Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure
Abstract
:1. Introduction
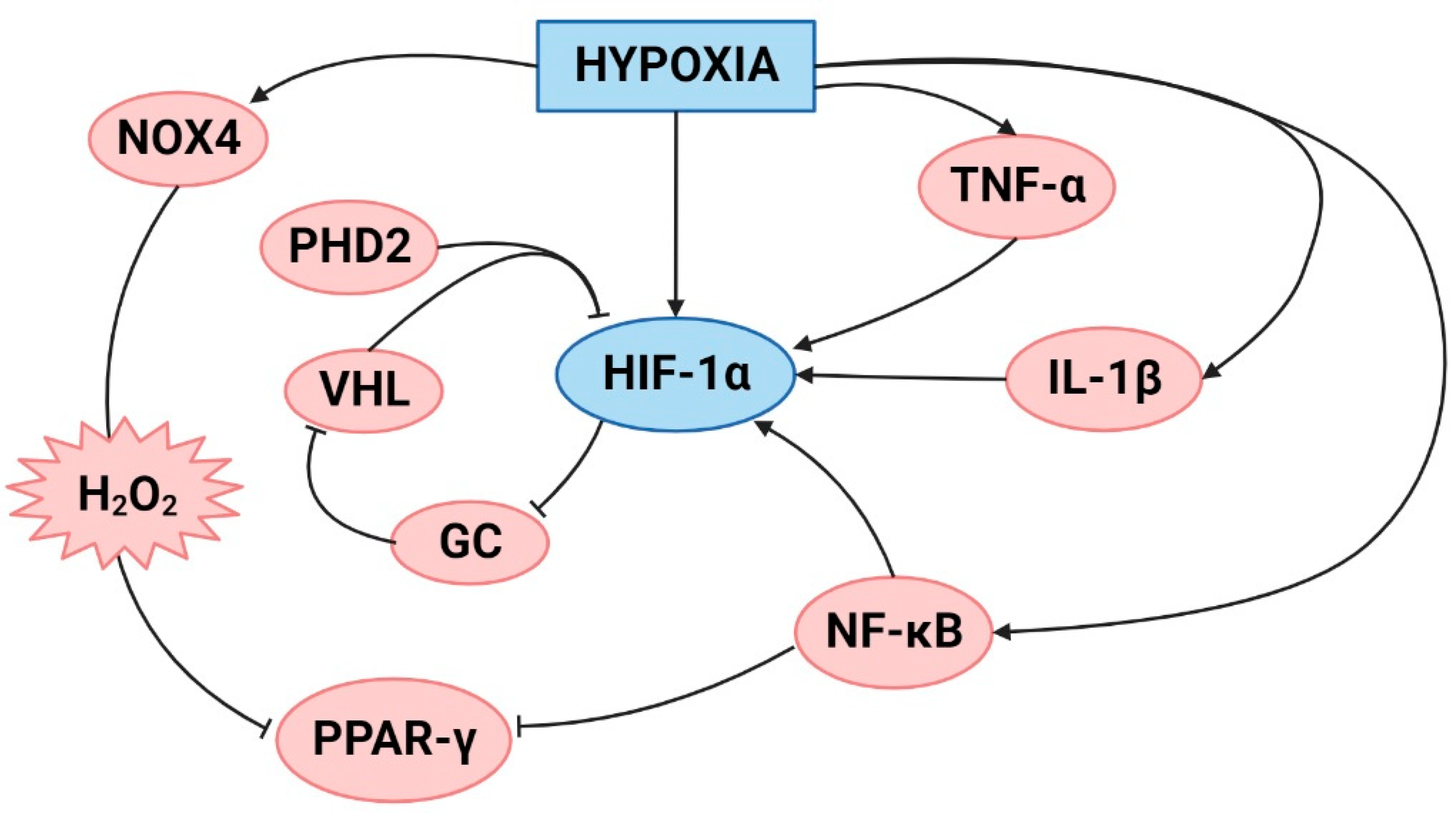
2. Hypoxia and Inflammation
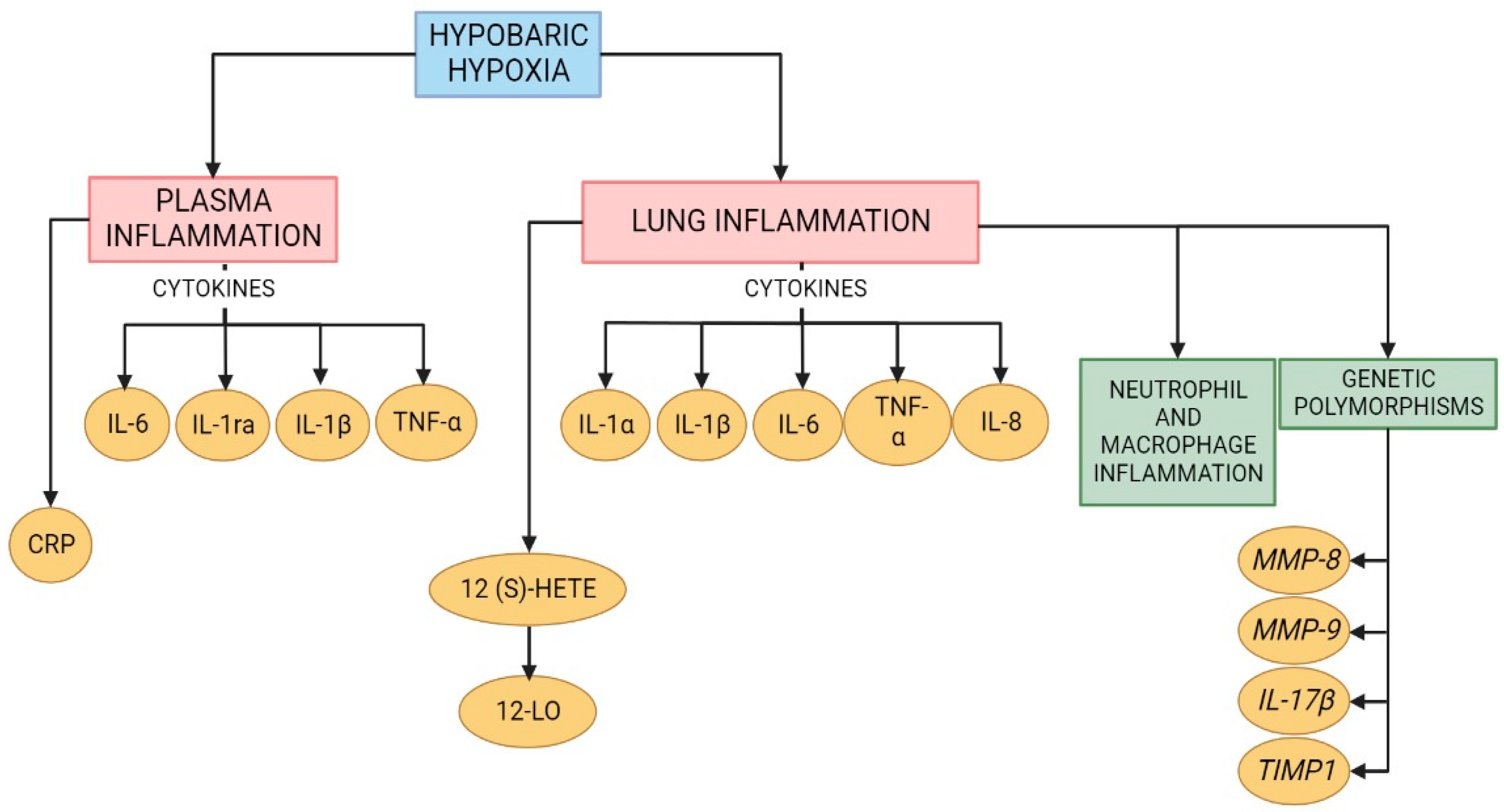
3. Lung Inflammation under Hypobaric Hypoxia
4. High Altitude Pulmonary Edema
5. High-Altitude Pulmonary Hypertension
6. Treatments
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HIF-1α | hypoxia-inducible factor-1α |
| GR | glucocorticoid receptor |
| MMP8 | matrix metalloproteinase 8 |
| MMP9 | matrix metalloproteinase 9 |
| IL-17β | interleukin-17β |
| TIMP1 | tissue inhibitor of metalloproteinase 1 |
| NR3C1 | nuclear receptor subfamily 3, group C, member 1 |
| ICAM-1 | intercellular adhesion molecule-1 |
| VCAM-1 | vascular cell adhesion molecule 1 |
| NF-κB | nuclear factor kappa B |
| TNF-α | tumor necrosis factor alpha |
| TLR4 | toll-like receptor 4 |
| 12(s)-HETE | 12(s)-hydroxyeicosatetraenoic acid |
| 12-LO | leukocyte-type 12 lipoxygenase |
| suPAR | soluble urokinase-type plasminogen activator receptor |
| MCP-1 | monocyte chemoattractant protein-1 |
| MIP-1α | macrophage inflammatory protein-1α |
| HSP70 | heat shock protein 70 |
| PDIA3 | protein disulfide isomerase associated 3 |
| MIF | macrophage migration inhibitory factor |
| ET-1 | endothelin 1 |
| PPARγ | proliferator-activated receptor γ |
| MIP-2 | macrophage inflammatory protein 2 |
References
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Agita, A.; Alsagaff, M.T. Inflammation, immunity, and hypertension. Acta Med. Indones. 2017, 49, 158–165. [Google Scholar] [PubMed]
- Pena, E.; Brito, J.; El Alam, S.; Siques, P. Oxidative stress, kinase activity and inflammatory implications in right ventricular hypertrophy and heart failure under hypobaric hypoxia. Int. J. Mol. Sci. 2020, 21, 6421. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Agarwal, S.; Shrimali, N.M.; Guchhait, P. Interplay between hypoxia and inflammation contributes to the progression and severity of respiratory viral diseases. Mol. Aspects Med. 2021, 81, 101000. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.E.; Simon, M.C. Hypoxia-inducible factors: Crosstalk between inflammation and metabolism. Semin. Cell Dev. Biol. 2012, 23, 389–394. [Google Scholar] [CrossRef]
- Patel, H.; Zaghloul, N.; Lin, K.; Liu, S.F.; Miller, E.J.; Ahmed, M. Hypoxia-induced activation of specific members of the NF-kB family and its relevance to pulmonary vascular remodeling. Int. J. Biochem. Cell Biol. 2017, 92, 141–147. [Google Scholar] [CrossRef]
- Hartmann, G.; Tschöp, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschöp, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef]
