Solvent- and Light-Sensitive AIEE-Active Azo Dye: From Spherical to 1D and 2D Assemblies
Abstract
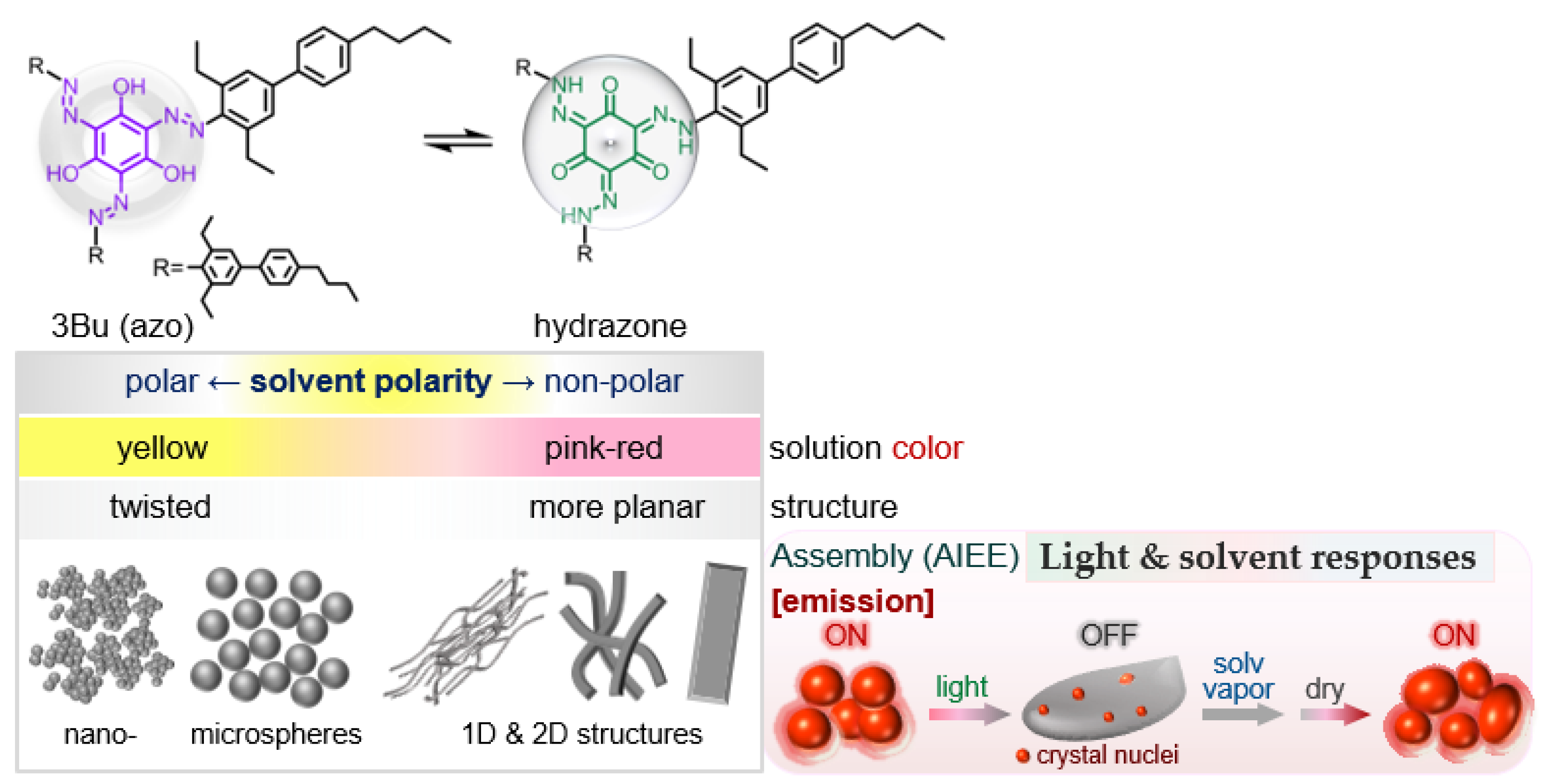
:1. Introduction
2. Results and Discussion
2.1. Solvent Effect
2.2. Theoretical Calculations
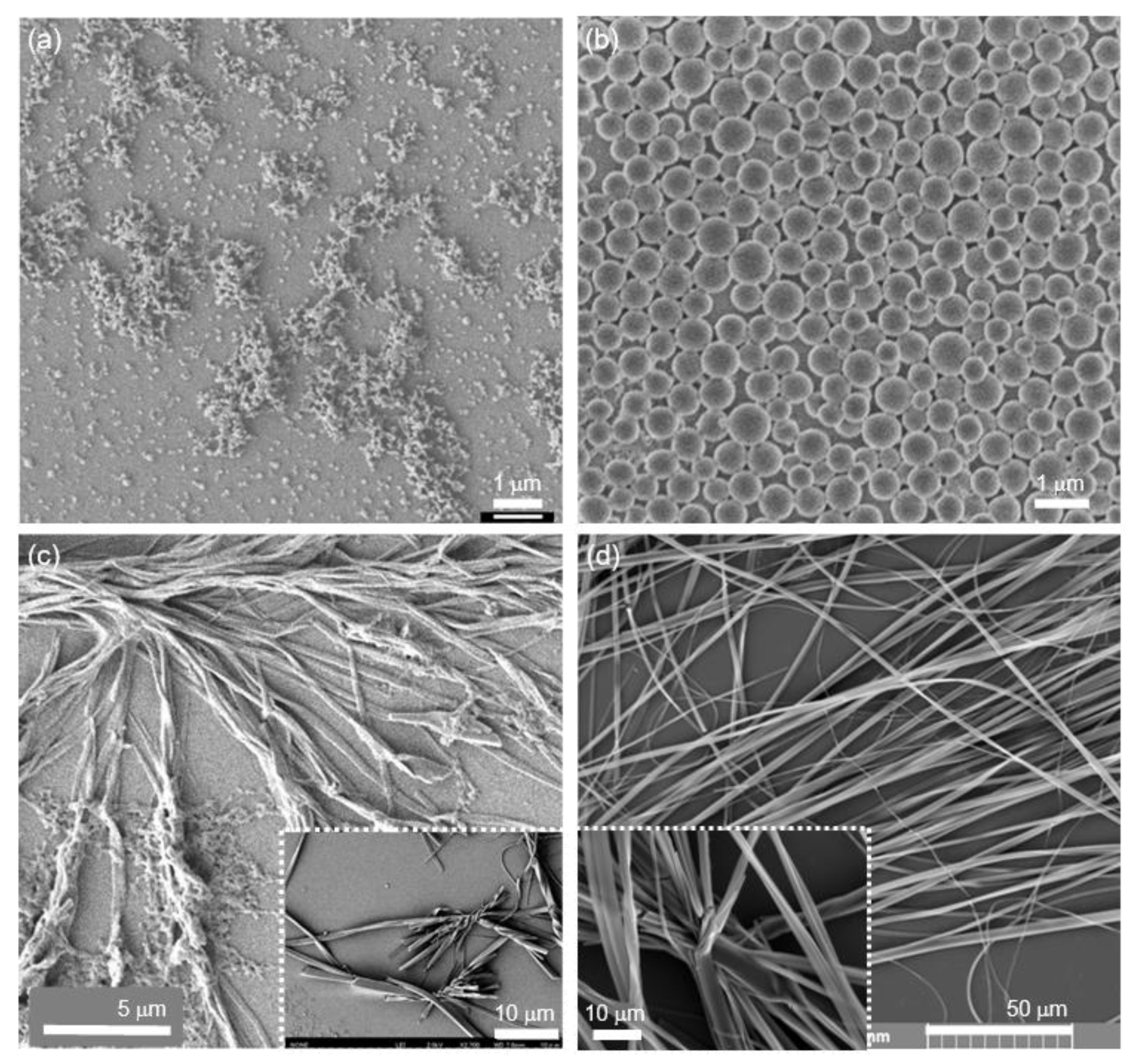
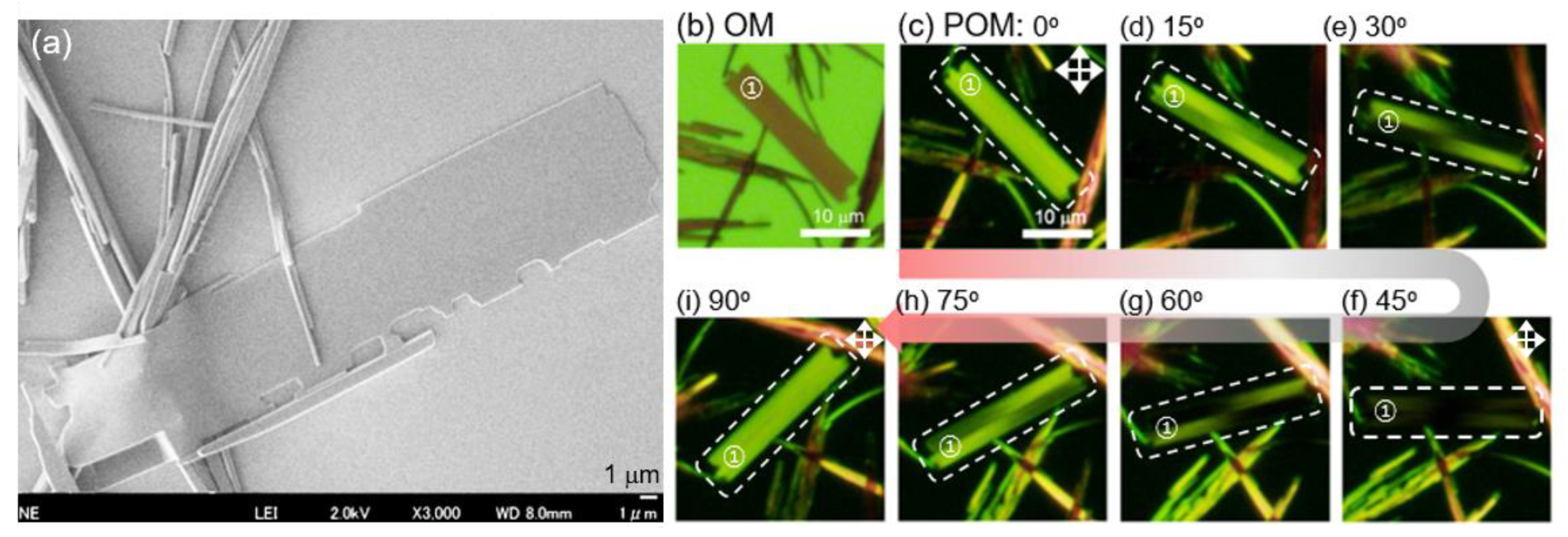
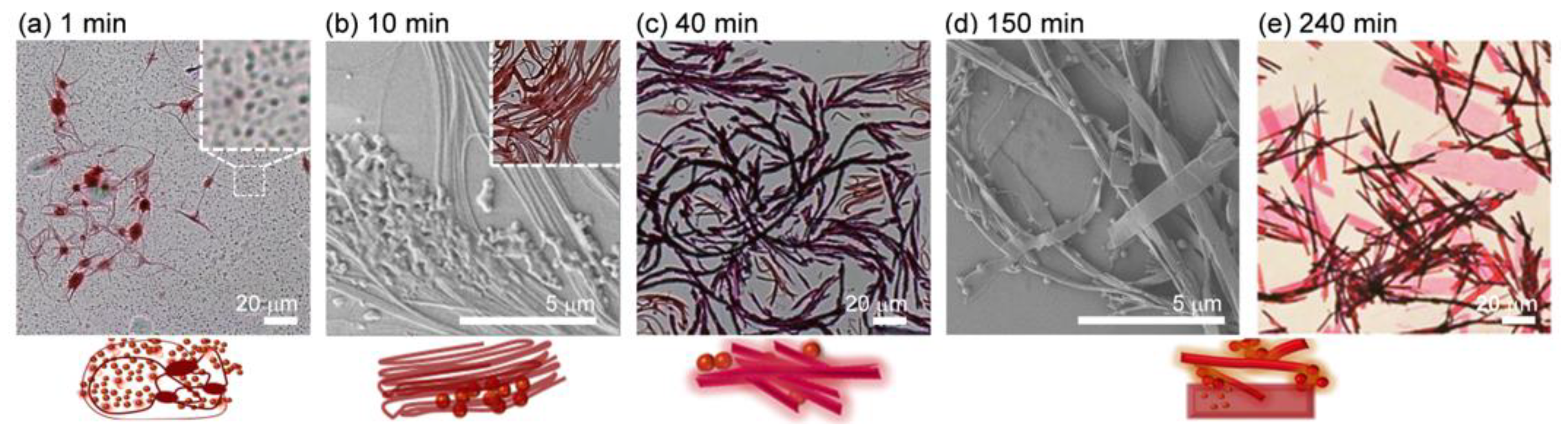
2.3. Self-Assembly into Spherical and 1D Structures
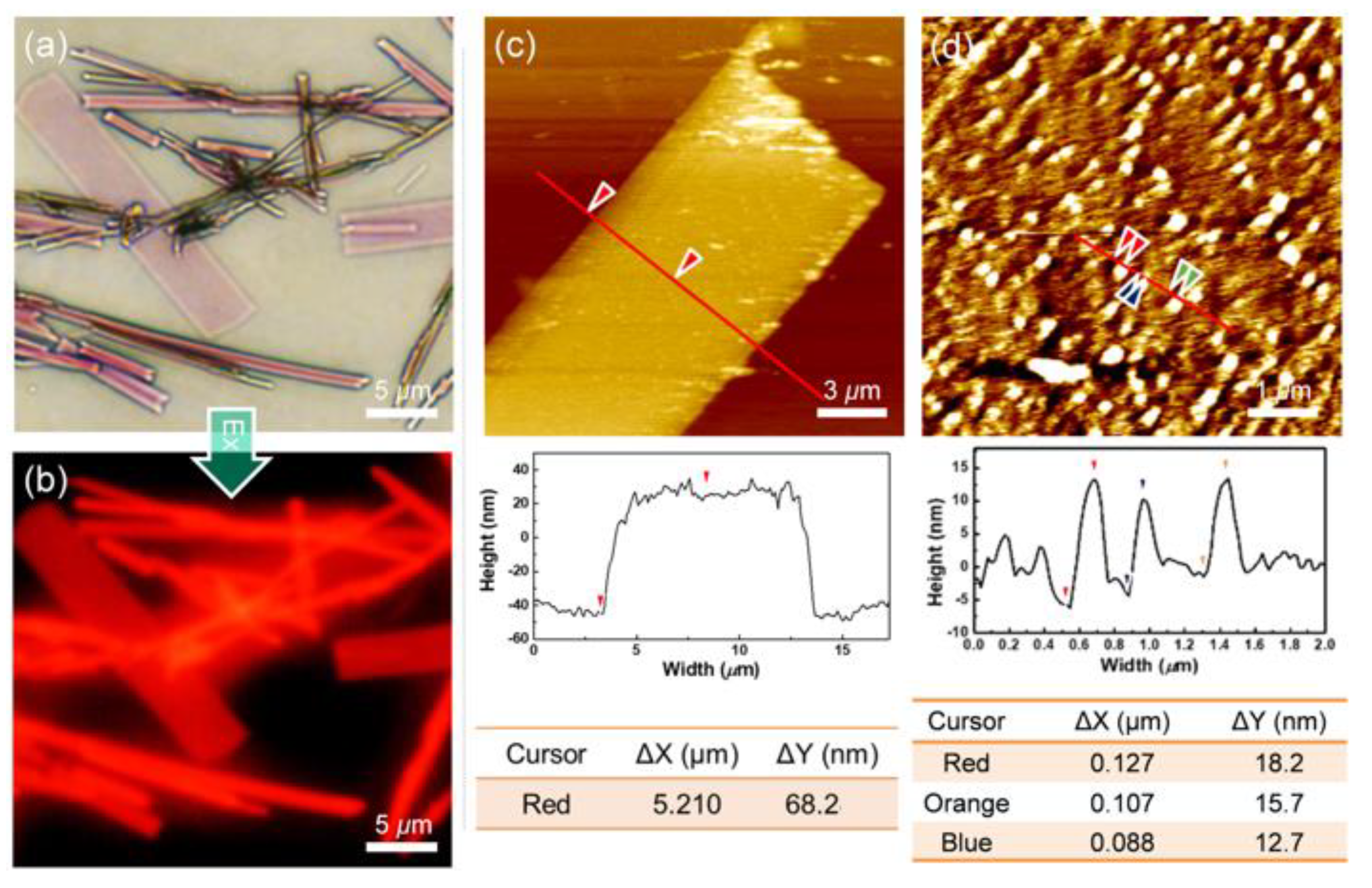
2.4. Coexistence of Spherical, 1D and 2D Structures
2.5. FT-IR and XRD Measurements
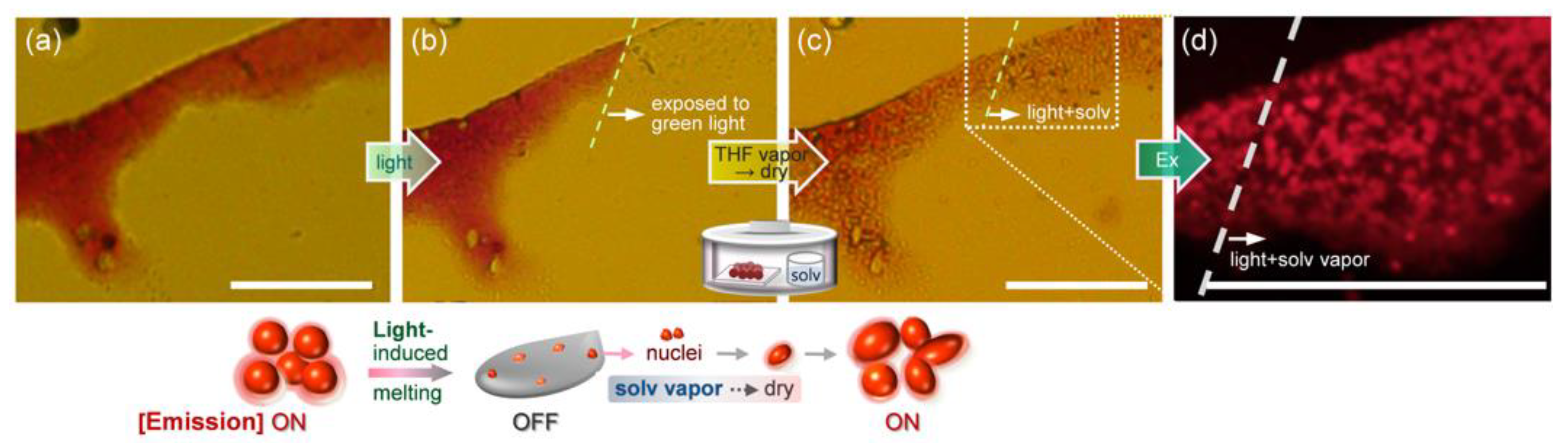
2.6. Fluorescence Switching Accompanied by Morphological Transition
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, L.; Stupp, S.I. Molecular Self-Assembly into One-Dimensional Nanostructures. Acc. Chem. Res. 2008, 41, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Liao, Q.; Yao, J. Construction and Optoelectronic Properties of Organic One-Dimensional Nanostructures. Acc. Chem. Res. 2010, 43, 409–418. [Google Scholar] [CrossRef]
- de Rooy, S.L.; El-Zahab, B.; Li, M.; Das, S.; Broering, E.; Chandler, L.; Warner, I.M. Fluorescent one-dimensional nanostructures from a group of uniform materials based on organic salts. Chem. Commun. 2011, 47, 8916–8918. [Google Scholar] [CrossRef]
- Cantekin, S.; Greef, T.F.A.; Palmans, A.R.A. Benzene-1,3,5-Tricarboxamide: A Versatile Ordering Moiety for Supramo-lecular Chemistry. Chem. Soc. Rev. 2012, 41, 6125–6137. [Google Scholar] [CrossRef]
- Kim, Y.; Li, W.; Shin, S.; Lee, M. Development of Toroidal Nanostructures by Self-Assembly: Rational Designs and Applications. Acc. Chem. Res. 2013, 46, 2888–2897. [Google Scholar] [CrossRef]
- Wei, R.; Song, P.; Tong, A. Reversible Thermochromism of Aggregation-Induced Emission-Active Benzophenone Azine Based on Polymorph-Dependent Excited-State Intramolecular Proton Transfer Fluorescence. J. Phys. Chem. C 2013, 117, 3467–3474. [Google Scholar] [CrossRef]
- Kim, H.-J.; Whang, D.R.; Gierschner, J.; Lee, C.H.; Park, S.Y. High-Contrast Red-Green-Blue Tricolor Fluorescence Switching in Bicomponent Molecular Film. Angew. Chem. Int. Ed. 2015, 54, 4330–4333. [Google Scholar] [CrossRef]
- Ito, S.; Yamada, T.; Taguchi, T.; Yamaguchi, Y.; Asami, M. N-Boc-Indolylbenzothiadiazole Derivatives: Efficient Full-Color Solid-State Fluorescence and Self-Recovering Mechanochromic Luminescence. Chem. Asian J. 2016, 11, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, C.; Lu, S.; Du, J.; Gao, Q.; Zhang, R.; Liu, Y.; Yang, C. Smart On-Off Switching Luminescence Materials with Reversible Piezochromism and Basichromism. ChemmistrySelect 2017, 2, 9215–9221. [Google Scholar] [CrossRef]
