Synergistic Antiviral Effects of Metal Oxides and Carbon Nanotubes
Abstract
1. Introduction
2. Results and Discussion
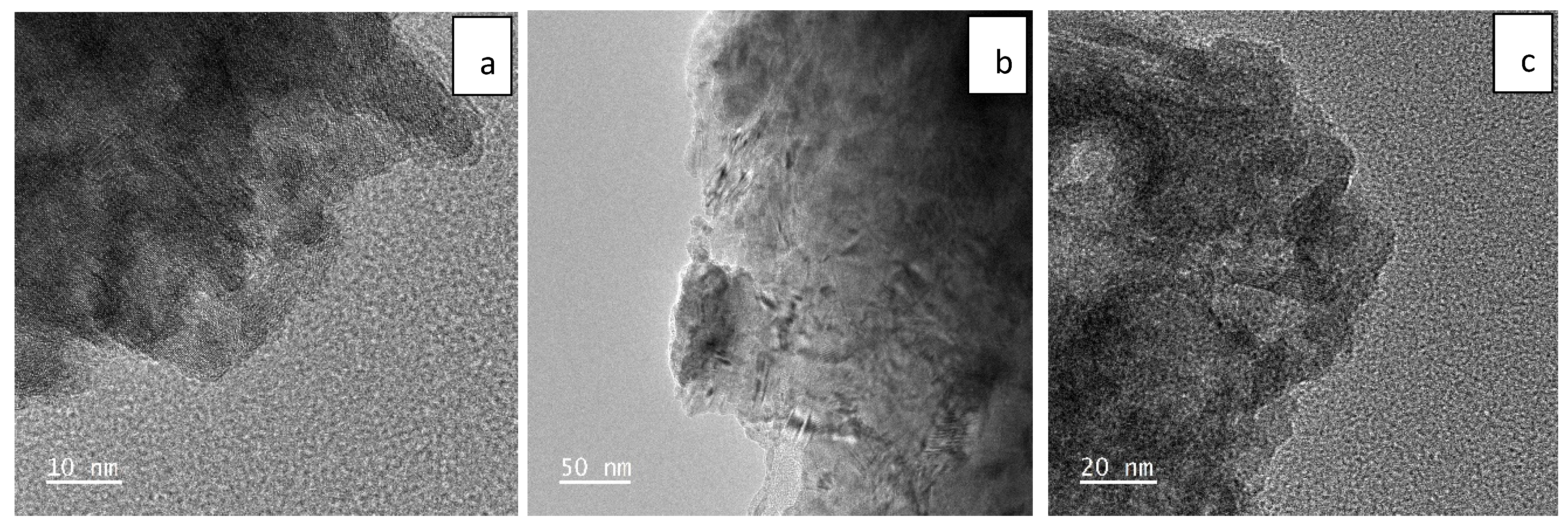
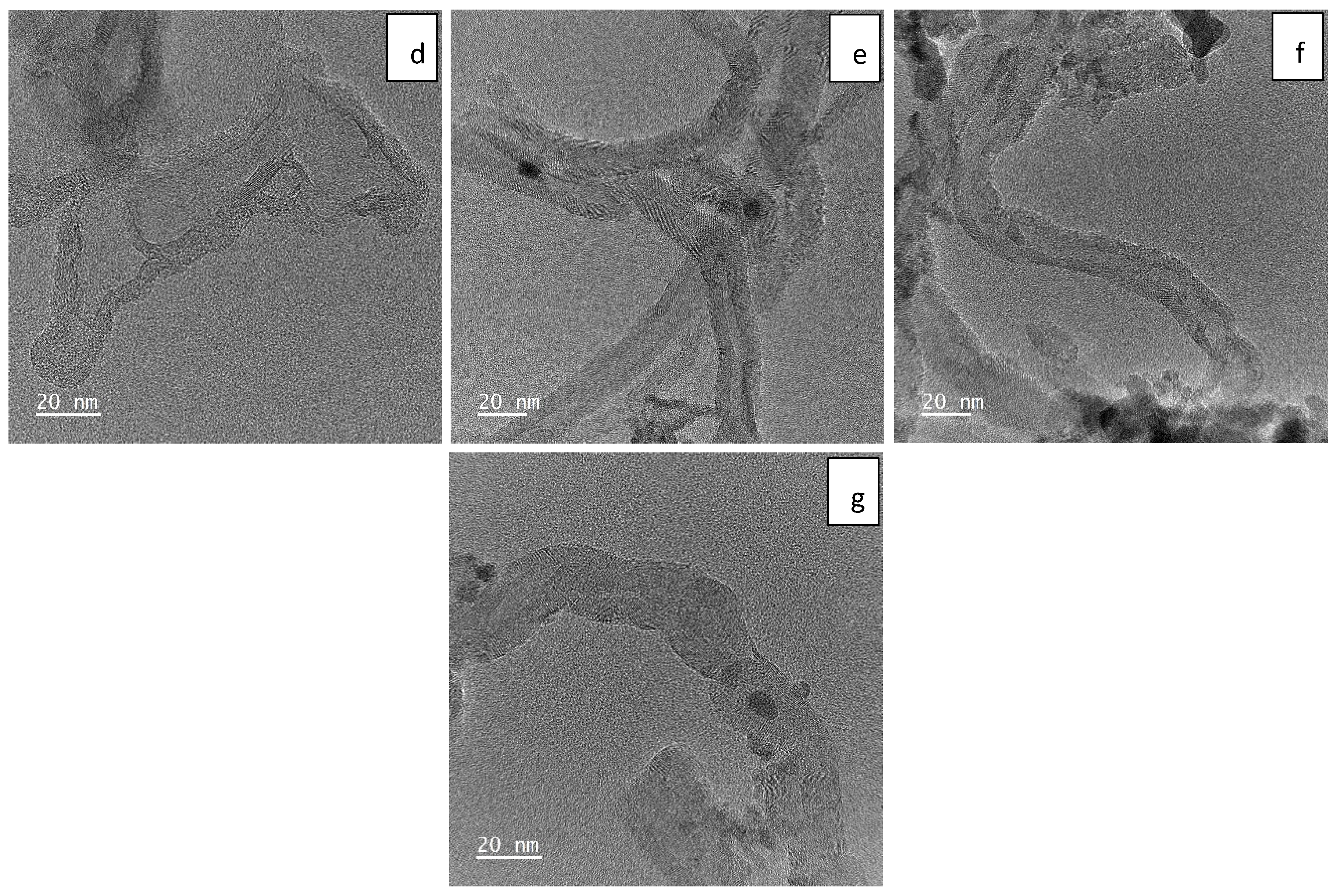
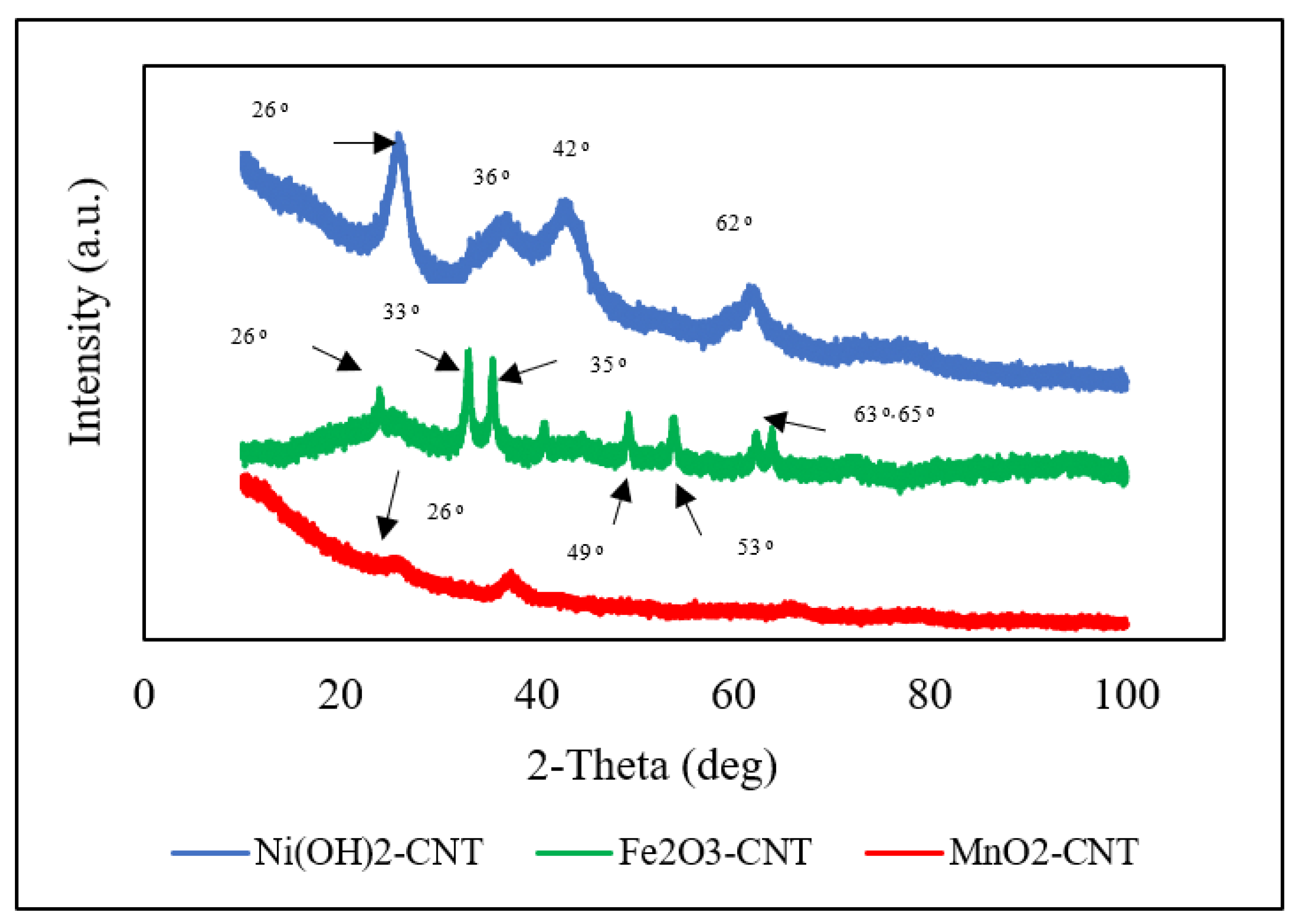
2.1. Sample Characterization
2.2. Antiviral Effects of MOs and MO-CNT Hybrids
2.3. Mechanism of Deactivation
3. Experimental Section
3.1. Chemicals and Materials
3.2. MS2 Bacteriophage Sample Preparation
3.3. Preparation of MOs and MO-CNTs
3.4. Phage Deactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashbolt, N.J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Patil, S.; Mullani, S.; Delekar, S. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Chamakura, K.; Perez-Ballestero, R.; Luo, Z.; Bashir, S.; Liu, J. Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloids Surf. B Biointerfaces 2011, 84, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; Del Real, G.; Aranda, P. Nanotechnology responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef]
- Turner, R.J. Metal-based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New antimicrobial strategies based on metal complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Metal-based nanoparticles for the treatment of infectious diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Parham, S.; Wicaksono, D.H.; Bagherbaigi, S.; Lee, S.L.; Nur, H. Antimicrobial treatment of different metal oxide nanoparticles: A critical review. J. Chin. Chem. Soc. 2016, 63, 385–393. [Google Scholar] [CrossRef]
- Jeżowska-Bojczuk, M.; Stokowa-Sołtys, K. Peptides having antimicrobial activity and their complexes with transition metal ions. Eur. J. Med. Chem. 2018, 143, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Martin-Benlloch, X.; Novodomska, A.; Jacquemin, D.; Davioud-Charvet, E.; Elhabiri, M. Iron (III) coordination properties of ladanein, a flavone lead with a broad-spectrum antiviral activity. New J. Chem. 2018, 42, 8074–8087. [Google Scholar] [CrossRef]