- Maston, L.D.; Jones, D.T.; Giermakowska, W.; Resta, T.C.; Ramiro-Diaz, J.; Howard, T.A.; Jernigan, N.L.; Herbert, L.; Maurice, A.A.; Gonzalez Bosc, L.V. Interleukin-6 trans-signaling contributes to chronic hypoxia-induced pulmonary hypertension. Pulm. Circ. 2018, 8, 2045894018780734. [Google Scholar] [CrossRef] [Green Version]
- Von Euler, U.S.; Liljestrand, G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol. Scand. 1946, 12, 301–320. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef]
- León-Velarde, F.; Maggiorini, M.; Reeves, J.T.; Aldashev, A.; Asmus, I.; Bernardi, L.; Ge, R.L.; Hackett, P.; Kobayashi, T.; Moore, L.G.; et al. Consensus statement on chronic and subacute high altitude diseases. High Alt. Med. Biol. 2005, 6, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, A.E.; Strohl, K.P. High-altitude pulmonary hypertension: An update on disease pathogenesis and management. Open Cardiovasc. Med. J. 2016, 10, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarada, S.; Himadri, P.; Mishra, C.; Geetali, P.; Ram, M.S.; Ilavazhagan, G. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp. Biol. Med. 2008, 233, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef]
- Maggiorini, M.; Rocca, H.P.B.L.; Peth, S.; Fischler, M.; Böhm, T.; Bernheim, A.; Kiencke, S.; Bloch, K.E.; Dehnert, C.; Naeije, R.; et al. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: A randomized trial. Ann. Intern. Med. 2006, 145, 497–506. [Google Scholar] [CrossRef]
- Woods, P.; Alcock, J. High-altitude pulmonary edema. Evol. Med. Public Health. 2021, 9, 118–119. [Google Scholar] [CrossRef]
- Siques, P.; Pena, E.; Brito, J.; El Alam, S. Oxidative Stress, Kinase Activation, and Inflammatory Pathways Involved in Effects on Smooth Muscle Cells During Pulmonary Artery Hypertension Under Hypobaric Hypoxia Exposure. Front. Physiol. 2021, 12, 690341. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, S.B.; Sarkar, S. Genome wide expression analysis suggests perturbation of vascular homeostasis during high altitude pulmonary edema. PLoS ONE 2014, 9, e85902. [Google Scholar] [CrossRef] [Green Version]
- Swenson, E.R.; Bärtsch, P. High-altitude pulmonary edema. Compr. Physiol. 2012, 2, 2753–2773. [Google Scholar] [CrossRef]
- Nijiati, Y.; Maimaitiyiming, D.; Yang, T.; Li, H.; Aikemu, A. Research on the improvement of oxidative stress in rats with high-altitude pulmonary hypertension through the participation of irbesartan in regulating intestinal flora. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4540–4553. [Google Scholar] [CrossRef]