- Rosbottom, I.; Ma, C.Y.; Turner, T.D.; O’Connell, R.A.; Loughrey, J.; Sadiq, G.; Davey, R.J.; Roberts, K.J. Influence of Solvent Composition on the Crystal Morphology and Structure of p Aminobenzoic Acid Crystallized from Mixed Ethanol and Ni-tromethane Solutions. Cryst. Growth Des. 2017, 17, 4151–4161. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Abe, I.; Matsuura, K.; Takeoka, Y.; Seki, T. Morphologically Diverse Micro- and Macrostructures Created via Solvent Evaporation-Induced Assembly of Fluorescent Spherical Particles in the Presence of Polyethylene Glycol Derivatives. Molecules 2021, 26, 4294. [Google Scholar] [CrossRef] [PubMed]
- Nador, F.; Wnuk, K.; Roscini, C.; Solorzano, R.; Faraudo, J.; Ruiz-Molina, D.; Novio, F. Solvent-Tuned Supramolecular Assembly of Fluorescent Catechol/Pyrene Amphiphilic Molecules. Chem. Eur. J. 2018, 24, 14724–14732. [Google Scholar] [CrossRef]
- Zheng, D.; Gu, Y.; Li, X.; Zhang, L.; Zhao, W.; Ma, J. Hydrogen Bonding Promoted Tautomerism between Azo and Hydrazone Forms in Calcon with Multistimuli Responsiveness and Biocompatibility. J. Chem. Inf. Model. 2019, 59, 2110–2122. [Google Scholar] [CrossRef]
- Peng, H.; Liu, B.; Liu, J.; Wei, P.; Zhang, H.; Han, T.; Qi, J.; Lam, J.W.Y.; Zhang, W.; Tang, B.Z. “Seeing” and Controlling Photoisomerization by (Z) /(E) Isomers with Aggregation-Induced Emission Characteristics. ACS Nano 2019, 13, 12120–12126. [Google Scholar] [CrossRef]
- Castillo-Vallés, M.; Martínez-Bueno, A.; Giménez, R.; Sierra, T.; Ros, M.B. Beyond liquid crystals: New research trends for mesogenic molecules in liquids. J. Mater. Chem. C 2019, 7, 14454–14470. [Google Scholar] [CrossRef]
- Shen, B.; Kim, Y.; Lee, M. Supramolecular Chiral 2D Materials and Emerging Functions. Adv. Mater. 2020, 32, e1905669. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, S.; Liu, W.; Yang, Q.; Sun, H.; Ding, R.; Qian, Z.; Feng, H. Rational design of reversibly photochromic molecules with aggregation-induced emission by introducing photoactive thienyl and benzothienyl groups. J. Mater. Chem. C 2020, 8, 13197–13204. [Google Scholar] [CrossRef]
- Yang, M.; Park, I.S.; Miyashita, Y.; Tanaka, K.; Yasuda, T. Mechanochromic Delayed Fluorescence Switching in Propeller-Shaped Carbazole–Isophthalonitrile Luminogens with Stimuli-Responsive Intramolecular Charge-Transfer Excited States. Angew. Chem. Int. Ed. 2020, 59, 13955–13961. [Google Scholar] [CrossRef]
- Kumar, R.; Aggarwal, H.; Srivastava, A. Of Twists and Curves: Electronics, Photophysics, and Upcoming Applications of Non-Planar Conjugated Organic Molecules. Chem. Eur. J. 2020, 26, 10653–10675. [Google Scholar] [CrossRef]