- Sikorska, K.; Romanowski, T.; Stalke, P.; Swieszewska, E.I.; Bielawski, K.P. Association of hepcidin mRNA expression with hepatocyte iron accumulation and effects of antiviral therapy in chronic hepatitis C infection. Hepat. Mon. 2014, 14, e21184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.; Topno, R.; Madhukar, M.; Das, P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- GÜVELİ, Ş.; Turan, K.; ÜLKÜSEVEN, B. Nickel (II)-PPh $ _ {3} $ complexes with ONS and ONN chelating thiosemicarbazones: Synthesis and inhibition potential on influenza A viruses. Turk. J. Chem. 2018, 42, 371–384. [Google Scholar] [CrossRef]
- Liu, J.; Mei, W.-J.; Xu, A.-W.; Tan, C.-P.; Shi, S.; Ji, L.-N. Synthesis, characterization and antiviral activity against influenza virus of a series of novel manganese-substituted rare earth borotungstates heteropolyoxometalates. Antivir. Res. 2004, 62, 65–71. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19. [Google Scholar]
- Jiang, Y.-W.; Gao, G.; Zhang, X.; Jia, H.-R.; Wu, F.-G. Antimicrobial carbon nanospheres. Nanoscale 2017, 9, 15786–15795. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Nanocarbon-immobilized membranes for separation of tetrahydrofuran from water via membrane distillation. ACS Appl. Nano Mater. 2020, 3, 6344–6353. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Low temperature recovery of acetone–butanol–ethanol (ABE) fermentation products via microwave induced membrane distillation on carbon nanotube immobilized membranes. Sustain. Energy Fuels 2020, 4, 3487–3499. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Microwave induced membrane distillation for enhanced ethanol–water separation on a carbon nanotube immobilized membrane. Ind. Eng. Chem. Res. 2019, 58, 18313–18319. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Enhanced membrane distillation of organic solvents from their aqueous mixtures using a carbon nanotube immobilized membrane. J. Membr. Sci. 2018, 568, 134–140. