Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium
Abstract
:1. Introduction
2. Results
2.1. Effects of PDRN, Se, and Their Association on Testis Weight
2.2. Effects of PDRN, Se, and Their Association on Testosterone Levels
2.3. Effects of PDRN, Se, and Their Association on Oxidative Stress Parameters
2.4. Effects of PDRN, Se, and Their Association on Nrf2 and pErk 1/2 Expression
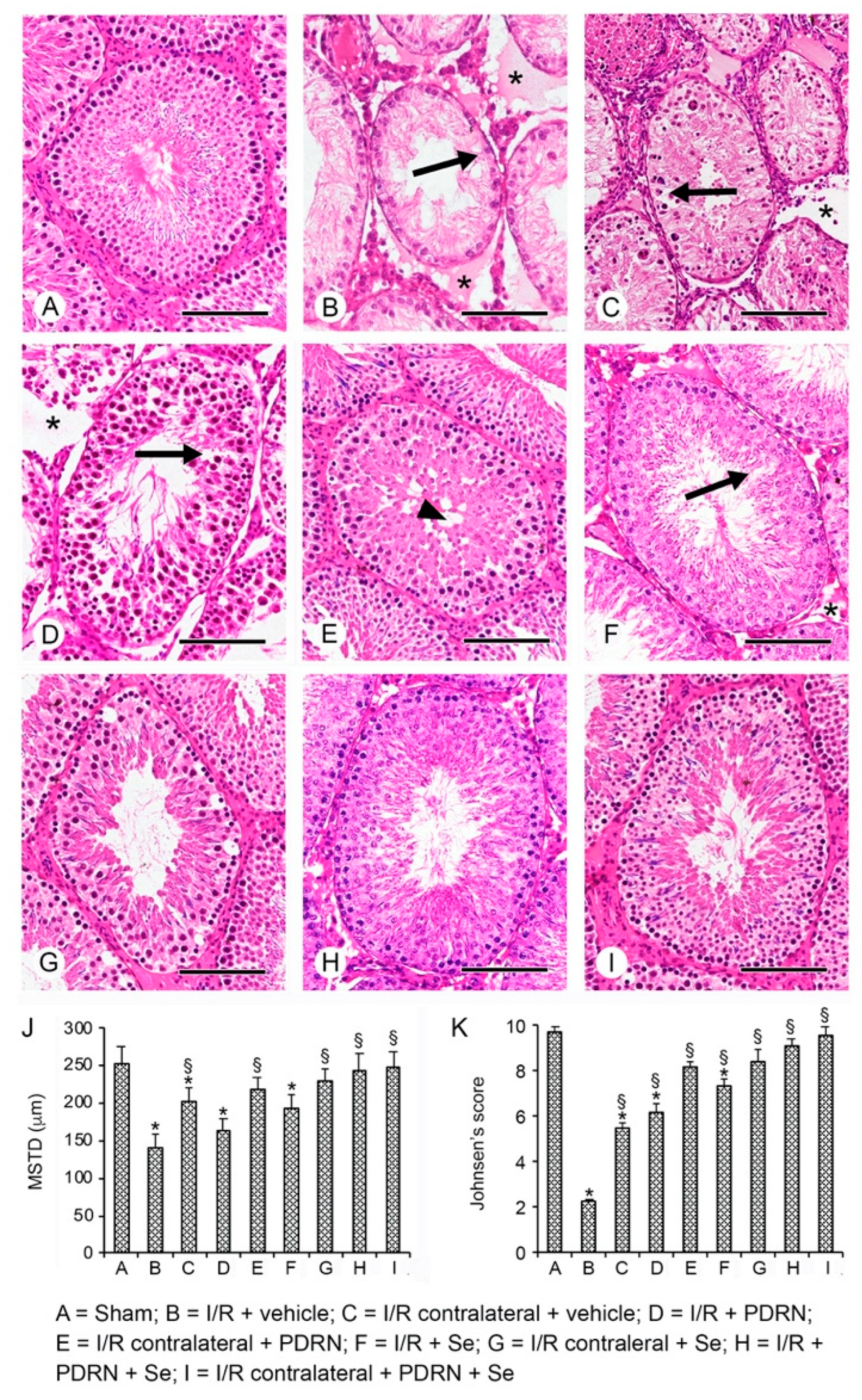
2.5. Effects of PDRN, Se, and Their Association on Testis Structure
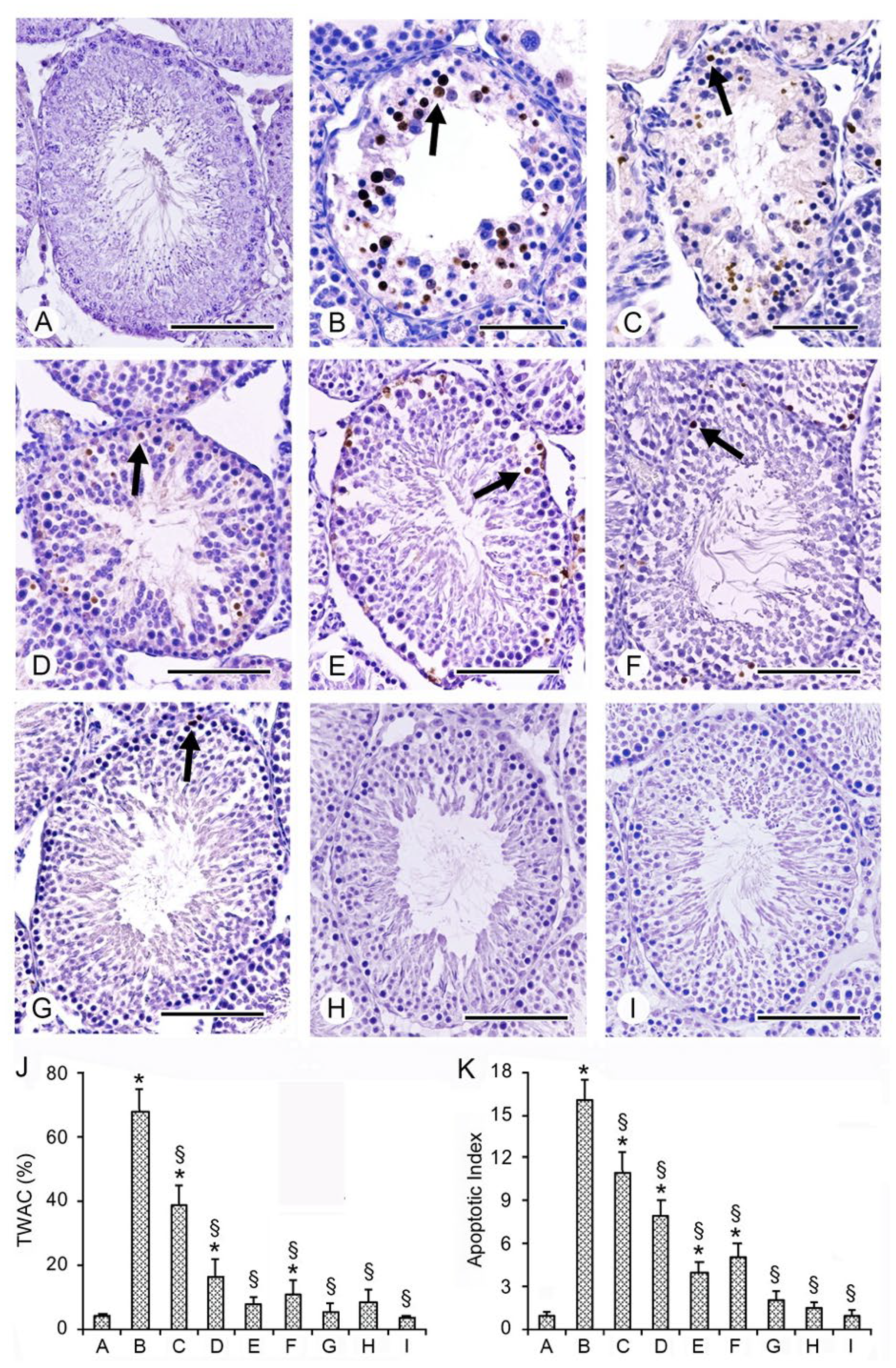
2.6. Effects of PDRN, Se, and Their Association on Apoptosis with TUNEL Assay
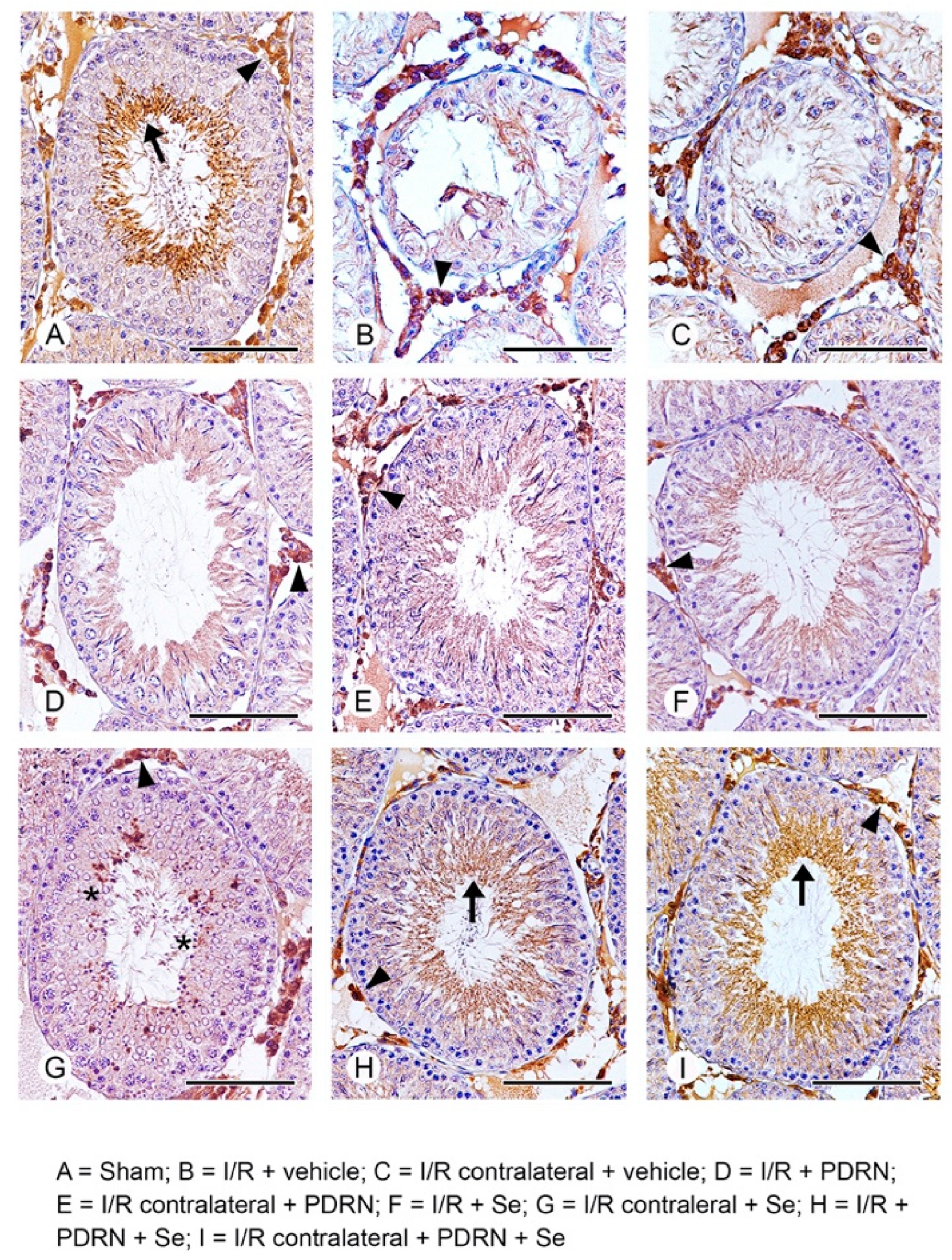
2.7. Effects of PDRN, Se, and Their Association on HIF-1α Activity
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Experimental Protocol
4.3. Isolation of Soluble Proteins
4.4. Determination of GSH and MDA Levels
4.5. Determination of Testosterone Levels
4.6. Determination of Nrf2 and pErk 1/2 by Western Blot Analysis
4.7. Histological Evaluation
4.8. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay for Apoptosis
4.9. Immunohistochemistry for HIF-1α
4.10. Drugs
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, R.; Zhang, J.; Cheng, L.; Lin, J. Testicular and paratesticular pathology in the pediatric population: A 20 year experience at Riley hospital for children. Pathol. Res. Pract. 2013, 209, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Chen, Y.F.; Chang, H.C.; Yang, T.K.; Hsieh, J.T.; Huang, K.H. The incidence rate and characteristics in patients with testicular torsion: A nationwide, population-based study. Acta Paediatr. 2013, 102, e363–e367. [Google Scholar] [CrossRef] [PubMed]
- Karaguzel, E.; Kadihasanoglu, M.; Kutlu, O. Mechanisms of testicular torsion and potential protective agents. Nat. Rev. Urol. 2014, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.W.; Torres, M.A.; Bordin, A.L.; Crezcynski-Pasa, T.B.; Boveris, A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol. Asp. Med. 2004, 25, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ikhlas, M.; Atherton, N.S. Vascular Reperfusion Injury. 2021. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Minutoli, L.; Antonuccio, P.; Squadrito, F.; Bitto, A.; Nicotina, P.A.; Fazzari, C.; Polito, F.; Marini, H.; Bonvissuto, G.; Arena, S.; et al. Effects of polydeoxyribonucleotide on the histological damage and the altered spermatogenesis induced by testicular ischaemia and reperfusion in rats. Int. J. Androl. 2012, 35, 133–144. [Google Scholar] [CrossRef]