- Fu, X.; Yang, C.; Chen, B.; Zeng, K.; Chen, S.; Fu, Y. Qi-Long-Tian formula extract alleviates symptoms of acute high-altitude diseases via suppressing the inflammation responses in rat. Respir. Res. 2021, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Gangwar, A.; Bhargava, K.; Ahmad, Y. D4F prophylaxis enables redox and energy homeostasis while preventing inflammation during hypoxia exposure. Biomed. Pharmacother. 2021, 133, 111083. [Google Scholar] [CrossRef] [PubMed]
- Cummins, E.P.; Keogh, C.E.; Crean, D.; Taylor, C.T. The role of HIF in immunity and inflammation. Mol. Asp. Med. 2016, 47–48, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Pena, E.; Siques, P.; Brito, J.; Arribas, S.M.; Böger, R.; Hannemann, J.; León-Velarde, F.; González, M.C.; López, M.R.; de Pablo, Á. Nox2 upregulation and p38α MAPK activation in right ventricular hypertrophy of rats exposed to long-term chronic intermittent hypobaric hypoxia. Int. J. Mol. Sci. 2020, 21, 8576. [Google Scholar] [CrossRef]
- Samanta, D.; Semenza, G.L. Maintenance of redox homeostasis by hypoxia-inducible factors. Redox Biol. 2017, 13, 331–335. [Google Scholar] [CrossRef]
- Pham, K.; Parikh, K.; Heinrich, E.C. Hypoxia and inflammation: Insights from high-altitude physiology. Front. Physiol. 2021, 12, 676782. [Google Scholar] [CrossRef]
- Li, J.; Yuan, W.; Jiang, S.; Ye, W.; Yang, H.; Shapiro, I.M.; Risbud, M.V. Prolyl-4-hydroxylase domain protein 2 controls NF-κB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J. Biol. Chem. 2015, 290, 7195–7207. [Google Scholar] [CrossRef] [Green Version]
- Shimba, A.; Ikuta, K. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 2020, 42, 669–680. [Google Scholar] [CrossRef]
- Vanderhaeghen, T.; Beyaert, R.; Libert, C. Bidirectional crosstalk between hypoxia inducible factors and glucocorticoid signalling in health and disease. Front. Immunol. 2021, 12, 684085. [Google Scholar] [CrossRef]
- Leonard, M.O.; Godson, C.; Brady, H.R.; Taylor, C.T. Potentiation of glucocorticoid activity in hypoxia through induction of the glucocorticoid receptor. J. Immunol. 2005, 174, 2250–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zhao, J.J.; Lv, Y.Y.; Ding, P.S.; Liu, R.Y. Hypoxia down-regulates glucocorticoid receptor alpha and attenuates the anti-inflammatory actions of dexamethasone in human alveolar epithelial A549 cells. Life Sci. 2009, 85, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fang, L.; Wu, H.; Ding, P.; Shen, Q.; Liu, R. Down-regulation of GRα expression and inhibition of its nuclear translocation by hypoxia. Life Sci. 2016, 146, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Vettori, A.; Greenald, D.; Wilson, G.K.; Peron, M.; Facchinello, N.; Markham, E.; Sinnakaruppan, M.; Matthews, L.C.; McKeating, J.A.; Argenton, F.; et al. Glucocorticoids promote Von Hippel Lindau degradation and Hif-1α stabilization. Proc. Natl. Acad. Sci. USA 2017, 114, 9948–9953. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Bijli, K.M.; Ramirez, A.; Murphy, T.C.; Kleinhenz, J.; Hart, C.M. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2013, 63, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Mrakic-Sposta, S.; Biagini, D.; Bondi, D.; Pietrangelo, T.; Vezzoli, A.; Lomonaco, T.; Di Francesco, F.; Verratti, V. OxInflammation at high altitudes: A proof of concept from the Himalayas. Antioxidants 2022, 11, 368. [Google Scholar] [CrossRef]
- Mishra, K.P.; Sharma, N.; Soree, P.; Gupta, R.K.; Ganju, L.; Singh, S.B. Hypoxia-induced inflammatory chemokines in subjects with a history of high-altitude pulmonary edema. Indian J. Clin. Biochem. 2016, 31, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Arya, A.; Sethy, N.K.; Singh, S.K.; Das, M.; Bhargava, K. Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int. J. Nanomed. 2013, 8, 4507–4520. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, Q.; Bai, J.; Chen, K.; Yang, H.; Wang, W.; Fan, F.; Zhang, Y.; Meng, X.; Kuang, T.; et al. The multiple organs insult and compensation mechanism in mice exposed to hypobaric hypoxia. Cell Stress Chaperones 2020, 25, 779–791. [Google Scholar] [CrossRef]
- Wu, G.; Xu, G.; Chen, D.W.; Gao, W.X.; Xiong, J.Q.; Shen, H.Y.; Gao, Y.Q. Hypoxia exacerbates inflammatory acute lung injury via the toll-like receptor 4 signaling pathway. Front. Immunol. 2018, 9, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.; Fahim, M.; Kotwani, A. Efficacy of tadalafil in chronic hypobaric hypoxia-induced pulmonary hypertension: Possible mechanisms. Fundam. Clin. Pharmacol. 2013, 27, 271–278. [Google Scholar] [CrossRef]
- Yu, Y.R.; Mao, L.; Piantadosi, C.A.; Gunn, M.D. CCR2 deficiency, dysregulation of Notch signaling, and spontaneous pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 48, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foll, M.; Gaggiotti, O.E.; Daub, J.T.; Vatsiou, A.; Excoffier, L. Widespread signals of convergent adaptation to high altitude in Asia and america. Am. J. Hum. Genet. 2014, 95, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Bigham, A.W.; Lee, F.S. Human high-altitude adaptation: Forward genetics meets the HIF pathway. Genes Dev. 2014, 28, 2189–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakurum, M.; Shreeniwas, R.; Chen, J.; Pinsky, D.; Yan, S.D.; Anderson, M.; Sunouchi, K.; Major, J.; Hamilton, T.; Kuwabara, K.; et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J. Clin. Investig. 1994, 93, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Yuhai, G.U.; Zhen, Z. Significance of the changes occurring in the levels of interleukins, SOD and MDA in rat pulmonary tissue following exposure to different altitudes and exposure times. Exp. Ther. Med. 2015, 10, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Preston, I.R.; Hill, N.S.; Warburton, R.R.; Fanburg, B.L. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L367–L374. [Google Scholar] [CrossRef]
