Systemic Lipopolysaccharide Challenge Induces Inflammatory Changes in Rat Dorsal Root Ganglia: An Ex Vivo Study
Abstract
:1. Introduction
2. Results
2.1. Systemic Inflammation Induces Increase in Circulating Cytokine Concentrations

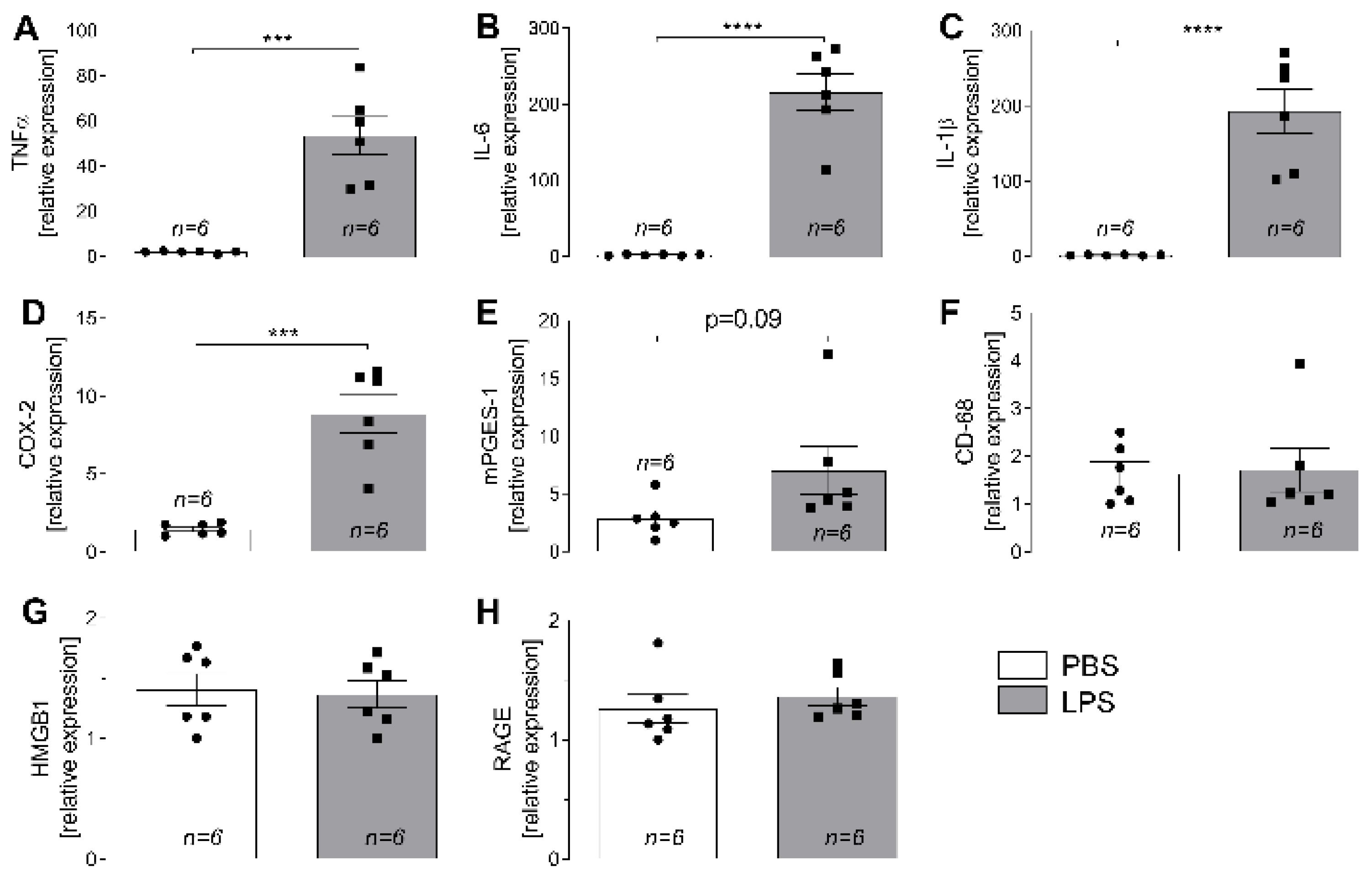
2.2. LPS-Induced Changes in Expression of Inflammatory Marker Genes in L4-L6 DRG
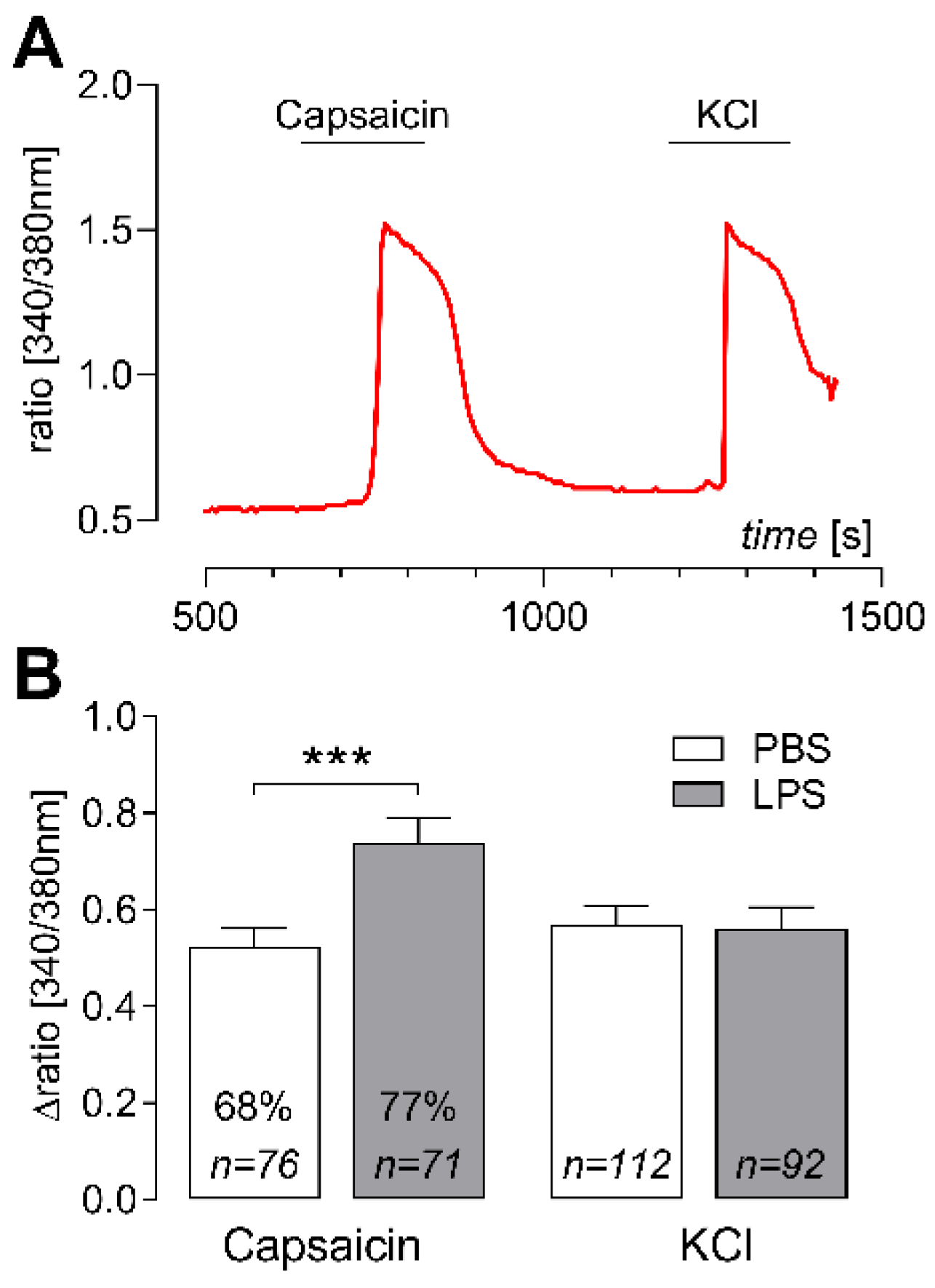
2.3. Elevated Capsaicin-Induced Ca2+ Responses in Cultured DRG Neurons of LPS-Treated Rats
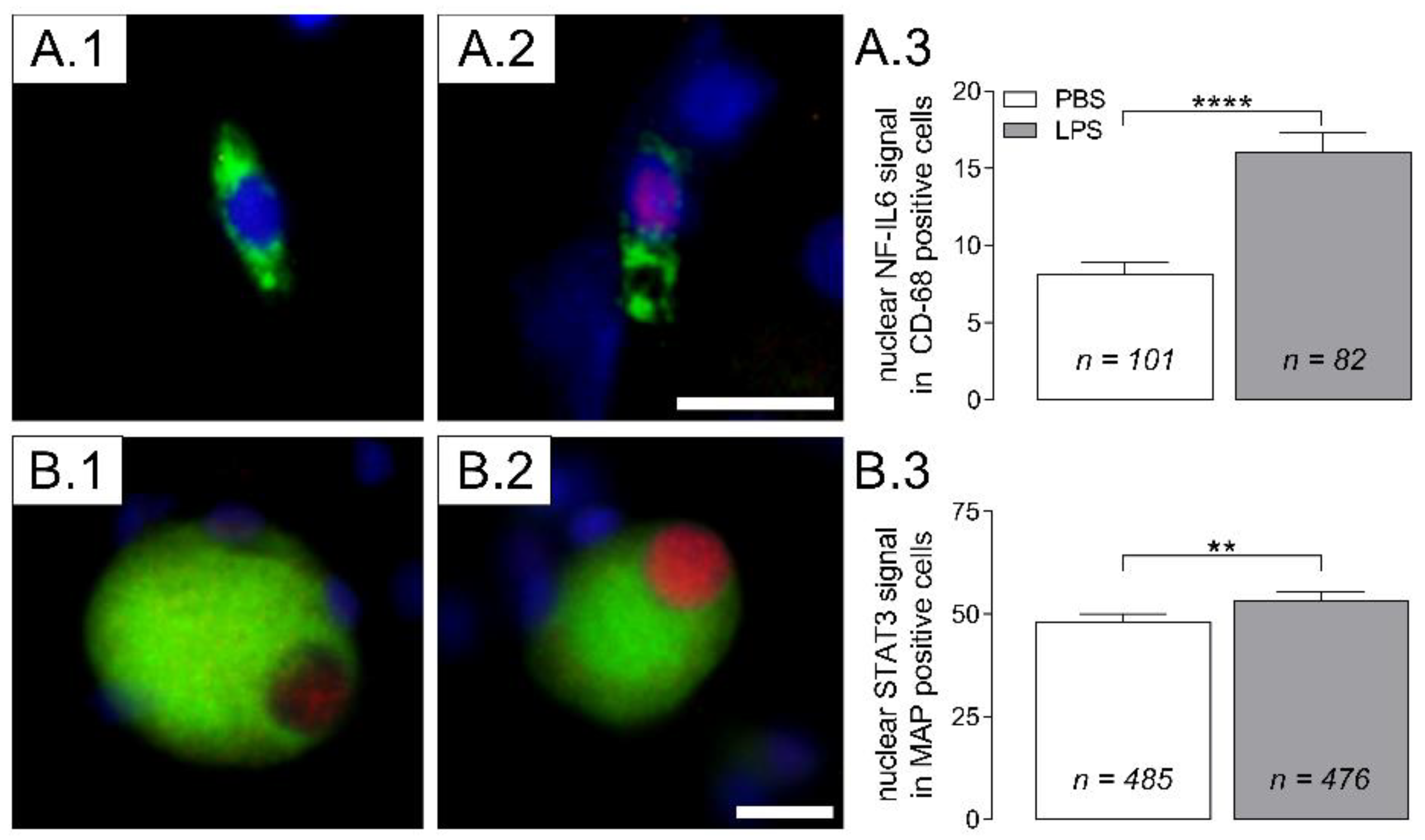
2.4. Nuclear Translocation of Inflammatory Transcription Factors (NF-IL6, STAT3) in Cultured DRG Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatments and Experimental Protocol
4.3. RT-qPCR
4.4. Preparation of DRG Primary Cell Cultures
4.5. Cytokine Measurements (TNFα, IL-6)
4.6. Measurement of Intracellular Calcium ([Ca2+]i)
4.7. Immunocytochemistry
4.8. Evaluation and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Bhave, G.; Gereau, R.W. Posttranslational mechanisms of peripheral sensitization. J. Neurobiol. 2004, 61, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cheng, J.; Han, J.-S.; Wan, Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund’s adjuvant in rats. NeuroReport 2004, 15, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yang, F.; Luo, H.; Liu, F.-Y.; Han, J.-S.; Xing, G.-G.; Wan, Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol. Pain 2008, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Dansereau, M.-A.; Midavaine, É.; Bégin-Lavallée, V.; Belkouch, M.; Beaudet, N.; Longpré, J.-M.; Mélik-Parsadaniantz, S.; Sarret, P. Mechanistic insights into the role of the chemokine CCL2/CCR2 axis in dorsal root ganglia to peripheral inflammation and pain hypersensitivity. J. Neuroinflamm. 2021, 18, 79. [Google Scholar] [CrossRef]
- Segond von Banchet, G.; Boettger, M.K.; Fischer, N.; Gajda, M.; Bräuer, R.; Schaible, H.-G. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain 2009, 145, 151–159. [Google Scholar] [CrossRef]
- Schaible, H.-G.; von Banchet, G.S.; Boettger, M.K.; Bräuer, R.; Gajda, M.; Richter, F.; Hensellek, S.; Brenn, D.; Natura, G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann. N. Y. Acad. Sci. 2010, 1193, 60–69. [Google Scholar] [CrossRef]