- Bašković, M.; Krsnik, D.; Perić, M.H.; Bojanac, A.K.; Sinčić, N.; Sonicki, Z.; Ježek, D. Astaxanthin Relieves Testicular Ischemia-Reperfusion Injury-Immunohistochemical and Biochemical Analyses. J. Clin. Med. 2022, 11, 1284. [Google Scholar] [CrossRef]
- Shimizu, S.; Saito, M.; Dimitriadis, F.; Kinoshita, Y.; Shomori, K.; Satoh, I.; Satoh, K. Protective effect of ischaemic post-conditioning on ipsilateral and contralateral testes after unilateral testicular ischaemia-reperfusion injury. Int. J. Androl. 2011, 34, 268–275. [Google Scholar] [CrossRef]
- Minutoli, L.; Irrera, N.; Squadrito, F.; Marini, H.; Nicotina, P.A.; Arena, S.; Romeo, C.; Antonuccio, P.; Altavilla, D. Effects of ischaemic post-conditioning on the early and late testicular damage after experimental testis ischaemia-reperfusion. Andrology 2014, 2, 76–82. [Google Scholar] [CrossRef]
- Palladino, M.A.; Pirlamarla, P.R.; McNamara, J.; Sottas, C.M.; Korah, N.; Hardy, M.P.; Hales, D.B.; Hermo, L. Normoxic expression of hypoxia-inducible factor 1 in rat Leydig cells in vivo and in vitro. J. Androl. 2011, 32, 307–323. [Google Scholar] [CrossRef]
- Vaos, G.; Zavras, N. Antioxidants in experimental ischemia-reperfusion injury of the testis: Where are we heading towards? World J. Methodol. 2017, 7, 37–45. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to Minimize Various Stress-Related Freeze-Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa. Biopreservation Biobanking 2019, 17, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and male infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taati, M.; Moghadasi, M.; Dezfoulian, O.; Asadian, P.; Zendehdel, M. Effects of Ghrelin on germ cell apoptosis and proinflammatory cytokines production in Ischemia-reperfusion of the rat testis. Iran. J. Reprod. 2015, 13, 85–92. [Google Scholar]
- Abdelzaher, W.Y.; Rofaeil, R.R.; Ali, D.M.E.; Attya, M.E. Protective effect of dipeptidyl peptidase-4 inhibitors in testicular torsion/detorsion in rats: A possible role of HIF-1α and nitric oxide. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.Z.; Morsy, M.A.; Mohamed, H.H.; Hafez, H.M. Paeonol protects against testicular ischaemia-reperfusion injury in rats through inhibition of oxidative stress and inflammation. Andrologia 2020, 52, e13599. [Google Scholar] [CrossRef] [PubMed]