- Barman, D.; Gogoi, R.; Narang, K.; Iyer, P.K. Recent Developments on Multi-Functional Metal-Free Mechanochromic Lumi-nescence and Thermally Activated Delayed Fluorescence Organic Materials. Front. Chem. 2020, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Nagai, S.; Ito, S.; Tachikawa, T. In Situ Exploration of Stimulus-Induced Emission Changes in Mechanochromic Dyes. J. Phys. Chem. Lett. 2021, 12, 7826–7831. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, F.; Iliş, M.; Madalan, A.; Manaila-Maximean, D.; Secu, M.; Cîrcu, V. Thermal and Emission Properties of a Series of Lanthanides Complexes with N-Biphenyl-Alkylated-4-Pyridone Ligands: Crystal Structure of a Terbium Complex with N-Benzyl-4-Pyridone. Molecules 2021, 26, 2017. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, L.F.; Ganea, P.C.; Manaila-Maximean, D.; Pasuk, I.; Circu, V. Synthesis and thermal, emission and dielectric properties of liquid crystalline Eu(III), Sm(III) and Tb(III) complexes based on mesogenic 4-pyridone ligands functionalized with cyano-biphenyl groups. J. Mol. Liq. 2019, 290, 111184. [Google Scholar] [CrossRef]
- Ilinca, T.A.; Manaila-Maximean, D.; Ganea, P.C.; Pasuk, I.; Circu, V. Polarized emission and dielectric studies of novel lanthanidomesogens based on 4-pyridone ligands. In Proceedings of the Advanced Topics in Optoelectronics, Microelectronics and Nanotechnologies X, Constanta, Romania, 20–23 August 2020; Volume 11718, p. 117182U. [Google Scholar]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Nigam, S.; Rutan, S. Principles and Applications of Solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, L.; Liu, M. Solvent-Polarity-Tuned Morphology and Inversion of Supramolecular Chirality in a Self-Assembled Pyridylpyrazole-Linked Glutamide Derivative: Nanofibers, Nanotwists, Nanotubes, and Microtubes. Chem. A Eur. J. 2013, 19, 9234–9241. [Google Scholar] [CrossRef]
- Niu, C.; You, Y.; Zhao, L.; He, D.; Na, N.; Ouyang, J. Solvatochromism, Reversible Chromism and Self-Assembly Effects of Heteroatom-Assisted Aggregation-Induced Enhanced Emission (AIEE) Compounds. Chem. Eur. J. 2015, 21, 13983–13990. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, J.; Simalou, O.; Wang, H.; Peng, J.; Zhai, L.; Xue, P.; Lu, R. Multi-stimuli-responsive fluorescent ami-nostyrylquinoxalines: Synthesis, solvatochromism, mechanofluorochromism and acidochromism. Dyes Pigm. 2018, 151, 296–302. [Google Scholar] [CrossRef]