- Imray, C.; Booth, A.; Wright, A.; Bradwell, A. Acute altitude illnesses. BMJ 2011, 343, d4943. [Google Scholar] [CrossRef] [Green Version]
- Swenson, E.R.; Maggiorini, M.; Mongovin, S.; Gibbs, J.S.; Greve, I.; Mairbäurl, H.; Bärtsch, P. Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. JAMA 2002, 287, 2228–2235. [Google Scholar] [CrossRef]
- Ran, Y.H.; Zhang, D.X.; Xiao, Z.H.; Zhang, Y.F.; Cui, W.Y.; Wang, Y.H.; Cui, J.H.; Wang, H. Changes of VEGF, TNF-alpha, IL-6 and NO in serum of patients with HAPE. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2011, 27, 201–203. [Google Scholar] [PubMed]
- Shukla, D.; Saxena, S.; Purushothaman, J.; Shrivastava, K.; Singh, M.; Shukla, S.; Malhotra, V.K.; Mustoori, S.; Bansal, A. Hypoxic preconditioning with cobalt ameliorates hypobaric hypoxia induced pulmonary edema in rat. Eur. J. Pharmacol. 2011, 656, 101–109. [Google Scholar] [CrossRef]
- Hilty, M.P.; Zügel, S.; Schoeb, M.; Auinger, K.; Dehnert, C.; Maggiorini, M. Soluble urokinase-type plasminogen activator receptor plasma concentration may predict susceptibility to high altitude pulmonary edema. Mediat. Inflamm. 2016, 2016, 1942460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Du, H.; Li, Y.; Guan, W.; Tang, F.; Ga, Q.; Ge, R.L. NR3C1 gene polymorphisms are associated with high-altitude pulmonary edema in Han Chinese. J. Physiol. Anthropol. 2019, 38, 4. [Google Scholar] [CrossRef] [Green Version]
- Kandel, R.S.; Mishra, R.; Gautam, J.; Alaref, A.; Hassan, A.; Jahan, N. Patchy vasoconstriction versus inflammation: A debate in the pathogenesis of high altitude pulmonary edema. Cureus 2020, 12, e10371. [Google Scholar] [CrossRef]
- Sydykov, A.; Mamazhakypov, A.; Maripov, A.; Kosanovic, D.; Weissmann, N.; Ghofrani, H.A.; Sarybaev, A.S.; Schermuly, R.T. Pulmonary hypertension in acute and chronic high altitude maladaptation disorders. Int. J. Environ. Res. Public Health 2021, 18, 1692. [Google Scholar] [CrossRef]
- Tan, X.; Feng, L.; Huang, X.; Yang, Y.; Yang, C.; Gao, Y. Histone deacetylase inhibitors promote eNOS expression in vascular smooth muscle cells and suppress hypoxia-induced cell growth. J. Cell. Mol. Med. 2017, 21, 2022–2035. [Google Scholar] [CrossRef]
- Ohata, Y.; Ogata, S.; Nakanishi, K.; Kanazawa, F.; Uenoyama, M.; Hiroi, S.; Tominaga, S.; Toda, T.; Kawai, T. Proteomic analysis of the lung in rats with hypobaric hypoxia-induced pulmonary hypertension. Histol. Histopathol. 2013, 28, 893–902. [Google Scholar] [CrossRef]