- Yeh, T.-Y.; Luo, I.-W.; Hsieh, Y.-L.; Tseng, T.-J.; Chiang, H.; Hsieh, S.-T. Peripheral Neuropathic Pain: From Experimental Models to Potential Therapeutic Targets in Dorsal Root Ganglion Neurons. Cells 2020, 9, 2725. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988, 12, 123–137. [Google Scholar] [CrossRef]
- Harden, L.M.; Kent, S.; Pittman, Q.J.; Roth, J. Fever and sickness behavior: Friend or foe? Brain Behav. Immun. 2015, 50, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.K.; Kavelaars, A.; Heijnen, C.J.; Dantzer, R. Neuroinflammation and comorbidity of pain and depression. Pharmacol. Rev. 2014, 66, 80–101. [Google Scholar] [CrossRef] [Green Version]
- Benson, S.; Engler, H.; Schedlowski, M.; Elsenbruch, S. Experimental endotoxemia as a model to study neuroimmune mechanisms in human visceral pain. Ann. N. Y. Acad. Sci. 2012, 1262, 108–117. [Google Scholar] [CrossRef]
- Wegner, A.; Elsenbruch, S.; Maluck, J.; Grigoleit, J.-S.; Engler, H.; Jäger, M.; Spreitzer, I.; Schedlowski, M.; Benson, S. Inflammation-induced hyperalgesia: Effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav. Immun. 2014, 41, 46–54. [Google Scholar] [CrossRef]
- Schedlowski, M.; Engler, H.; Grigoleit, J.-S. Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain Behav. Immun. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Roth, J.; de Souza, G.E. Fever induction pathways: Evidence from responses to systemic or local cytokine formation. Braz. J. Med. Biol. Res. 2001, 34, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Roth, J.; Blatteis, C.M. Mechanisms of fever production and lysis: Lessons from experimental LPS fever. Compr. Physiol. 2014, 4, 1563–1604. [Google Scholar] [CrossRef]
- Lasselin, J.; Lekander, M.; Benson, S.; Schedlowski, M.; Engler, H. Sick for science: Experimental endotoxemia as a translational tool to develop and test new therapies for inflammation-associated depression. Mol. Psychiatry 2021, 26, 3672–3683. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Steiner, A.A.; Romanovsky, A.A. Fever and hypothermia in systemic inflammation. Handb. Clin. Neurol. 2018, 157, 565–597. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Bredehöft, J.; Perniss, A.; Fuchs, F.; Roth, J.; Rummel, C. Age Dependent Hypothalamic and Pituitary Responses to Novel Environment Stress or Lipopolysaccharide in Rats. Front. Behav. Neurosci. 2018, 12, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Töllner, B.; Roth, J.; Störr, B.; Martin, D.; Voigt, K.; Zeisberger, E. The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflugers Arch. 2000, 440, 925–932. [Google Scholar] [CrossRef]
- Roth, J.; Harré, E.-M.; Rummel, C.; Gerstberger, R.; Hübschle, T. Signaling the brain in systemic inflammation: Role of sensory circumventricular organs. Front. Biosci. 2004, 9, 290–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rummel, C.; Bredehöft, J.; Damm, J.; Schweighöfer, H.; Peek, V.; Harden, L.M. Obesity Impacts Fever and Sickness Behavior during Acute Systemic Inflammation. Physiology 2016, 31, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Blum, E.; Procacci, P.; Conte, V.; Hanani, M. Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience 2014, 274, 209–217. [Google Scholar] [CrossRef]
- Blum, E.; Procacci, P.; Conte, V.; Sartori, P.; Hanani, M. Long term effects of lipopolysaccharide on satellite glial cells in mouse dorsal root ganglia. Exp. Cell Res. 2017, 350, 236–241. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Patel, D.; Dougherty, P.M. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience 2012, 221, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Leisengang, S.; Ott, D.; Murgott, J.; Gerstberger, R.; Rummel, C.; Roth, J. Primary Cultures from Rat Dorsal Root Ganglia: Responses of Neurons and Glial Cells to Somatosensory or Inflammatory Stimulation. Neuroscience 2018, 394, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leisengang, S.; Ott, D.; Murgott, J.; Nürnberger, F.; Gerstberger, R.; Rummel, C.; Schmidt, M.; Roth, J. Effects of gabapentinoids on responses of primary cultures from rat dorsal root ganglia to inflammatory or somatosensory stimuli. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190261. [Google Scholar] [CrossRef]