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Enrichment of 1, 4-dioxane from water by sweep gas membrane distillation on nano-carbon immobilized membranes. Sep. Purif. Technol. 2021, 276, 119360. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Synergistic effect of air sparging in direct contact membrane distillation to control membrane fouling and enhancing flux. Sep. Purif. Technol. 2021, 272, 118681. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Reduction and Elimination of Humic Acid Fouling in Air Sparged Membrane Distillation Using Nanocarbon Immobilized Membrane. Molecules 2022, 27, 2896. [Google Scholar] [CrossRef]
- Paul, S.; Bhoumick, M.C.; Roy, S.; Mitra, S. Carbon nanotube enhanced membrane filtration for trace level dewatering of hydrocarbons. Sep. Purif. Technol. 2022, 292, 121047. [Google Scholar] [CrossRef]
- Paul, S.; Bhoumick, M.C.; Roy, S.; Mitra, S. Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene. Membranes 2022, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An Overview of Antimicrobial Properties of Carbon Nanotubes-Based Nanocomposites. Adv. Pharm. Bull. 2021, 12, 449. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhao, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. Iscience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B. Antibacterial carbon-based nanomaterials. Adv. Mater. 2019, 31, 1804838. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Nanocarbon immobilized membranes for generating bacteria and endotoxin free water via membrane distillation. Sep. Purif. Technol. 2021, 259, 118133. [Google Scholar] [CrossRef]
- Gupta, I.; Chakraborty, J.; Roy, S.; Farinas, E.T.; Mitra, S. Synergistic Effects of Microwave Radiation and Nanocarbon Immobilized Membranes in the Generation of Bacteria-Free Water via Membrane Distillation. Ind. Eng. Chem. Res. 2021, 61, 1453–1463. [Google Scholar] [CrossRef]
- Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Antiviral properties of select carbon nanostructures and their functionalized analogs. Mater. Today Commun. 2021, 29, 102743. [Google Scholar] [CrossRef]
