32, 603–606. [Google Scholar] [CrossRef]
- Minniti, A.N.; Cataldo, R.; Trigo, C.; Vasquez, L.; Mujica, P.; Leighton, F.; Inestrosa, N.C.; Aldunate, R. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging Cell 2009, 8, 690–705. [Google Scholar] [CrossRef]
- Wu, P.-F.; Xie, N.; Zhang, J.-J.; Guan, X.-L.; Zhou, J.; Long, L.-H.; Li, Y.-L.; Xiong, Q.-J.; Zeng, J.-H.; Wang, F.; et al. Resveratrol preconditioning increases methionine sulfoxide reductases A expression and enhances resistance of human neuroblastoma cells to neurotoxins. J. Nutr. Biochem. 2013, 24, 1070–1077. [Google Scholar] [CrossRef]
- Liu, Y.; Chong, L.; Li, X.; Tang, P.; Liu, P.; Hou, C.; Zhang, X.; Li, R. Astragaloside IV rescues MPP(+)-induced mitochondrial dysfunction through upregulation of methionine sulfoxide reductase A. Exp. Ther. Med. 2017, 14, 2650–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiribau, C.B.; Cheng, L.; Cucoranu, I.C.; Yu, Y.S.; Clempus, R.E.; Sorescu, D. FOXO3A regulates peroxiredoxin III expression in human cardiac fibroblasts. J. Biol. Chem. 2008, 283, 8211–8217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom. J. Morphol. Embryol. 2011, 52, 1173–1185. [Google Scholar]