- Al-Maghrebi, M.; Renno, W.M.; Al-Somali, H.F.; Botras, M.S.; Qadhi, I.N. Lutein modulates transcription dysregulation of adhesion molecules and spermatogenesis transcription factors induced by testicular ischemia reperfusion injury: It could be SAFE. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Mishra, D.P.; Shaha, C. Male germ cell development: Turning on the apoptotic pathways. J. Reprod. Immunol. 2009, 83, 31–35. [Google Scholar] [CrossRef]
- Blanco-Rodríguez, J. A matter of death and life: The significance of germ cell death during spermatogenesis. Int. J. Androl. 1998, 21, 236–248. [Google Scholar] [CrossRef]
- Minutoli, L.; Antonuccio, P.; Irrera, N.; Rinaldi, M.; Bitto, A.; Marini, H.; Pizzino, G.; Romeo, C.; Pisani, A.; Santoro, G.; et al. NLRP3 Inflammasome Involvement in the Organ Damage and Impaired Spermatogenesis Induced by Testicular Ischemia and Reperfusion in Mice. J. Pharmacol. Exp. Ther. 2015, 355, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Yurtçu, M.; Abasiyanik, A.; Avunduk, M.C.; Muhtaroğlu, S. Effects of melatonin on spermatogenesis and testicular ischemia-reperfusion injury after unilateral testicular torsion-detorsion. J. Pediatr. Surg. 2008, 43, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Abbaszadeh, A.; Khayat, Z.K.; Anbari, K.; Baharvand, P.; Gharravi, A.M. Honey improves spermatogenesis and hormone secretion in testicular ischaemia-reperfusion-induced injury in rats. Andrologia 2018, 50, e12804. [Google Scholar] [CrossRef] [PubMed]
- Palladino, M.A.; Shah, A.; Tyson, R.; Horvath, J.; Dugan, C.; Karpodinis, M. Myeloid cell leukemia-1 (Mc1-1) is a candidate target gene of hypoxia-inducible factor-1 (HIF-1) in the testis. Reprod. Biol. Endocrinol. 2012, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koksal, M.; Oğuz, E.; Baba, F.; Eren, M.A.; Ciftci, H.; Demir, M.E.; Kurcer, Z.; Take, G.; Aral, F.; Ocak, A.R.; et al. Effects of melatonin on testis histology, oxidative stress and spermatogenesis after experimental testis ischemia-reperfusion in rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 582–588. [Google Scholar]