- Budipramana, Y.; Ersam, T.; Kurniawan, F. Synthesis nickel hydroxide by electrolysis at high voltage. ARPN J. Eng. Appl. Sci 2014, 9, 2074–2077. [Google Scholar]
- Amuanyena, M.O.; Kandawa-Schulz, M.; Kwaambwa, H.M. Magnetic iron oxide nanoparticles modified with Moringa seed proteins for recovery of precious metal ions. J. Biomater. Nanobiotechnol. 2019, 10, 142. [Google Scholar] [CrossRef]
- Raul, P.K.; Devi, R.R.; Umlong, I.M.; Banerjee, S.; Singh, L.; Purkait, M. Removal of fluoride from water using iron oxide-hydroxide nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.R.T.; Silva, M.F.; de Andrade, M.B.; Vieira, M.F.; Bergamasco, R. Magnetic coagulant based on Moringa oleifera seeds extract and super paramagnetic nanoparticles: Optimization of operational conditions and reuse evaluation. Desalin Water Treat 2018, 106, 226–237. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Ma, H.; Guo, S.; Cao, F.; Gong, J.; Deng, Y. Manganese oxide nanocomposite fabricated by a simple solid-state reaction and its ultraviolet photoresponse property. Chem. Commun. 2011, 47, 2619–2621. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Z.; Nejad, R.-A.K.; Mahmoodzadeh, H.; Satari, T.N. Investigating of a wide range of concentrations of multi-walled carbon nanotubes on germination and growth of castor seeds (Ricinus communis L.). J. Plant Prot. Res. 2017, 57, 228–236. [Google Scholar] [CrossRef]
- Bakather, O.Y.; Kayvani Fard, A.; Khraisheh, M.; Nasser, M.S.; Atieh, M.A. Enhanced adsorption of selenium ions from aqueous solution using iron oxide impregnated carbon nanotubes. Bioinorg. Chem. Appl. 2017, 2017, 4323619. [Google Scholar] [CrossRef]
- Yi, H.; Wang, H.; Jing, Y.; Peng, T.; Wang, Y.; Guo, J.; He, Q.; Guo, Z.; Wang, X. Advanced asymmetric supercapacitors based on CNT@ Ni (OH) 2 core–shell composites and 3D graphene networks. J. Mater. Chem. A 2015, 3, 19545–19555. [Google Scholar] [CrossRef]
- Ma, S.-B.; Ahn, K.-Y.; Lee, E.-S.; Oh, K.-H.; Kim, K.-B. Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45, 375–382. [Google Scholar] [CrossRef]