- Balam-Villarreal, J.A.; López-Mayorga, B.J.; Gallardo-Rosas, D.; Toscano, R.A.; Carreón-Castro, M.P.; Basiuk, V.A.; Cor-tés-Guzmán, F.; López-Cortés, J.G.; Ortega-Alfaro, M.C. π-Extended push-pull azo-pyrrole photoswitches: Synthesis, solva-tochromism and optical band gaps. Org. Biomol. Chem. 2020, 18, 1657–1670. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harada, T.; Kawabata, H.; Komori, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and Light Control of the Sol-Gel Phase Transition in Cholesterol-Based Organic Gels. Novel Helical Aggregation Modes as Detected by Circular Dichroism and Electron Microscopic Observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Abendroth, J.M.; Bushuyev, O.S.; Weiss, P.S.; Barrett, C.J. Controlling Motion at the Nanoscale: Rise of the Molecular Ma-chines. ACS Nano 2015, 9, 7746–7768. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.C.; Soares, N.D.F.F.; Da Silva, L.H.M.; Da Silva, M.C.H.; Mageste, A.B.; Soares, R.F.; Teixeira, A.V.N.C.; Andrade, N.J. Thermodynamic Study of Colorimetric Transitions in Polydiacetylene Vesicles Induced by the Solvent Effect. J. Phys. Chem. B 2010, 114, 13365–13371. [Google Scholar] [CrossRef]
- Kaszyńska, J.; Łapiński, A.; Bielejewski, M.; Luboradzki, R.; Tritt-Goc, J. On the relation between the solvent parameters and the physical properties of methyl-4,6-O-benzylidene-α-d-glucopyranoside organogels. Tetrahedron 2012, 68, 3803–3810. [Google Scholar] [CrossRef]
- Zhu, N.; Yan, Q.; Luo, Z.; Zhai, Y.; Zhao, D. Helical Folding of Conjugated Oligo(phenyleneethynylene): Chain-Length De-pendence, Solvent Effects, and Intermolecular Assembly. Chem. Asian J. 2012, 7, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F. Solvent Effects in Supramolecular Chemistry: Linear Free Energy Relationships for Common Intermolecular Interactions. J. Org. Chem. 2021. [Google Scholar] [CrossRef]
- Rau, H. Photochromism, Molecules and Systems; Dürr, H., Buuas-Laurent, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Sekkat, Z.; Knoll, W. Photoreactive Organic thin Films in the Light of Bound Electromagnetic Waves; John and Wiley and Sons: Hoboken, NJ, USA, 1997; pp. 117–195. [Google Scholar]
- Rau, H. Spectroscopic properties of organic azo compounds, Angew. Chem. Int. Ed. Engl. 1973, 12, 224. [Google Scholar] [CrossRef]
- Morgante, C.G.; Struve, W.S. S2→S0 fluorescence in trans-azobenzene. Chem. Phys. Lett. 1979, 68, 267–271. [Google Scholar] [CrossRef]
- Azuma, J.; Tamai, N.; Shishido, A.; Ikeda, T. Femtosecond dynamics and stimulated emission from the S2 state of a liquid crystalline trans-azobenzene. Chem. Phys. Lett. 1998, 288, 77–82. [Google Scholar] [CrossRef]