- Zhang, M.; Zhang, Q.; Hu, Y.; Xu, L.; Jiang, Y.; Zhang, C.; Ding, L.; Jiang, R.; Sun, J.; Sun, H.; et al. miR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death Dis. 2017, 8, e3088. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Su, J.; Zhou, H.; Liu, X.; Nilsson, J.; Fredrikson, G.N.; Zhao, M. oxLDL antibody inhibits MCP-1 release in monocytes/macrophages by regulating Ca2+/K+ channel flow. J. Cell. Mol. Med. 2017, 21, 929–940. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C.; Zhang, J.; Wen, J.; Yan, S.; Li, C.; Zhang, F.; Zhang, J. Oxidized LDL Disrupts Metabolism and Inhibits Macrophage Survival by Activating a miR-9/Drp1/Mitochondrial Fission Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8848930. [Google Scholar] [CrossRef]
- Xu, H.; An, H.; Yu, Y.; Zhang, M.; Qi, R.; Cao, X. Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J. Biol. Chem. 2003, 278, 36334–36340. [Google Scholar] [CrossRef]

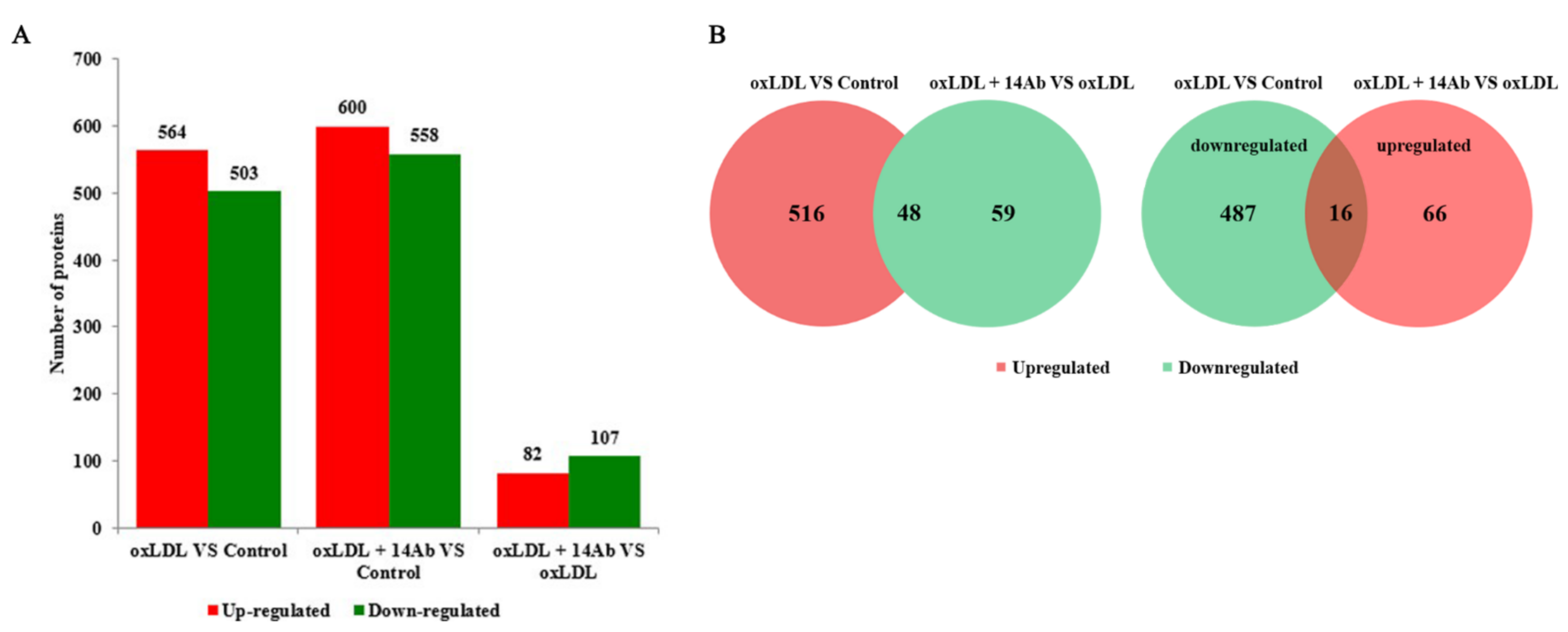

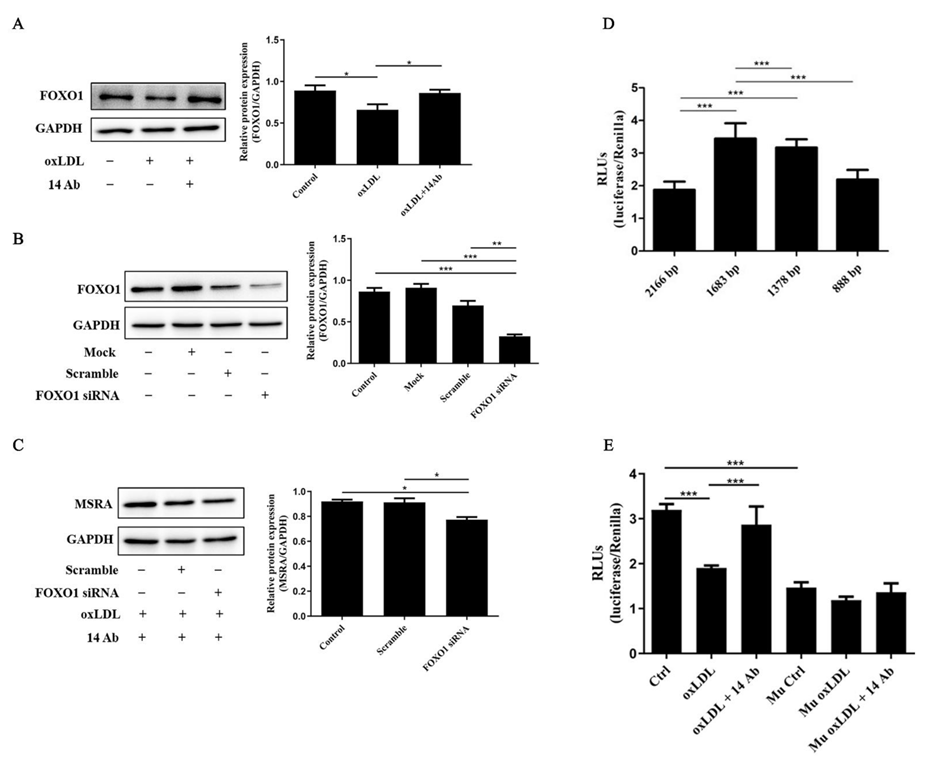

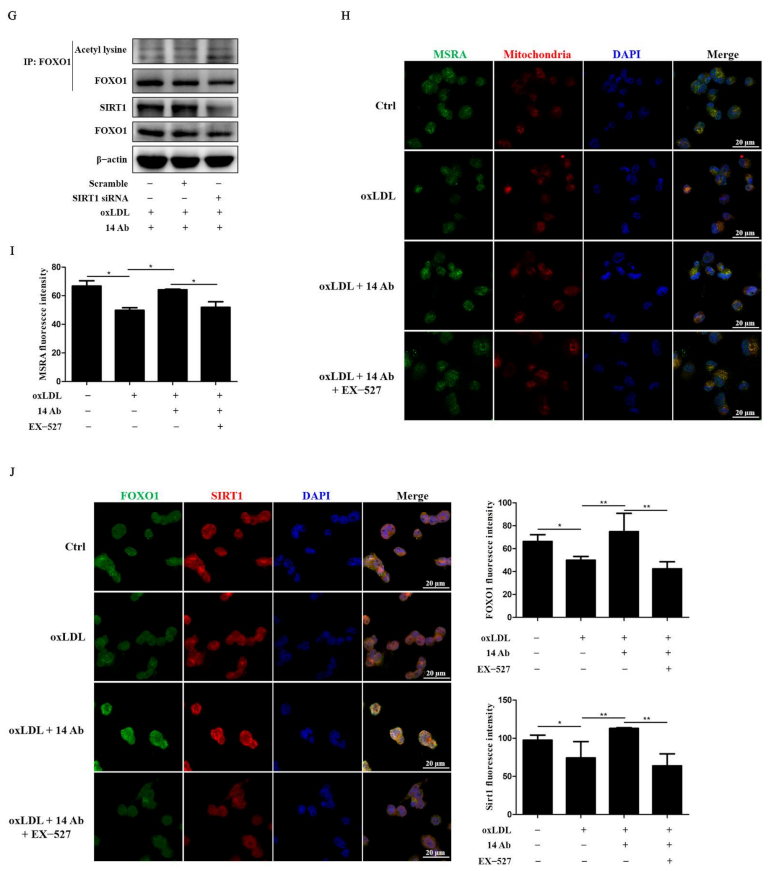
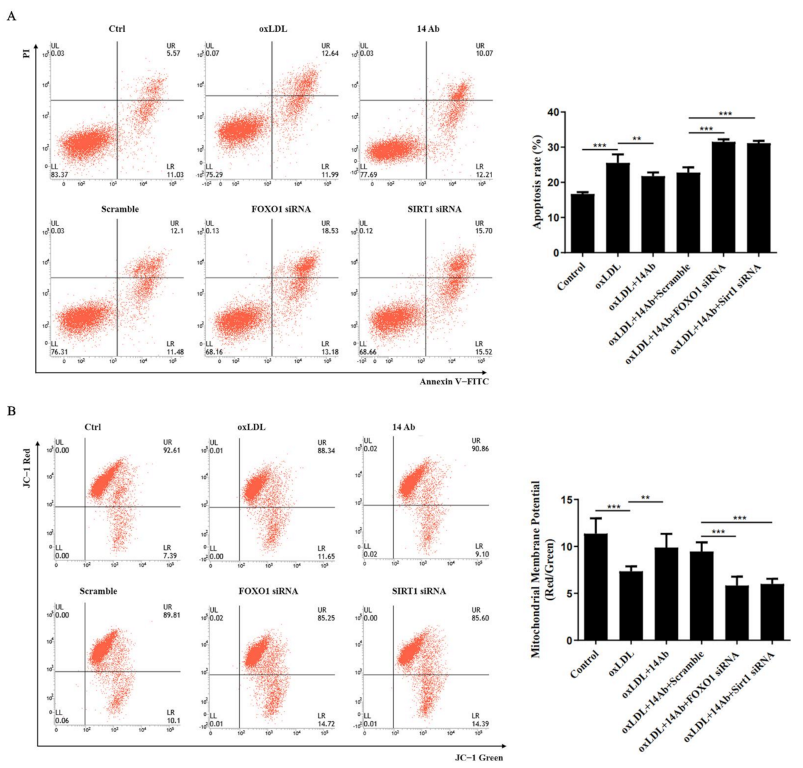