- Chen, W.; Pan, X.; Willinger, M.-G.; Su, D.S.; Bao, X. Facile autoreduction of iron oxide/carbon nanotube encapsulates. J. Am. Chem. Soc. 2006, 128, 3136–3137. [Google Scholar] [CrossRef]
- Huiqun, C.; Meifang, Z.; Yaogang, L. Decoration of carbon nanotubes with iron oxide. J. Solid State Chem. 2006, 179, 1208–1213. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Enhanced antimicrobial activity of silver nanoparticles with controlled particle size by pH variation. Powder Technol. 2015, 269, 110–117. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Pecson, B.M.; Sigstam, T.; Bosshard, F.; Kohn, T. Virus inactivation mechanisms: Impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012, 46, 12069–12078. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Soliman, M.Y.; Medema, G.; Bonilla, B.E.; Brouns, S.J.; van Halem, D. Inactivation of RNA and DNA viruses in water by copper and silver ions and their synergistic effect. Water Res. X 2020, 9, 100077. [Google Scholar] [CrossRef] [PubMed]
- Hewett, K.B.; Anderson, L.C.; Rosynek, M.P.; Lunsford, J.H. Formation of hydroxyl radicals from the reaction of water and oxygen over basic metal oxides. J. Am. Chem. Soc. 1996, 118, 6992–6997. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Hu, J.; Ong, S.; Song, L.; Feng, Y.; Liu, W.; Tan, T.; Lee, L.; Ng, W. Removal of MS2 bacteriophage using membrane technologies. Water Sci. Technol. 2003, 47, 163–168. [Google Scholar] [CrossRef]
- Boudaud, N.; Machinal, C.; David, F.; Fréval-Le Bourdonnec, A.; Jossent, J.M.; Bakanga, F.; Arnal, C.; Jaffrezic, M.P.; Oberti, S.; Gantzer, C. Removal of MS2, Qβ and GA bacteriophages during drinking water treatment at pilot scale. Water Res. 2012, 46, 2651–2664. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, X.; Chen, K.; Mitra, S. Synthesis of carbon nanotube incorporated metal oxides for the fabrication of printable, flexible nickel-zinc batteries. Adv. Mater. Interfaces 2018, 5, 1701036. [Google Scholar] [CrossRef]