- Kostakis, I.D.; Zavras, N.; Damaskos, C.; Sakellariou, S.; Korkolopoulou, P.; Misiakos, E.P.; Tsaparas, P.; Vaos, G.; Karatzas, T. Erythropoietin and sildenafil protect against ischemia/reperfusion injury following testicular torsion in adult rats. Exp. Ther. Med. 2017, 13, 3341–3347. [Google Scholar] [CrossRef] [Green Version]
- Kölükçü, E.; Atılgan, D.; Uluocak, N.; Deresoy, F.A.; Katar, M.; Unsal, V. Milrinone ameliorates ischaemia-reperfusion injury in experimental testicular torsion/detorsion rat model. Andrologia 2021, 53, e14128. [Google Scholar] [CrossRef] [PubMed]
- Balci, C.N.; Firat, T.; Acar, N.; Kukner, A. Carvacrol treatment opens Kir6.2 ATP-dependent potassium channels and prevents apoptosis on rat testis following ischemia-reperfusion injury model. Rom. J. Morphol. Embryol. 2021, 62, 179–190. [Google Scholar] [CrossRef]
- Xu, L.Z.; He, K.X.; Ning, J.Z.; Cheng, F. Oleuropein attenuates testicular ischemia-reperfusion by inhibiting apoptosis and inflammation. Tissue Cell 2022, 78, 101876. [Google Scholar] [CrossRef]
- Wei, S.M.; Huang, Y.M.; Qin, Z.Q. Salidroside Exerts Beneficial Effect on Testicular Ischemia-Reperfusion Injury in Rats. Oxidative Med. Cell. Longev. 2022, 2022, 8069152. [Google Scholar] [CrossRef]
- Poltronieri, R.; Cevese, A.; Sbarbati, A. Protective effect of selenium in cardiac ischemia and reperfusion. Cardioscience 1992, 3, 155–160. [Google Scholar]
- Kaur, S.; Bansal, M.P. Protective role of dietary-supplemented selenium and vitamin E in heat-induced apoptosis and oxidative stress in mice testes. Andrologia 2015, 47, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Avlan, D.; Erdouğan, K.; Cimen, B.; Apa, D.D.; Cinel, I.; Aksöyek, S. The protective effect of selenium on ipsilateral and contralateral testes in testicular reperfusion injury. Pediatr. Surg. Int. 2005, 21, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Kara, Ö.; Sari, E.; Akşit, H.; Yay, A.; Akşit, D.; Dönmez, M.I. Effects of selenium on ischaemia-reperfusion injury in a rat testis model. Andrologia 2016, 48, 1267–1273. [Google Scholar] [CrossRef]
- Tunçkiran, A.; Cayan, S.; Bozlu, M.; Yilmaz, N.; Acar, D.; Akbay, E. Protective effect of vascular endothelial growth factor on histologic changes in testicular ischemia-reperfusion injury. Fertil. Steril. 2005, 84, 468–473. [Google Scholar] [CrossRef]