| Protein Accession | Protein Description | oxLDL vs. Ctrl | oxLDL + 14 Ab vs. oxLDL | oxLDL + 14 Ab vs. Ctrl |
|---|---|---|---|---|
| P14222 | Perforin-1 | 0.819 | 1.209 | 0.991 |
| Q92890 | Ubiquitin recognition factor in ER-associated degradation protein 1 | 0.77 | 1.284 | 0.988 |
| Q9Y385 | Ubiquitin-conjugating enzyme E2 J1 | 0.785 | 1.331 | 1.044 |
| Q8NCW5 | NAD(P)H-hydrate epimerase | 0.653 | 1.442 | 0.941 |
| P01034 | Cystatin-C | 0.601 | 1.255 | 0.754 |
| P17050 | Alpha-N-acetylgalactosaminidase | 0.811 | 1.237 | 1.003 |
| P22694 | cAMP-dependent protein kinase catalytic subunit beta | 0.548 | 1.28 | 0.701 |
| Q9GIY3 | HLA class II histocompatibility antigen, DRB1-14 beta chain | 0.674 | 1.281 | 0.864 |
| Q13409 | Cytoplasmic dynein 1 intermediate chain 2 | 0.818 | 1.307 | 1.07 |
| Q9Y2E5 | Epididymis-specific alpha-mannosidase | 0.768 | 1.231 | 0.945 |
| O75822 | Eukaryotic translation initiation factor 3 subunit J | 0.715 | 1.202 | 0.859 |
| P28161 | Glutathione S-transferase Mu 2 | 0.66 | 1.215 | 0.803 |
| Q9GZP4 | PITH domain-containing protein 1 | 0.774 | 1.231 | 0.953 |
| Q9BUE0 | Mediator of RNA polymerase II transcription subunit 18 | 0.649 | 1.307 | 0.848 |
