Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase
Abstract
:1. Introduction
2. Results and Discussion
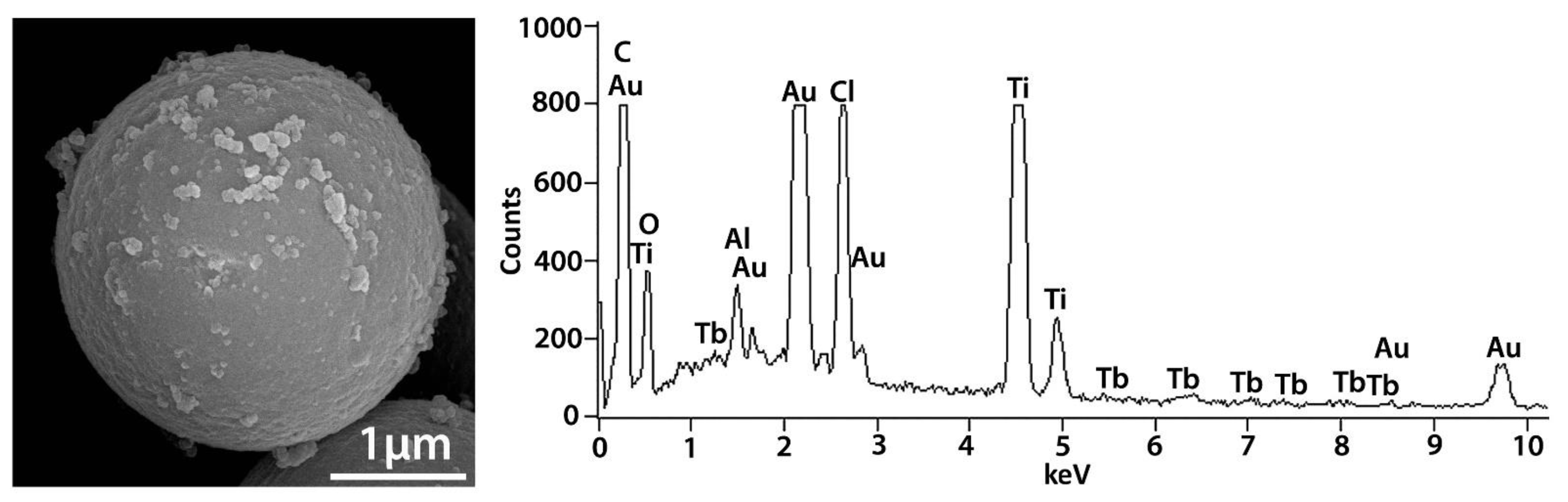
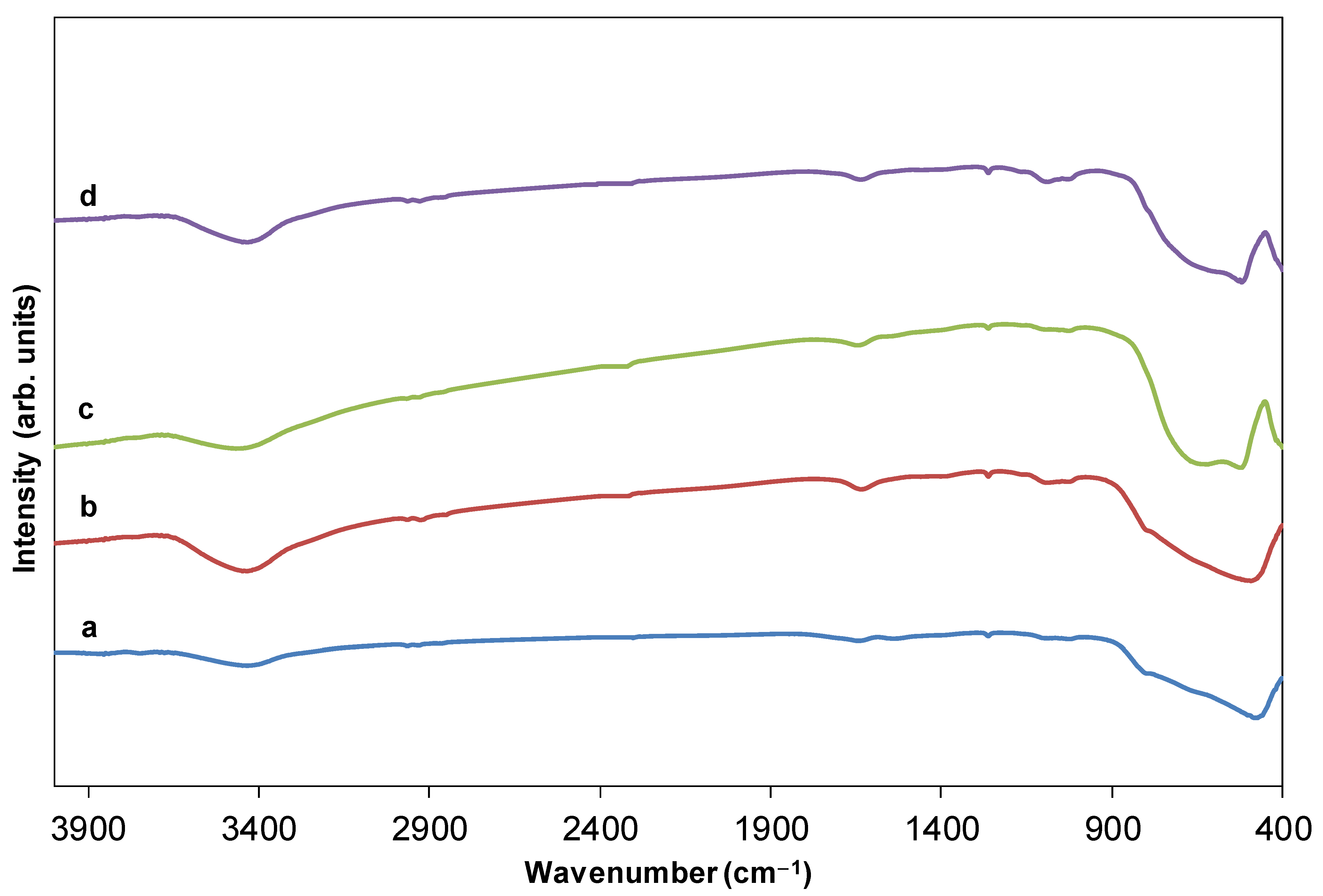