- Nürnberger, F.; Leisengang, S.; Ott, D.; Murgott, J.; Gerstberger, R.; Rummel, C.; Roth, J. Sensitization of primary cultures from rat dorsal root ganglia with lipopolysaccharide (LPS) requires a robust inflammatory response. Inflamm. Res. 2022, 71, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Ott, D.; Simm, B.; Pollatzek, E.; Gerstberger, R.; Rummel, C.; Roth, J. Prostaglandin D2 modulates calcium signals induced by prostaglandin E2 in neurons of rat dorsal root ganglia. Neurosci. Lett. 2015, 597, 159–163. [Google Scholar] [CrossRef]
- Tse, K.-H.; Chow, K.B.S.; Leung, W.K.; Wong, Y.H.; Wise, H. Lipopolysaccharide differentially modulates expression of cytokines and cyclooxygenases in dorsal root ganglion cells via Toll-like receptor-4 dependent pathways. Neuroscience 2014, 267, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, F.; Leisengang, S.; Ott, D.; Murgott, J.; Gerstberger, R.; Rummel, C.; Roth, J. Manifestation of lipopolysaccharide-induced tolerance in neuro-glial primary cultures of the rat afferent somatosensory system. Inflamm. Res. 2021, 70, 429–444. [Google Scholar] [CrossRef]
- Rummel, C.; Gerstberger, R.; Roth, J.; Hübschle, T. Parthenolide attenuates LPS-induced fever, circulating cytokines and markers of brain inflammation in rats. Cytokine 2011, 56, 739–748. [Google Scholar] [CrossRef]
- Harré, E.-M.; Roth, J.; Gerstberger, R.; Hübschle, T. Interleukin-6 mediates lipopolysaccharide-induced nuclear STAT3 translocation in astrocytes of rat sensory circumventricular organs. Brain Research 2003, 980, 151–155. [Google Scholar] [CrossRef]
- Rummel, C. Inflammatory transcription factors as activation markers and functional readouts in immune-to-brain communication. Brain Behav. Immun. 2016, 54, 1–14. [Google Scholar] [CrossRef]
- Schneiders, J.; Fuchs, F.; Damm, J.; Herden, C.; Gerstberger, R.; Soares, D.M.; Roth, J.; Rummel, C. The transcription factor nuclear factor interleukin 6 mediates pro- and anti-inflammatory responses during LPS-induced systemic inflammation in mice. Brain Behav. Immun. 2015, 48, 147–164. [Google Scholar] [CrossRef]