- Antonuccio, P.; Minutoli, L.; Romeo, C.; Nicòtina, P.A.; Bitto, A.; Arena, S.; Altavilla, D.; Zuccarello, B.; Polito, F.; Squadrito, F. Lipid peroxidation activates mitogen-activated protein kinases in testicular ischemia-reperfusion injury. J. Urol. 2006, 176, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddas, A.; Dashti-Khavidaki, S. L-Carnitine and Potential Protective Effects Against Ischemia-Reperfusion Injury in Noncardiac Organs: From Experimental Data to Potential Clinical Applications. J. Diet. Suppl. 2018, 15, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Azizollahi, S.; Babaei, H.; Derakhshanfar, A.; Oloumi, M.M. Effects of co-administration of dopamine and vitamin C on ischaemia-reperfusion injury after experimental testicular torsion-detorsion in rats. Andrologia 2011, 43, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Bitto, A.; Squadrito, F.; Irrera, N.; Rinaldi, M.; Nicotina, P.A.; Arena, S.; Magno, C.; Marini, H.; Spaccapelo, L.; et al. Melanocortin 4 receptor activation protects against testicular ischemia-reperfusion injury by triggering the cholinergic antiinflammatory pathway. Endocrinology 2011, 152, 3852–3861. [Google Scholar] [CrossRef] [Green Version]
- Ozbal, S.; Ergur, B.U.; Erbil, G.; Tekmen, I.; Bagrıyanık, A.; Cavdar, Z. The effects of α-lipoic acid against testicular ischemia-reperfusion injury in Rats. Sci. World J. 2012, 2012, 489248. [Google Scholar] [CrossRef] [Green Version]
- Taati, M.; Moghadasi, M.; Dezfoulian, O.; Asadian, P. Effects of Ghrelin on Testicular Ischemia/Reperfusion-Induced Injury. Acta Med. Iran. 2016, 54, 32–38. [Google Scholar] [PubMed]
- Mirhoseini, M.; Amiri, F.T.; Malekshah, A.A.K.; Gatabi, Z.R.; Ghaffari, E. Protective effects of melatonin on testis histology following acute torsion-detorsion in rats. Int. J. Reprod. Biomed. 2017, 15, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohsaka, T.; Yoneda, Y.; Yoshida, T.; Minagawa, I.; Pitia, A.M.; Iwasawa, A.; Ikegaya, N. Relaxin exerts a protective effect during ischemia-reperfusion in the rat model. Andrology 2022, 10, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fokam, D.; Hoskin, D. Instrumental role for reactive oxygen species in the inflammatory response. Front. Biosci. 