- Wang, W.T.; Sun, L.; Sun, C.H. PDIA3-regulted inflammation and oxidative stress contribute to the traumatic brain injury (TBI) in mice. Biochem. Biophys. Res. Commun. 2019, 518, 657–663. [Google Scholar] [CrossRef]
- Chamberlain, N.; Korwin-Mihavics, B.R.; Nakada, E.M.; Bruno, S.R.; Heppner, D.E.; Chapman, D.G.; Hoffman, S.M.; van der Vliet, A.; Suratt, B.T.; Dienz, O.; et al. Lung epithelial protein disulfide isomerase A3 (PDIA3) plays an important role in influenza infection, inflammation, and airway mechanics. Redox Biol. 2019, 22, 101129. [Google Scholar] [CrossRef]
- Tanaka, K.; Tanaka, Y.; Namba, T.; Azuma, A.; Mizushima, T. Heat shock protein 70 protects against bleomycin-induced pulmonary fibrosis in mice. Biochem. Pharmacol. 2010, 80, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Lin, H.J.; Cheng, B.C.; Lin, M.T.; Chang, C.P. Attenuating systemic inflammatory markers in simulated high-altitude exposure by heat shock protein 70-mediated hypobaric hypoxia preconditioning in rats. J. Formos. Med. Assoc. 2015, 114, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.J.; Wang, C.T.; Niu, K.C.; Gao, C.; Li, Z.; Lin, M.T.; Chang, C.P. Hypobaric hypoxia preconditioning attenuates acute lung injury during high-altitude exposure in rats via up-regulating heat-shock protein 70. Clin. Sci. 2011, 121, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Shen, M.; Xu, M.; Liu, L.L.; Luo, Y.; Xu, D.Q.; Wang, Y.X.; Liu, M.L.; Liu, Y.; Dong, H.Y.; et al. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediat. Inflamm. 2012, 2012, 840737. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, Y.; Luo, Y.; Niu, W.; Dong, M.; Liu, M.; Dong, H.; et al. Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 2016, 13, 942–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehra, S.; Bhardwaj, V.; Bansal, A.; Saraswat, D. Nanocurcumin accords protection against acute hypobaric hypoxia induced lung injury in rats. J. Physiol. Biochem. 2016, 72, 763–779. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, B.; Sagi, S.S.K. Prophylactic efficacy of Quercetin in ameliorating the hypoxia induced vascular leakage in lungs of rats. PLoS ONE 2019, 14, e0219075. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Z.; Li, M.; Guo, J.; Chen, L.; Ding, L.; Ding, X.; Zhou, T.; Zhang, J. Polysaccharide from Potentilla anserina L ameliorate pulmonary edema induced by hypobaric hypoxia in rats. Biomed. Pharmacother. 2021, 139, 111669. [Google Scholar] [CrossRef]
- Ko, C.L.; Lin, J.A.; Chen, K.Y.; Hsu, A.C.; Wu, S.Y.; Tai, Y.T.; Lin, K.H.; Chung, W.C.; Li, M.H. Netrin-1 dampens hypobaric hypoxia-induced lung injury in mice. High Alt. Med. Biol. 2019, 20, 293–302. [Google Scholar] [CrossRef]