- Rummel, C.; Voss, T.; Matsumura, K.; Korte, S.; Gerstberger, R.; Roth, J.; Hübschle, T. Nuclear STAT3 translocation in guinea pig and rat brain endothelium during systemic challenge with lipopolysaccharide and interleukin-6. J. Comp. Neurol. 2005, 491, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bredehöft, J.; Dolga, A.M.; Honrath, B.; Wache, S.; Mazurek, S.; Culmsee, C.; Schoemaker, R.G.; Gerstberger, R.; Roth, J.; Rummel, C. SK-Channel Activation Alters Peripheral Metabolic Pathways in Mice, but Not Lipopolysaccharide-Induced Fever or Inflammation. J. Inflamm. Res. 2022, 15, 509–531. [Google Scholar] [CrossRef] [PubMed]
- Peek, V.; Harden, L.M.; Damm, J.; Aslani, F.; Leisengang, S.; Roth, J.; Gerstberger, R.; Meurer, M.; von Köckritz-Blickwede, M.; Schulz, S.; et al. LPS Primes Brain Responsiveness to High Mobility Group Box-1 Protein. Pharmaceuticals 2021, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.; Hübschle, T.; Gerstberger, R.; Roth, J. Nuclear translocation of the transcription factor STAT3 in the guinea pig brain during systemic or localized inflammation. J. Physiol. 2004, 557, 671–687. [Google Scholar] [CrossRef]
- Rummel, C.; Barth, S.W.; Voss, T.; Korte, S.; Gerstberger, R.; Hübschle, T.; Roth, J. Localized vs. systemic inflammation in guinea pigs: A role for prostaglandins at distinct points of the fever induction pathways? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R340–R347. [Google Scholar] [CrossRef]
- Benson, S.; Engler, H.; Wegner, A.; Rebernik, L.; Spreitzer, I.; Schedlowski, M.; Elsenbruch, S. What Makes You Feel Sick After Inflammation? Predictors of Acute and Persisting Physical Sickness Symptoms Induced by Experimental Endotoxemia. Clin. Pharmacol. Ther. 2017, 102, 141–151. [Google Scholar] [CrossRef]
- Lasselin, J.; Schedlowski, M.; Karshikoff, B.; Engler, H.; Lekander, M.; Konsman, J.P. Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: Relevance for symptoms of anxiety and depression. Neurosci. Biobehav. Rev. 2020, 115, 15–24. [Google Scholar] [CrossRef]
- Rummel, C.; Matsumura, K.; Luheshi, G.N. Circulating IL-6 contributes to peripheral LPS-induced mPGES-1 expression in the rat brain. Brain Res. Bull. 2011, 86, 319–325. [Google Scholar] [CrossRef]
- Eskilsson, A.; Mirrasekhian, E.; Dufour, S.; Schwaninger, M.; Engblom, D.; Blomqvist, A. Immune-induced fever is mediated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J. Neurosci. 2014, 34, 15957–15961. [Google Scholar] [CrossRef] [Green Version]