2.1. Monitoring Ti and Tb Simultaneous Implantation and Sol-Gel Synthesis of Ti[(OH)4−yCly]@PST–PDVB, Ti1−xTbx[(OH)4−uClv]@PST–PDVB, TiO2 and (Ti,Tb)O2−w′ HSs (IR, FEG-SEM-XEDS and XPS)
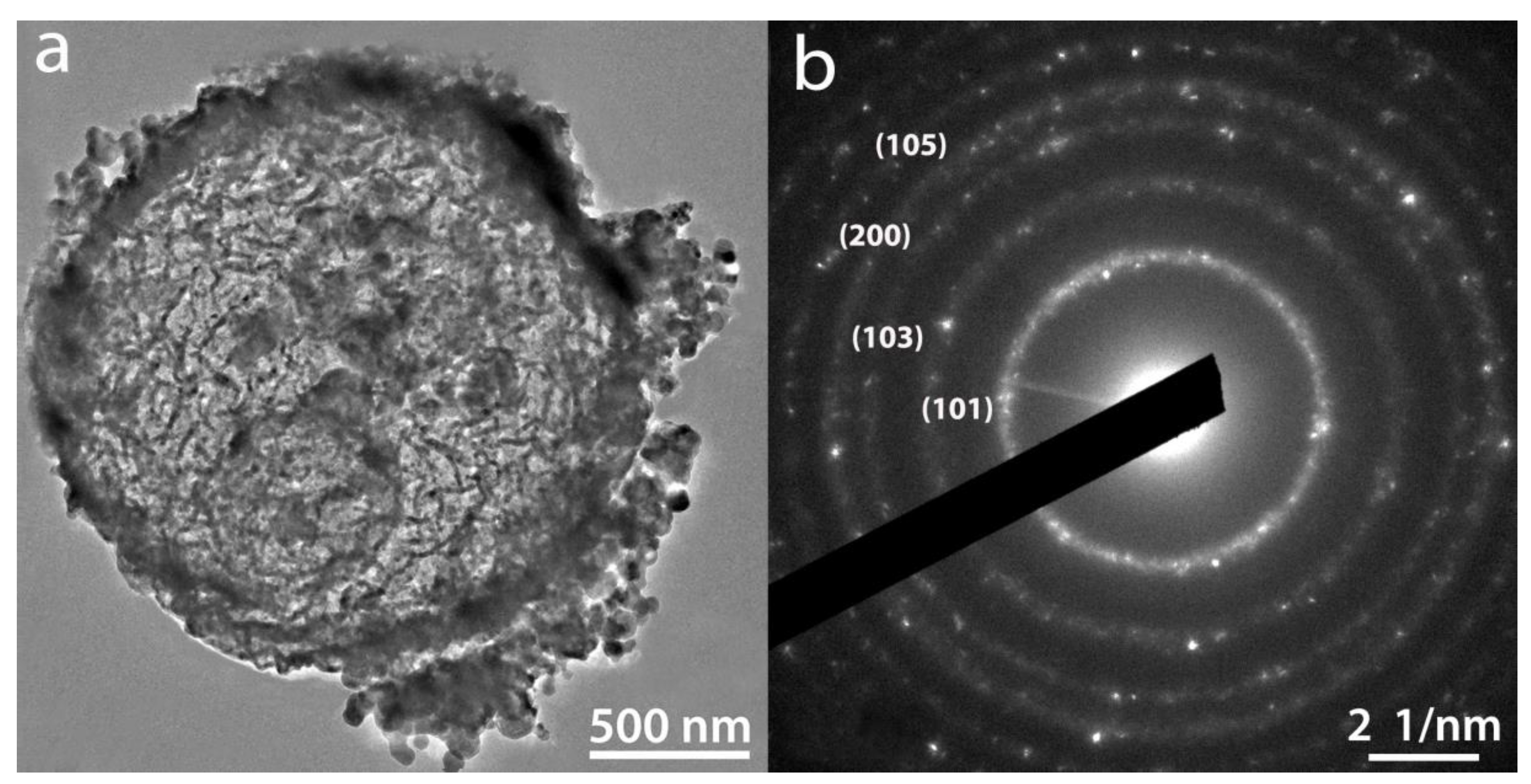
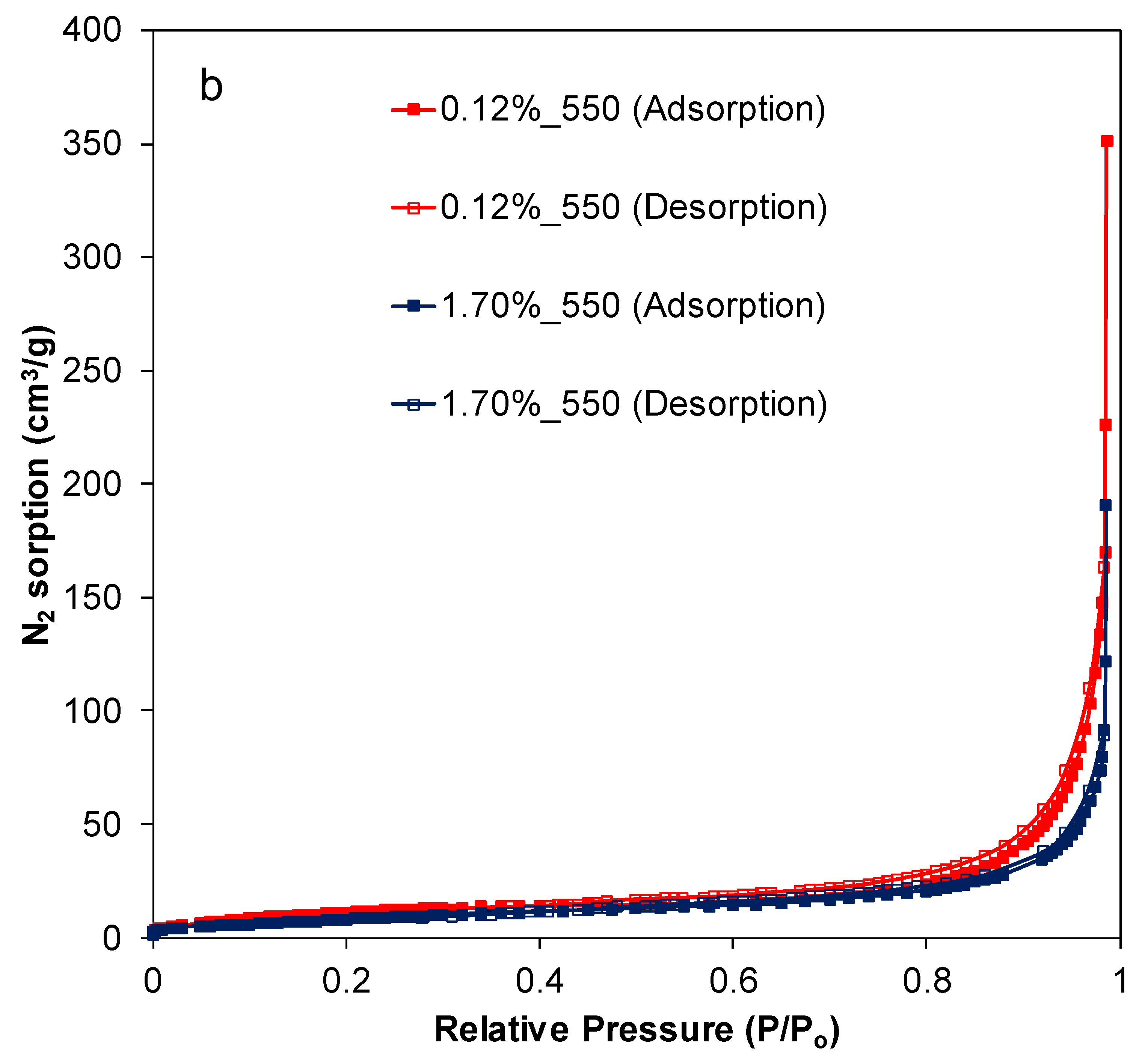
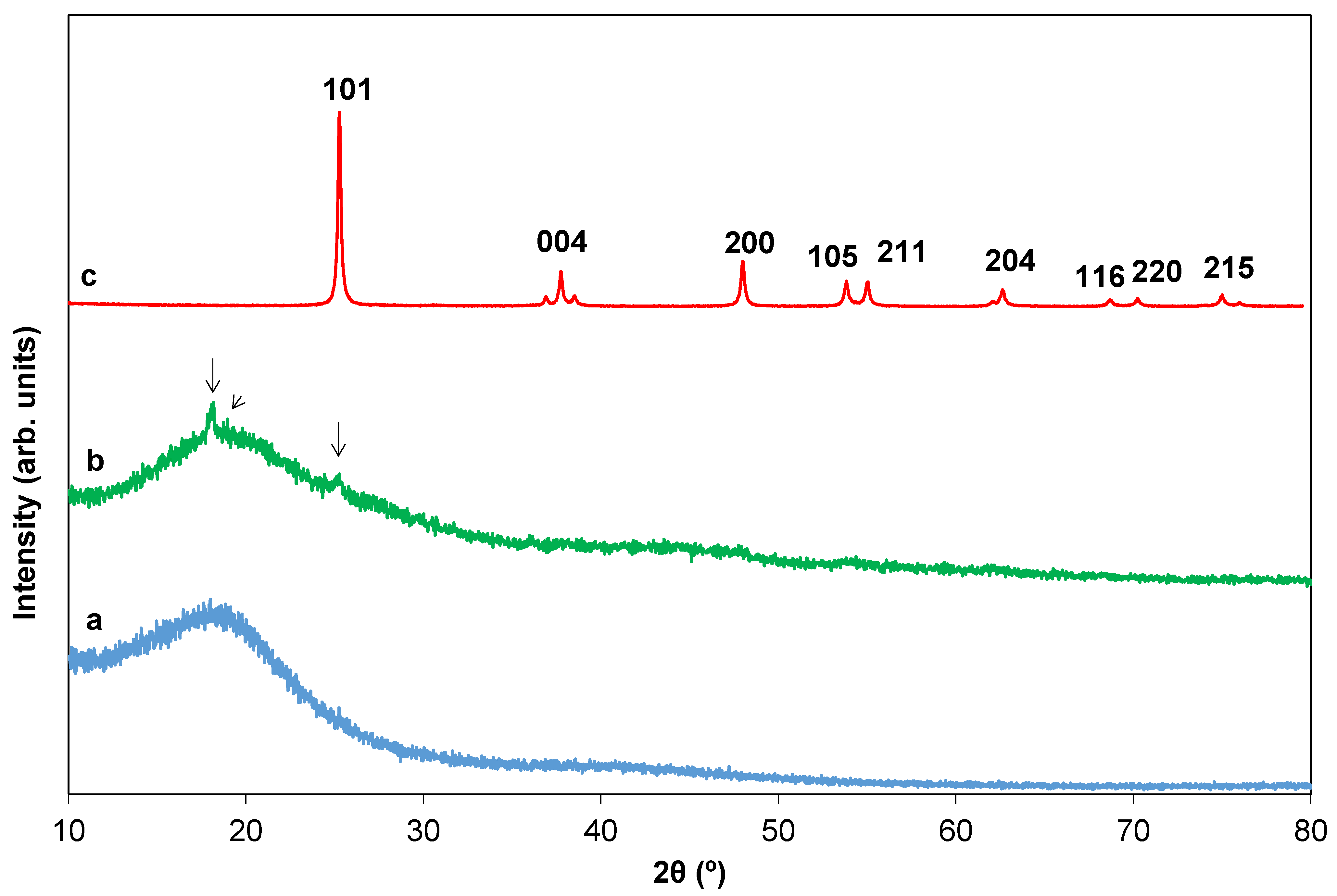
2.2. Texture and Structure of Ti[(OH)4−yCly]@PST–PDVB, Ti1−xTbx[(OH)4−uClv]@PST–PDVB, TiO2 and (Ti,Tb)O2−w′
2.3. Thermal Evolution of the Ti1−xTbx[(OH)4−uClv]@PST–PDVB followed by DTA-TG Analysis
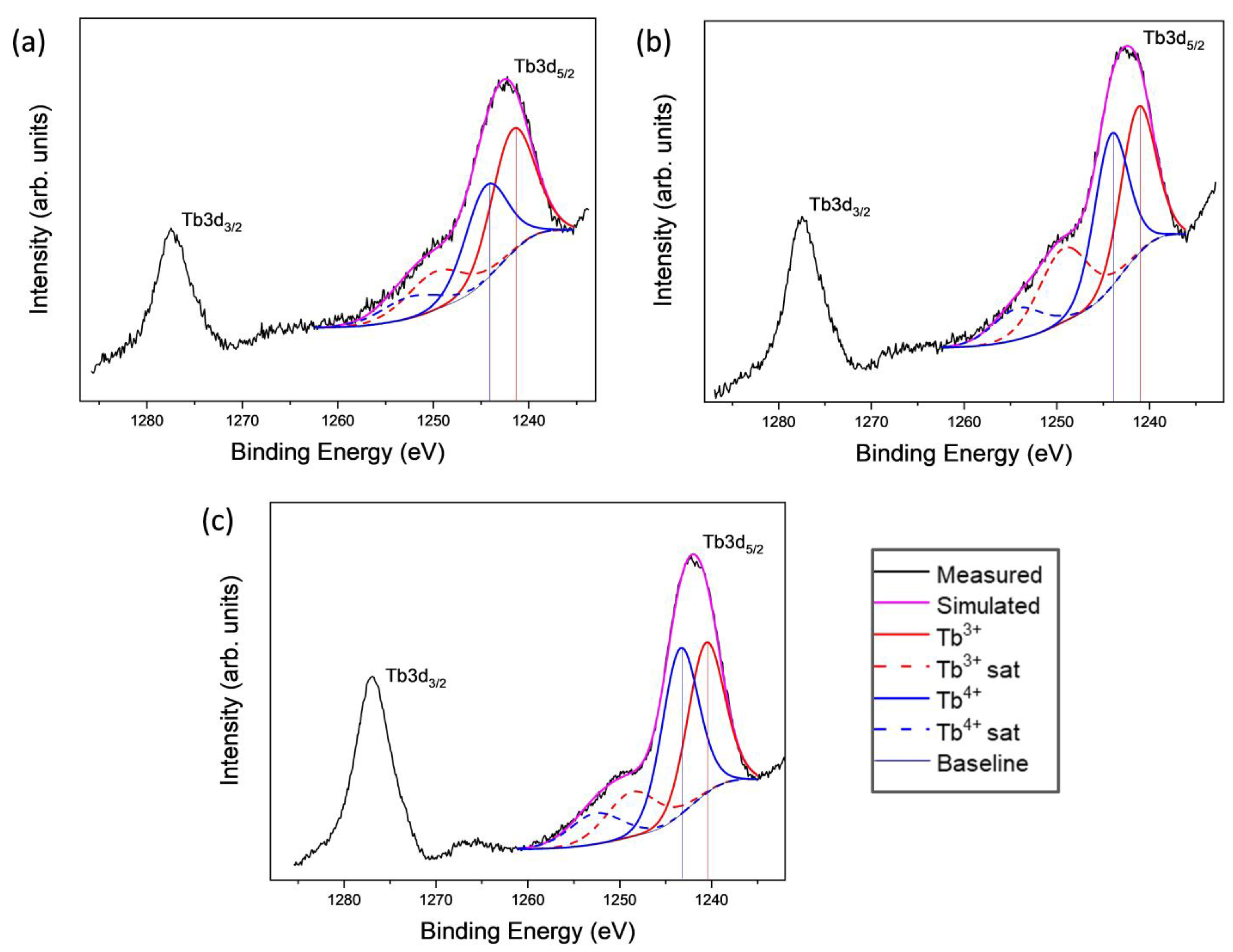