2020, 25, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, F.; Taheri, S.; Raisi, A.; Rajabzadeh, A.; Ahmadvand, H.; Hablolvarid, M.H.; Zakian, A. Investigating the sperm parameters, oxidative stress and histopathological effects of salvia miltiorrhiza hydroalcoholic extract in the prevention of testicular ischemia reperfusion damage in rats. Theriogenology 2020, 144, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z. Oxidative damage of sulfur dioxide on various organs of mice: Sulfur dioxide is a systemic oxidative damage agent. Inhal. Toxicol. 2003, 15, 181–195. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Lysiak, J.J.; Nguyen, Q.A.; Turner, T.T. Fluctuations in rat testicular interstitial oxygen tensions are linked to testicular vasomotion: Persistence after repair of torsion. Biol. Reprod. 2000, 63, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Jo, A.; Yoo, H.J.; Lee, M. Robustaflavone Isolated from Nandina domestica Using Bioactivity-Guided Fractionation Downregulates Inflammatory Mediators. Molecules 2019, 24, 1789. [Google Scholar] [CrossRef] [Green Version]
- Antonuccio, P.; Micali, A.G.; Romeo, C.; Freni, J.; Vermiglio, G.; Puzzolo, D.; Squadrito, F.; Irrera, N.; Marini, H.R.; Rana, R.A.; et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. Int. J. Mol. Sci. 2021, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.E.; Berra, E.; Gothié, E.; Roux, D.; Pouysségur, J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999, 274, 32631–32637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marti, H.H.; Katschinski, D.M.; Wagner, K.F.; Schäffer, L.; Stier, B.; Wenger, R.H. Isoform-specific expression of hypoxia-inducible factor-1alpha during the late stages of mouse spermiogenesis. Mol. Endocrinol. 2002, 16, 234–243. [Google Scholar] [PubMed]
- Takahashi, N.; Davy, P.M.; Gardner, L.H.; Mathews, J.; Yamazaki, Y.; Allsopp, R.C. Hypoxia Inducible Factor 1 Alpha Is Expressed in Germ Cells throughout the Murine Life Cycle. PLoS ONE 2016, 11, e0154309. [Google Scholar] [CrossRef] [Green Version]
- Reyes, J.G.; Farias, J.G.; Henríquez-Olavarrieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The hypoxic testicle: Physiology and pathophysiology. Oxidative Med. Cell. Longev. 2012, 2012, 929285. [Google Scholar] [CrossRef] [Green Version]