- Maba, I.K.; Cruz, J.V.; Zampronio, A.R. Change in prostaglandin signaling during sickness syndrome hyperalgesia after ovariectomy in female rats. Behav. Brain Res. 2021, 410, 113368. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Lee, Y.-J.; Lee, J.W.; Lu, S.; Tucci, M.A.; Dai, X.; Ojeda, N.B.; Lee, H.J.; Fan, L.-W.; Tien, L.-T. Interleukin-1 receptor antagonist ameliorates the pain hypersensitivity, spinal inflammation and oxidative stress induced by systemic lipopolysaccharide in neonatal rats. Neurochem. Int. 2020, 135, 104686. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.D.; Christensen, A.D.; Tewari, D.; McMahon, S.B.; Hamilton, J.A. Immune Cytokines and Their Receptors in Inflammatory Pain. Trends Immunol. 2018, 39, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ji, A.; Weihe, E.; Schäfer, M.K.-H. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J. Neurosci. 2004, 24, 9623–9631. [Google Scholar] [CrossRef] [Green Version]
- Obreja, O.; Schmelz, M.; Poole, S.; Kress, M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain 2002, 96, 57–62. [Google Scholar] [CrossRef]
- Obreja, O.; Biasio, W.; Andratsch, M.; Lips, K.S.; Rathee, P.K.; Ludwig, A.; Rose-John, S.; Kress, M. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain 2005, 128, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Obreja, O.; Rathee, P.K.; Lips, K.S.; Distler, C.; Kress, M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: Involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002, 16, 1497–1503. [Google Scholar] [CrossRef]
- Pitchford, S.; Levine, J.D. Prostaglandins sensitize nociceptors in cell culture. Neurosci. Lett. 1991, 132, 105–108. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; McNaughton, P.A. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008, 59, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef]
- Schuligoi, R.; Ulcar, R.; Peskar, B.; Amann, R. Effect of endotoxin treatment on the expression on cyclooxygenase-2 and prostaglandin synthases in spinal cord, dorsal root ganglia, and skin of rats. Neuroscience 2003, 116, 1043–1052. [Google Scholar] [CrossRef]