- Satzger, H.; Spörlein, S.; Root, C.; Wachtveitl, J.; Zinth, W.; Gilch, P. Fluorescence spectra of trans- and cis-azobenzene—emission from the Franck–Condon state. Chem. Phys. Lett. 2003, 372, 216–223. [Google Scholar] [CrossRef]
- Yoshino, J.; Kano, N.; Kawashima, T. Synthesis of the most intensely fluorescent azobenzene by utilizing the B–N interaction. Chem. Commun. 2007, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Furuta, A.; Kambe, T.; Itoi, H.; Kano, N.; Kawashima, T.; Ito, Y.; Asashima, M. Intensely Fluorescent Azobenzenes: Synthesis, Crystal Structures, Effects of Substituents, and Application to Fluorescent Vital Stain. Chem. Eur. J. 2010, 16, 4951. [Google Scholar] [CrossRef]
- Yoshino, J.; Kano, N.; Kawashima, T. Fluorescent azobenzenes and aromatic aldimines featuring an N–B interaction. Dalton Trans. 2013, 42, 15826. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 174–1741. [Google Scholar] [CrossRef]
- An, B.-K.; Kwon, S.-K.; Jung, S.-D.; Park, S.Y. Enhanced Emission and Its Switching in Fluorescent Organic Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- An, B.-K.; Gierschner, J.; Park, S.Y. π-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-Naphthalimide AIE/AIEEgens: Recent Advances and Prospects. ACS Appl. Mater. Interfaces 2018, 10, 12081–12111. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Xie, H.; Tang, B.Z. Aggregation-Induced Emission-Active Gels: Fabrications, Functions, and Applications. Adv. Mater. 2021, 33, 2100021. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Jo, S.; Park, S.J.; Kim, H.M.; Kim, D.; Lee, T.S. Unusual fluorescence of o-phenylazonaphthol derivatives with aggregation-induced emission and their use in two-photon cell imaging. Chem. Commun. 2019, 55, 6747–6750. [Google Scholar] [CrossRef]
- Yamauchi, M.; Yokoyama, K.; Aratani, N.; Yamada, H.; Masuo, S. Crystallization-Induced Emission of Azobenzene Deriva-tives. Angew. Chem. Int. Ed. 2019, 58, 14173–14178. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Piotto, S.; Concilio, S.; Shikler, R.; Panunzi, B. Spectroscopic Behaviour of Two Novel Azobenzene Fluorescent Dyes and Their Polymeric Blends. Molecules 2020, 25, 1368. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Song, X.; Park, H.; Baik, M.-H.; Lee, D. Torsionally Responsive C3-Symmetric Azo Dyes: Azo−Hydrazone Tautomerism, Conformational Switching, and Application for Chemical Sensing. J. Am. Chem. Soc. 2010, 132, 12133–12144. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.-J.; An, B.-K.; Jung, S.D.; Chung, M.-A.; Park, S.Y. Photoswitchable Organic Nanoparticles and a Polymer Film Employing Multifunctional Molecules with Enhanced Fluorescence Emission and Bistable Photochromism. Angew. Chem. Int. Ed. 2004, 43, 6346–6350. [Google Scholar] [CrossRef] [PubMed]