- Faria, M.J.; Simões, Z.L.; Lunardi, L.O.; Hartfelder, K. Apoptosis process in mouse Leydig cells during postnatal development. Microsc. Microanal. 2003, 9, 68–73. [Google Scholar] [CrossRef]
- Tsounapi, P.; Saito, M.; Dimitriadis, F.; Kitatani, K.; Kinoshita, Y.; Shomori, K.; Takenaka, A.; Satoh, K. The role of K ATP channels on ischemia-reperfusion injury in the rat testis. Life Sci. 2012, 90, 649–656. [Google Scholar] [CrossRef]
- Wang, X.; Jin, L.; Jiang, S.; Wang, D.; Lu, Y.; Zhu, L. Transcription regulation of NRF1 on StAR reduces testosterone synthesis in hypoxemic murine. J. Steroid Biochem. Mol. Biol. 2019, 191, 105370. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Minutoli, L.; Antonuccio, P.; Polito, F.; Bitto, A.; Squadrito, F.; Di Stefano, V.; Nicotina, P.A.; Fazzari, C.; Maisano, D.; Romeo, C.; et al. Mitogen-activated protein kinase 3/mitogen-activated protein kinase 1 activates apoptosis during testicular ischemia-reperfusion injury in a nuclear factor-kappaB-independent manner. Eur. J. Pharmacol. 2009, 604, 27–35. [Google Scholar] [CrossRef]
- Minutoli, L.; Altavilla, D.; Marini, H.; Rinaldi, M.; Irrera, N.; Pizzino, G.; Bitto, A.; Arena, S.; Cimino, S.; Squadrito, F.; et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: Effects of serenoa repens, selenium and lycopene. J. Biomed. Sci. 2014, 21, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.; Zee, R.S.; Omidi, N.; Bayne, C.; Hester, A.; Mukherjee, E.; Sims-Lucas, S.; Mbanefo, E.C.; Hsieh, M.H.; Casella, D.P. Varenicline limits ischemia reperfusion injury following testicular torsion in mice. J. Pediatr. Urol. 2021, 17, 631.e1–631.e8. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.; El-Adl, M.; Rezk, S.; Marghani, B.; Eldomany, W.; Eldesoky, A.; Elmetwally, M.A. The potential protective and therapeutic effects of platelet-rich plasma on ischemia/reperfusion injury following experimental torsion/detorsion of testis in the Albino rat model. Life Sci. 2020, 256, 117982. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Altavilla, D.; Bitto, A.; Polito, F.; Bellocco, E.; Laganà, G.; Fiumara, T.; Magazù, S.; Migliardo, F.; Venuti, F.S.; et al. Trehalose: A biophysics approach to modulate the inflammatory response during endotoxic shock. Eur. J. Pharmacol. 