- Araldi, D.; Ferrari, L.F.; Lotufo, C.M.; Vieira, A.S.; Athié, M.C.P.; Figueiredo, J.G.; Duarte, D.B.; Tambeli, C.H.; Ferreira, S.H.; Parada, C.A. Peripheral inflammatory hyperalgesia depends on the COX increase in the dorsal root ganglion. Proc. Natl. Acad. Sci. USA 2013, 110, 3603–3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehrenbacher, J.C.; Burkey, T.H.; Nicol, G.D.; Vasko, M.R. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain 2005, 113, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sculptoreanu, A.; Kullmann, F.A.; Artim, D.E.; Bazley, F.A.; Schopfer, F.; Woodcock, S.; Freeman, B.A.; de Groat, W.C. Nitro-oleic acid inhibits firing and activates TRPV1- and TRPA1-mediated inward currents in dorsal root ganglion neurons from adult male rats. J. Pharmacol. Exp. Ther. 2010, 333, 883–895. [Google Scholar] [CrossRef]
- Diogenes, A.; Ferraz, C.C.R.; Akopian, A.N.; Henry, M.A.; Hargreaves, K.M. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res. 2011, 90, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Wadachi, R.; Hargreaves, K.M. Trigeminal nociceptors express TLR-4 and CD14: A mechanism for pain due to infection. J. Dent. Res. 2006, 85, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Diogenes, A.; Jeske, N.A.; Henry, M.A.; Akopian, A.; Hargreaves, K.M. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience 2008, 155, 503–509. [Google Scholar] [CrossRef]
- Donnerer, J.; Liebmann, I.; Schicho, R. ERK and STAT3 phosphorylation in sensory neurons during capsaicin-induced impairment and nerve growth factor treatment. Pharmacology 2005, 75, 116–121. [Google Scholar] [CrossRef]
- Dubový, P.; Hradilová-Svíženská, I.; Klusáková, I.; Brázda, V.; Joukal, M. Interleukin-6 contributes to initiation of neuronal regeneration program in the remote dorsal root ganglia neurons after sciatic nerve injury. Histochem. Cell Biol. 2019, 152, 109–117. [Google Scholar] [CrossRef]
- Rummel, C.; Inoue, W.; Sachot, C.; Poole, S.; Hübschle, T.; Luheshi, G.N. Selective contribution of interleukin-6 and leptin to brain inflammatory signals induced by systemic LPS injection in mice. J. Comp. Neurol. 2008, 511, 373–395. [Google Scholar] [CrossRef]