- Wang, Y.; Duo, D.; Yan, Y.; He, R.; Wu, X. Magnesium lithospermate B ameliorates hypobaric hypoxia-induced pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition and its potential targets. Biomed. Pharmacother. 2020, 130, 110560. [Google Scholar] [CrossRef]
- Gao, L.; Liu, J.; Hao, Y.; Zhao, Z.; Tan, H.; Zhang, J.; Meng, N.; Zheng, Q.; Wang, Z.; Zhang, Y. Chronic intermittent hypobaric hypoxia attenuates monocrotaline-induced pulmonary arterial hypertension via modulating inflammation and suppressing NF-κB/p38 pathway. Iran J. Basic Med. Sci. 2018, 21, 244–252. [Google Scholar] [CrossRef]
- Modesti, P.A.; Vanni, S.; Morabito, M.; Modesti, A.; Marchetta, M.; Gamberi, T.; Sofi, F.; Savia, G.; Mancia, G.; Gensini, G.F.; et al. Role of endothelin-1 in exposure to high altitude: Acute mountain sickness and endothelin-1 (ACME-1) study. Circulation 2006, 114, 1410–1416. [Google Scholar] [CrossRef] [Green Version]
- Satwiko, M.G.; Ikeda, K.; Nakayama, K.; Yagi, K.; Hocher, B.; Hirata, K.; Emoto, N. Targeted activation of endothelin-1 exacerbates hypoxia-induced pulmonary hypertension. Biochem. Biophys. Res. Commun. 2015, 465, 356–362. [Google Scholar] [CrossRef]
- Hocher, B.; Schwarz, A.; Fagan, K.A.; Thöne-Reineke, C.; El-Hag, K.; Kusserow, H.; Elitok, S.; Bauer, C.; Neumayer, H.H.; Rodman, D.M.; et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am. J. Respir. Cell Mol. Biol. 2000, 23, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Donaubauer, B.; Busch, T.; Lachmann, R.; Deja, M.; Petersen, B.; Francis, R.; Träger, A.; Ebsen, M.; Boemke, W.; Kaisers, U. Low-dose inhalation of an endothelin-A receptor antagonist in experimental acute lung injury: ET-1 plasma concentration and pulmonary inflammation. Exp. Biol. Med. 2006, 231, 960–966. [Google Scholar]
- Kassuya, C.A.; Rogerio, A.P.; Calixto, J.B. The role of ET(A) and ET(B) receptor antagonists in acute and allergic inflammation in mice. Peptides 2008, 29, 1329–1337. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Ouerd, S.; Trindade, M.; Gornitsky, J.; Rehman, A.; Barhoumi, T.; Offermanns, S.; Gonzalez, F.J.; Neves, M.F.; Paradis, P.; et al. Vascular smooth muscle cell peroxisome proliferator-activated receptor γ protects against endothelin-1-induced oxidative stress and inflammation. J. Hypertens. 2017, 35, 1390–1401. [Google Scholar] [CrossRef]
- Ito, T.; Zhang, E.; Omori, A.; Kabwe, J.; Kawai, M.; Maruyama, J.; Okada, A.; Yokochi, A.; Sawada, H.; Mitani, Y.; et al. Model difference in the effect of cilostazol on the development of experimental pulmonary hypertension in rats. BMC Pulm. Med. 2021, 21, 377. [Google Scholar] [CrossRef]
- Nesterov, Y.V. Age features of the reaction of the lung tissue and surfactant to hypobaric hypoxia and hyperbaric hyperoxia in the experiment. Adv. Gerontol. 2019, 32, 985–989. [Google Scholar]