2008, 589, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Doğan, G.; İpek, H. The protective effect of Ganoderma lucidum on testicular torsion/detorsion-induced ischemia-reperfusion (I/R) injury. Acta Cirúrgica Bras. 2020, 35, e202000103. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testis: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar]
- Erdemir, F.; Atilgan, D.; Markoc, F.; Boztepe, O.; Suha-Parlaktas, B.; Sahin, S. The effect of diet induced obesity on testicular tissue and serum oxidative stress parameters. Actas Urológicas Españolas 2012, 36, 153–159. [Google Scholar] [CrossRef]

| Groups | Testis Weight (g) | Testosterone (ng/mL) | GSH (µmol/g Tissue) | MDA (nmol/g Tissue) |
|---|---|---|---|---|
| Sham | 1.71 ± 0.21 | 5.7 ± 0.4 | 344 ± 14 | 5.4 ± 0.3 |
| I/R + vehicle | 0.93 ± 0.32 a | 2.5 ± 0.4 a | 262 ± 15 a | 11.8 ± 0.4 a |
| I/R CL + vehicle | 1.38 ± 0.35 a | 271 ± 13 a | 9.8 ± 0.3 a | |
| I/R + PDRN | 1.40 ± 0.29 a,b | 4.1 ± 0.3 a,b | 294 ± 16 a | 8.7 ± 0.5 a |
| I/R CL + PDRN | 1.55 ± 0.27 a,b | 305 ± 14 a,b | 7.2 ± 0.3 a,b | |
| I/R + Se | 1.44 ± 0.33 a,b | 4.4 ± 0.5 a,b | 303 ± 13 a,b | 8.1 ± 0.5 a,b |
| I/R CL + Se | 1.59 ± 0.25 a,b | 316 ± 15 a,b | 6.8 ± 0.4 a,b | |
| I/R + PDRN + Se | 1.62 ± 0.27 b | 5.3 ± 0.4 b | 331 ± 15 b | 5.9 ± 0.2 b |
| I/R CL + PDRN + Se | 1.70 ± 0.26 b | 337 ± 13 b | 5.5 ± 0.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonuccio, P.; Pallio, G.; Marini, H.R.; Irrera, N.; Romeo, C.; Puzzolo, D.; Freni, J.; Santoro, G.; Pirrotta, I.; Squadrito, F.; et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. Int. J. Mol. Sci. 2022, 23, 13144. https://doi.org/10.3390/ijms232113144
Antonuccio P, Pallio G, Marini HR, Irrera N, Romeo C, Puzzolo D, Freni J, Santoro G, Pirrotta I, Squadrito F, et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. International Journal of Molecular Sciences. 2022; 23(21):13144. https://doi.org/10.3390/ijms232113144
Chicago/Turabian StyleAntonuccio, Pietro, Giovanni Pallio, Herbert Ryan Marini, Natasha Irrera, Carmelo Romeo, Domenico Puzzolo, Jose Freni, Giuseppe Santoro, Igor Pirrotta, Francesco Squadrito, and et al. 2022. "Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium" International Journal of Molecular Sciences 23, no. 21: 13144. https://doi.org/10.3390/ijms232113144