- Lebel, E.; Vallières, L.; Rivest, S. Selective involvement of interleukin-6 in the transcriptional activation of the suppressor of cytokine signaling-3 in the brain during systemic immune challenges. Endocrinology 2000, 141, 3749–3763. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Kong, L.-Y.; Cai, J.; Li, S.; Liu, X.-D.; Han, J.-S.; Xing, G.-G. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: Roles in the development of bone cancer pain in a rat model. Pain 2015, 156, 1124–1144. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Salgado, M.; Vidal-Taboada, J.M.; Saura, J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS. Prog. Neurobiol. 2015, 132, 1–33. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, E.M.; Hu, P. Inflammation in dorsal root ganglia after peripheral nerve injury: Effects of the sympathetic innervation. Auton. Neurosci. 2014, 182, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; de Carvalho-Barbosa, M.; Kavelaars, A.; Heijnen, C.J.; Albrecht, P.J.; Dougherty, P.M. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J. Pain 2016, 17, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Aarden, L.A.; de Groot, E.R.; Schaap, O.L.; Lansdorp, P.M. Production of hybridoma growth factor by human monocytes. Eur. J. Immunol. 1987, 17, 1411–1416. [Google Scholar] [CrossRef]
- Espevik, T.; Nissen-Meyer, J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J. Immunol. Methods 1986, 95, 99–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nürnberger, F.; Ott, D.; Claßen, R.; Rummel, C.; Roth, J.; Leisengang, S. Systemic Lipopolysaccharide Challenge Induces Inflammatory Changes in Rat Dorsal Root Ganglia: An Ex Vivo Study. Int. J. Mol. Sci. 2022, 23, 13124. https://doi.org/10.3390/ijms232113124
Nürnberger F, Ott D, Claßen R, Rummel C, Roth J, Leisengang S. Systemic Lipopolysaccharide Challenge Induces Inflammatory Changes in Rat Dorsal Root Ganglia: An Ex Vivo Study. International Journal of Molecular Sciences. 2022; 23(21):13124. https://doi.org/10.3390/ijms232113124
Chicago/Turabian StyleNürnberger, Franz, Daniela Ott, Rebecca Claßen, Christoph Rummel, Joachim Roth, and Stephan Leisengang. 2022. "Systemic Lipopolysaccharide Challenge Induces Inflammatory Changes in Rat Dorsal Root Ganglia: An Ex Vivo Study" International Journal of Molecular Sciences 23, no. 21: 13124. https://doi.org/10.3390/ijms232113124