- Herbert, K.; Schrettl, S.; Rowan, S.J.; Weder, C. 50th Anniversary Perspective: Solid-State Multistimuli, Multiresponsive Polymeric Materials. Macromolecules 2017, 50, 8845–8870. [Google Scholar] [CrossRef]
- Han, T.; Yao, Z.; Qiu, Z.; Zhao, Z.; Wu, K.; Wang, J.; Poon, A.W.; Lam, J.W.Y.; Tang, B.Z. Photoresponsive spiro-polymers generated in situ by C–H-activated polyspiroannulation. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, I.; Hara, M.; Seki, T.; Cho, S.J.; Shimizu, M.; Matsuura, K.; Cheong, H.-K.; Kim, J.Y.; Oh, J.; Jung, J.; et al. A trigonal molecular assembly system with the dual light-driven functions of phase transition and fluorescence switching. J. Mater. Chem. C 2019, 7, 2276–2282. [Google Scholar] [CrossRef]
- Luo, W.; Wang, G. Photo-Responsive Fluorescent Materials with Aggregation-Induced Emission Characteristics. Adv. Opt. Mater. 2020, 8, 2001362. [Google Scholar] [CrossRef]
- Xue, L.; Pan, Y.; Zhang, S.; Chen, Y.; Yu, H.; Yang, Y.; Mo, L.; Sun, Z.; Li, L.; Yang, H. Fluorescent Azobenzene-Containing Compounds: From Structure to Mechanism. Crystals 2021, 11, 840. [Google Scholar] [CrossRef]
- Forber, C.L.; Kelusky, E.C.; Bunce, N.J.; Zerner, M.C. Electronic spectra of cis- and trans-azobenzenes: Consequences of ortho substitution. J. Am. Chem. Soc. 1985, 107, 5884–5890. [Google Scholar] [CrossRef]
- Beharry, A.A.; Sadovski, O.; Woolley, G.A. Azobenzene Photoswitching without Ultraviolet Light. J. Am. Chem. Soc. 2011, 133, 19684–19687. [Google Scholar] [CrossRef]
- Han, M.; Cho, S.J.; Norikane, Y.; Shimizu, M.; Kimura, A.; Tamagawa, T.; Seki, T. Multistimuli-responsive azobenzene nan-ofibers with aggregation-induced emission enhancement characteristics. Chem. Commun. 2014, 50, 15815–15818. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Gabor, G.; Frei, Y.; Gegiou, D.; Kaganowitch, M.; Fischer, E. Tautomerism and Geometric Isomerism in Arylazo-Phenols and Naphthols. Part III. Orthohydroxy Derivatives and their Reversible Photochemical Reactions. Isr. J. Chem. 1967, 5, 193–211. [Google Scholar] [CrossRef]
- Douhal, A.; Sanz, M.; Tormo, L. Femtochemistry of orange II in solution and in chemical and biological nanocavities. Proc. Natl. Acad. Sci. USA 2005, 102, 18807–18812. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Guan, P.-J.; Fang, W.-H. Photoinduced Proton Transfer and Isomerization in a Hydrogen-Bonded Aromatic Azo Compound: A CASPT2//CASSCF Study. J. Phys. Chem. A 2014, 118, 4732–4739. [Google Scholar] [CrossRef]