- Davis, C.; Hackett, P. Advances in the Prevention and Treatment of High Altitude Illness. Emerg. Med. Clin. North Am. 2017, 35, 241–260. [Google Scholar] [CrossRef]
- Patir, H.; Sarada, S.K.; Singh, S.; Mathew, T.; Singh, B.; Bansal, A. Quercetin as a prophylactic measure against high altitude cerebral edema. Free Radic. Biol. Med. 2012, 53, 659–668. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Lung Injury | Animal Model | Anti-Inflammatory Effects | References |
|---|---|---|---|---|
| Cobalt | HAPE |
Sprague Dawley rats | Decrease TNF-α, TNF-β, NF-κB, MCP-1, and IL-6/Increase HO-1 and MT | Shukla et al. [52] |
| Curcumin | HAPE |
Sprague Dawley rats | Decrease NF-κB | Sarada et al. [13] |
| Nanocurcumin | HAPE |
Sprague Dawley rats | Decrease TNF-α, TNF-β, IL-6, and ET-1 | Nehra et al. [66] |
| Quercetin | HAPE |
Sprague Dawley rats | Downregulate NF-κB and TNF-α Decreased ICAM-1, VCAM-1, and P-selectin Increase TGF-β and IL-4 | Tripathi et al. [67] |
| Potentilla anserina L polysaccharide | HAPE | Wistar rats | Decrease IL-1β, TNF-α, IL-6 Inhibition HIF-1α and NF-κB | Shi et al. [68] |
| Hypobaric hypoxia preconditioning | HAPE |
Sprague Dawley rats | Increase HSP70 | Lin et al. [63] |
| Netrine-1 | HAPI | Mice | Reduced neutrophil infiltration Decrease MIP-2 | Ko et al. [69] |
| Cerium oxide nanoparticles | HAPI | Sprague Dawley rats | Decrease IL-1β, IL-6, and TNF-α | Arya et al. [39] |
| Tadalafil | HAPH | Wistar rats | Decrease TNF-α Decrease inflammatory cells infiltration | Rashid et al. [42] |
| Magnesium lithospermate B | HAPH | Sprague Dawley rats | Downregulated HIF-1α MCP-1 and NF-κB | Wang et al. [70] |
| Intermittent Hypobaric hypoxia treatment | HAPH |
Sprague Dawley rats | Decrease NF-κB, TNF-α, and IL-6 and macrophage infiltration | Gao et al. [71] |
| Resveratrol | HAPH |
Sprague Dawley rats | Decrease IL-6, IL-1β, TNF-α, VEGF, and HIF-1α | Xu et al. [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Alam, S.; Pena, E.; Aguilera, D.; Siques, P.; Brito, J. Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure. Int. J. Mol. Sci. 2022, 23, 12656. https://doi.org/10.3390/ijms232012656
El Alam S, Pena E, Aguilera D, Siques P, Brito J. Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure. International Journal of Molecular Sciences. 2022; 23(20):12656. https://doi.org/10.3390/ijms232012656
Chicago/Turabian StyleEl Alam, Samia, Eduardo Pena, Diego Aguilera, Patricia Siques, and Julio Brito. 2022. "Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure" International Journal of Molecular Sciences 23, no. 20: 12656. https://doi.org/10.3390/ijms232012656