- Wang, Y.; Iqbal, Z.; Mitra, S. Microwave-induced rapid chemical functionalization of single-walled carbon nanotubes. Carbon 2005, 43, 1015–1020. [Google Scholar] [CrossRef]




| Sample | Elemental Composition | ||||
|---|---|---|---|---|---|
| C (%) | O (%) | Ni (%) | Fe (%) | Mn (%) | |
| Ni(OH)2 | N/A | 42.4 | 57.6 | N/A | N/A |
| Fe2O3 | N/A | 11.3 | N/A | 88.7 | N/A |
| MnO2 | N/A | 34.9 | N/A | N/A | 65.1 |
| Ni(OH)2-CNT | 64 | 20 | 15 | N/A | N/A |
| Fe2O3-CNT | 55 | 36 | N/A | 8 | N/A |
| MnO2-CNT | 54 | 36 | N/A | N/A | 9 |
| Nanomaterials | T50 (min) | T80 (min) | LD50 (µg/mL) | Specific Growth Rate (SGR) (hr−1) | Initial Deactivation Rate (IRD) (hr−1) |
|---|---|---|---|---|---|
| Ni(OH)2 | 50.6 | 83.4 | 278 | −0.013 | −3.52 |
| Fe2O3 | 53.8 | 86.8 | 348 | −0.012 | −3.48 |
| MnO2 | 56.9 | 90.5 | 357 | −0.011 | −3.42 |
| CNT | 31.8 | 54.6 | 63 | −0.025 | −5.42 |
| Ni(OH)2-CNT | 22.7 | 44.2 | 16 | −0.038 | −5.46 |
| Fe2O3-CNT | 24.3 | 46.0 | 24 | −0.035 | −5.44 |
| MnO2-CNT | 25.1 | 46.3 | 33 | −0.032 | −5.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, I.; Azizighannad, S.; Farinas, E.T.; Mitra, S. Synergistic Antiviral Effects of Metal Oxides and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 11957. https://doi.org/10.3390/ijms231911957
Gupta I, Azizighannad S, Farinas ET, Mitra S. Synergistic Antiviral Effects of Metal Oxides and Carbon Nanotubes. International Journal of Molecular Sciences. 2022; 23(19):11957. https://doi.org/10.3390/ijms231911957
Chicago/Turabian StyleGupta, Indrani, Samar Azizighannad, Edgardo T. Farinas, and Somenath Mitra. 2022. "Synergistic Antiviral Effects of Metal Oxides and Carbon Nanotubes" International Journal of Molecular Sciences 23, no. 19: 11957. https://doi.org/10.3390/ijms231911957
APA StyleGupta, I., Azizighannad, S., Farinas, E. T., & Mitra, S. (2022). Synergistic Antiviral Effects of Metal Oxides and Carbon Nanotubes. International Journal of Molecular Sciences, 23(19), 11957. https://doi.org/10.3390/ijms231911957