- Ghasemian, M.; Kakanejadifard, A.; Azarbani, F.; Zabardasti, A.; Kakanejadifard, S. Spectroscopy and solvatochromism studies along with antioxidant and antibacterial activities investigation of azo–azomethine compounds 2-(2-hydroxyphenylimino)methyl)-4-phenyldiazenyl)phenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Hisaindee, S.; Saleh, N. Spectroscopic studies of keto–enol tautomeric equilibrium of azo dyes. RSC Adv. 2015, 5, 18097–18110. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Pacific Northwest National Laboratory (PNNL), Richland, WA (US), Environmental Molecular Sciences Laboratory (EMSL) Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378. [Google Scholar]
- Polarized Light Microscopy. Available online: https://www.microscopyu.com/techniques/polarized-light/polarized-light-microscopy (accessed on 10 December 2021).
- Fukuhara, K.; Nagano, S.; Hara, M.; Seki, T. Free-surface molecular command systems for photoalignment of liquid crystalline materials. Nat. Commun. 2014, 5, 3320. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Hirade, T. Anisotropic two-dimensional sheets assembled from rod-shaped metal complexes. Chem. Commun. 2012, 48, 100–102. [Google Scholar] [CrossRef]
- Bäcklund, F.G.; Elfwing, A.; Musumeci, C.; Ajjan, F.; Babenko, V.; Dzwolak, W.; Solin, N.; Inganäs, O. Conducting microhelices from self-assembly of protein fibrils. Soft Matter 2017, 13, 4412–4417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavia, D.L.; Lampan, G.M.; Kriz, G.S. Introduction to Spectroscopy; W.B. Saunders Company: Philadelphia, PA, USA, 1979. [Google Scholar]
- Guenzler, H.; Gremlich, H.; Bluemich, M. IR Spectroscopy: An Introduction; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Han, M.; Ichimura, K. In-Plane and Tilt Reorientation of p-Methoxyazobenzene Side Chains Tethered to Liquid Crystalline Polymethacrylates by Irradiation with 365 nm Light. Macromolecules 2001, 34, 90–98. [Google Scholar] [CrossRef]
- Iijima, S.; Ichibashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603. [Google Scholar] [CrossRef]
- Davis, R.; Rath, N.P.; Das, S. Thermally reversible fluorescent polymorphs of alkoxy-cyano-substituted diphenylbutadienes: Role of crystal packing in solid state fluorescence. Chem. Commun. 2004, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N.; Aida, T. Self-Assembled Hexa- peri -hexabenzocoronene Graphitic Nanotube. Science 2004, 304, 1481–1483. [Google Scholar] [CrossRef] [Green Version]