| Q9UJ68 | Mitochondrial peptide methionine sulfoxide reductase | 0.625 | 1.274 | 0.796 |
| Q15661 | Tryptase alpha/beta-1 | 0.826 | 1.678 | 1.386 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, Z.; Liu, X.; Zhao, M. Recombinant Humanized IgG1 Antibody Protects against oxLDL-Induced Oxidative Stress and Apoptosis in Human Monocyte/Macrophage THP-1 Cells by Upregulation of MSRA via Sirt1-FOXO1 Axis. Int. J. Mol. Sci. 2022, 23, 11718. https://doi.org/10.3390/ijms231911718
Zhang Q, Li Z, Liu X, Zhao M. Recombinant Humanized IgG1 Antibody Protects against oxLDL-Induced Oxidative Stress and Apoptosis in Human Monocyte/Macrophage THP-1 Cells by Upregulation of MSRA via Sirt1-FOXO1 Axis. International Journal of Molecular Sciences. 2022; 23(19):11718. https://doi.org/10.3390/ijms231911718
Chicago/Turabian StyleZhang, Qi, Zhonghao Li, Xianyan Liu, and Ming Zhao. 2022. "Recombinant Humanized IgG1 Antibody Protects against oxLDL-Induced Oxidative Stress and Apoptosis in Human Monocyte/Macrophage THP-1 Cells by Upregulation of MSRA via Sirt1-FOXO1 Axis" International Journal of Molecular Sciences 23, no. 19: 11718. https://doi.org/10.3390/ijms231911718