- Mena-Osteritz, E. Superstructures of Self-Organizing Thiophenes. Adv. Mater. 2002, 14, 609–616. [Google Scholar] [CrossRef]
- Bunce, N.J.; Ferguson, G.; Forber, C.L.; Stachnyk, G.J. Sterically Hindered Azobenzenes: Isolation of Cis Isomers and Kinetics of Thermal Cis → Trans Isomerization. J. Org. Chem. 1987, 52, 394–398. [Google Scholar] [CrossRef]
- Han, M.R.; Hashizume, D.; Hara, M. 1-[(E)-3-sec-Butyl-4-(4′-ethoxy-3,5-diethylbiphenyl-4-yldiazenyl)phenoxy]hexadecane. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3001–o3003. [Google Scholar] [CrossRef]
- Hirade, T.; Okui, Y.; Han, M. A design strategy for stable light-sensitive palladium complexes. J. Mater. Chem. C 2013, 1, 2672–2679. [Google Scholar] [CrossRef]
- Lameijer, L.N.; Budzak, S.; Simeth, N.A.; Hansen, M.J.; Feringa, B.L.; Jacquemin, D.; Szymanski, W. General Principles for the Design of Visible-Light-Responsive Photoswitches: Tetra-ortho-Chloro-Azobenzenes. Angew. Chem. Int. Ed. 2020, 59, 21663–21670. [Google Scholar] [CrossRef] [PubMed]
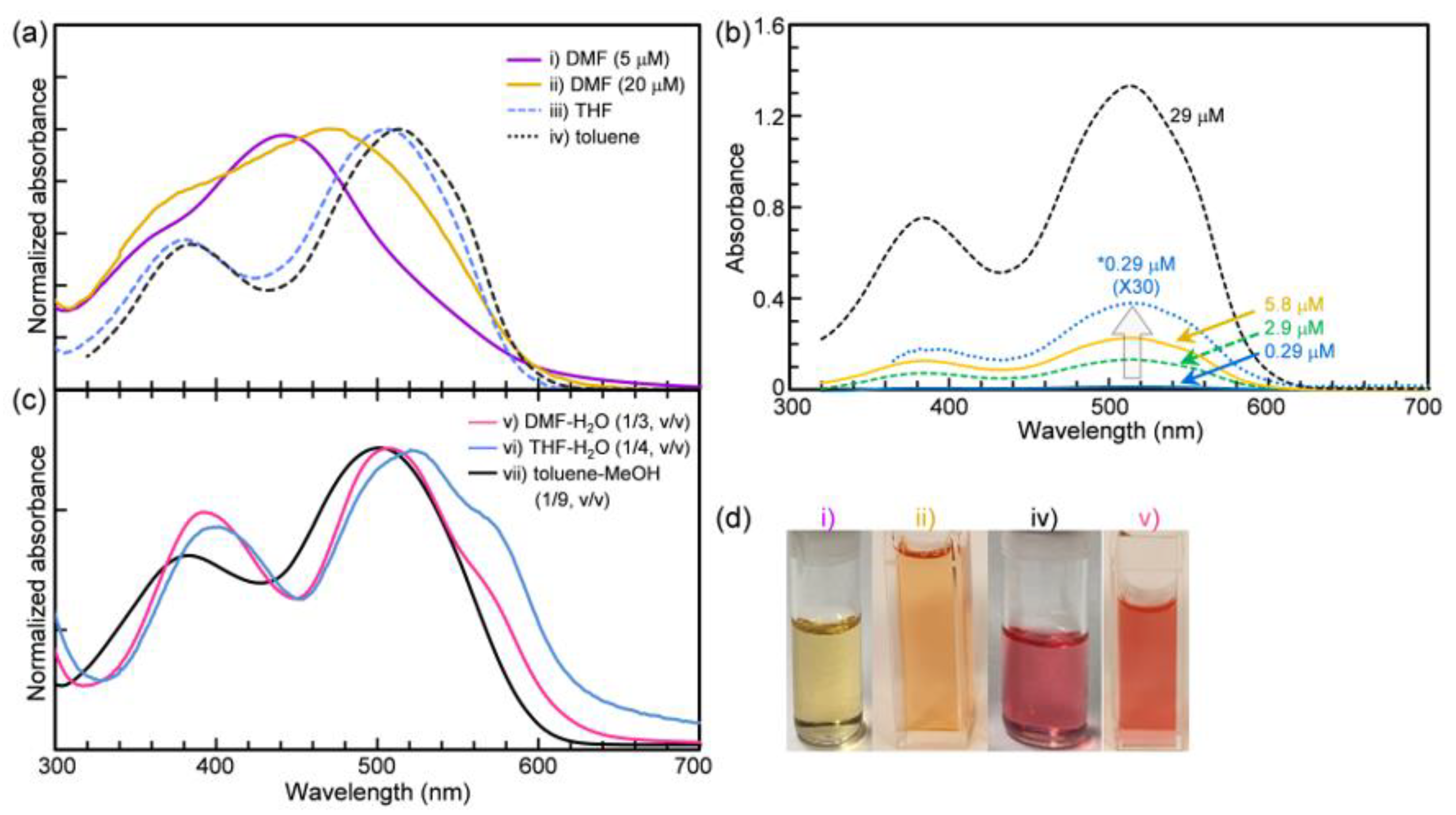



| Solvent | DMF | DMF-H2O | THF | THF-H2O | Toluene | Toluene-MeOH |
|---|---|---|---|---|---|---|
| polarity | 13.7 | - | 5.7 | - | 1.4 | - |
| dielectric constant | 38.2 | - | 7.5 | - | 2.4 | - |
| 442→470–480 | 392, 505 | 381, 504 | 400, 517 | 384, 515 | 383, 502 | |
| 608 | 632 | 610 | 629 | 616 | 617 | |
| aggregate morphology | - | nanoparticle→1D structures | - | sphere (1D→2D structures) | - | 1D structures |
| AIEE property | - | ○ | - | ○ | - | ○ |
| Solvent | DMF | THF | Toluene | |
|---|---|---|---|---|
| Average dihedral angle (°) | ||||
 | θ1 | 27.1 | 18.5 | 13.3 |
| θ2 | −34.2 | −34.8 | −35.4 | |
| θ3 | 89.7 | 89.7 | 88.9 | |
| HOMO-LUMO gap (eV) | 3.12 | 2.35 | 2.25 | |
| Abs. wavelength (λmax, nm) | 473.9 | 491.8 | 497.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Abe, I.; Oh, J.; Jung, J.; Son, Y.J.; Noh, J.; Hara, M.; Seki, T. Solvent- and Light-Sensitive AIEE-Active Azo Dye: From Spherical to 1D and 2D Assemblies. Int. J. Mol. Sci. 2022, 23, 965. https://doi.org/10.3390/ijms23020965
Han M, Abe I, Oh J, Jung J, Son YJ, Noh J, Hara M, Seki T. Solvent- and Light-Sensitive AIEE-Active Azo Dye: From Spherical to 1D and 2D Assemblies. International Journal of Molecular Sciences. 2022; 23(2):965. https://doi.org/10.3390/ijms23020965
Chicago/Turabian StyleHan, Mina, Ikue Abe, Jihun Oh, Jaehoon Jung, Young Ji Son, Jaegeun Noh, Mitsuo Hara, and Takahiro Seki. 2022. "Solvent- and Light-Sensitive AIEE-Active Azo Dye: From Spherical to 1D and 2D Assemblies" International Journal of Molecular Sciences 23, no. 2: 965. https://doi.org/10.3390/ijms23020965