2.4. Compositional Analysis of the Calcined HSs by XPS
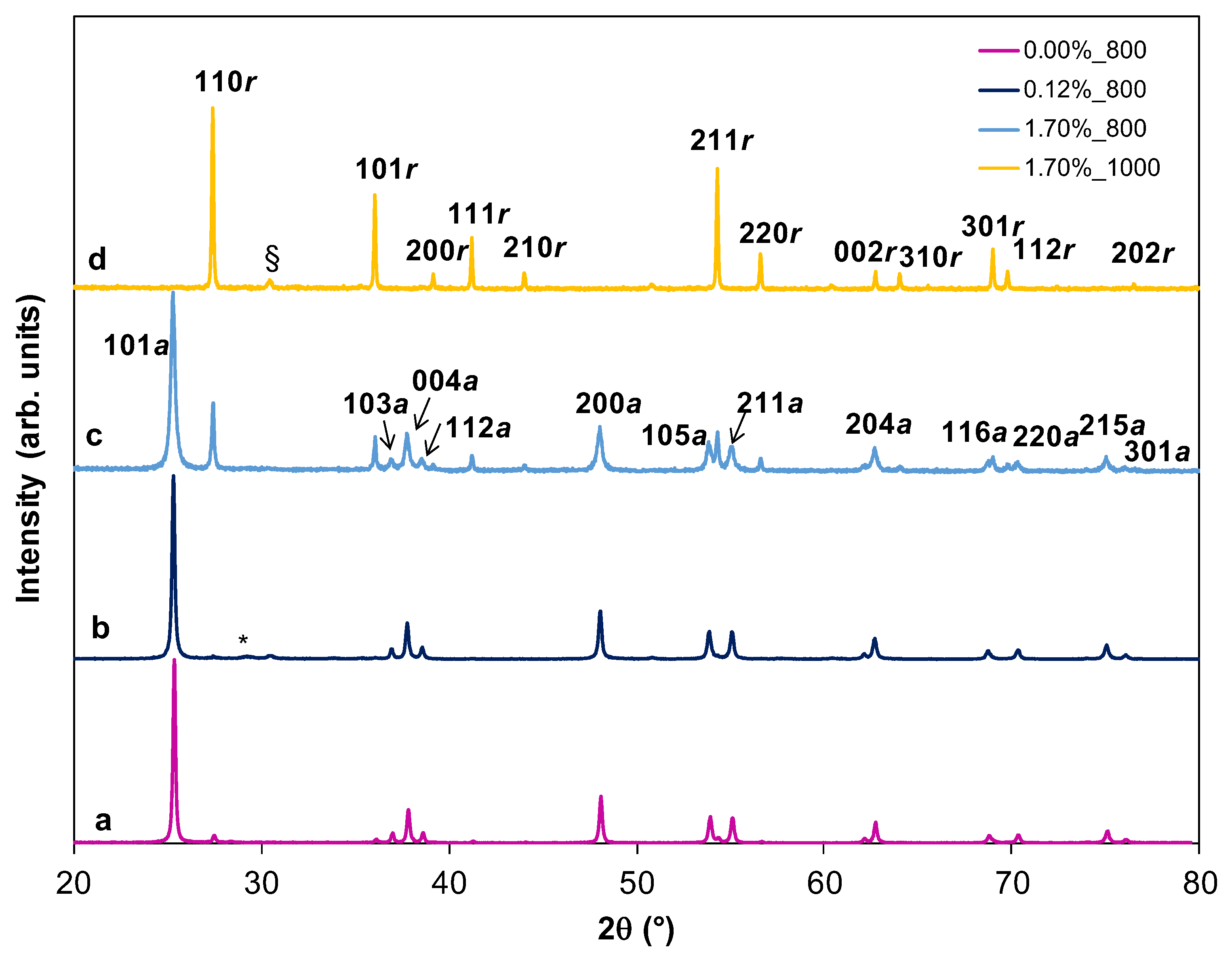
2.5. Effect of Tb Doping and Temperature on the Structure and ART of the HSs
3. Materials and Methods
3.1. Synthesis of Ti[(OH)4−yCly]@PST–PDVB, Ti1−xTbx[(OH)4−uClv]@PST–PDVB, TiO2 or (Ti,Tb)O2−w′ HSs
3.2. Characterization Techniques
3.2.1. IR
3.2.2. N2 Adsorption-Desorption
3.2.3. DTA-TG Studies
3.2.4. C and N Analysis
3.2.5. XRD Studies
3.2.6. XPS
3.2.7. FEG-SEM-XEDS and TEM-SAED-XEDS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozman, N.; Tobaldi, D.M.; Cvelbar, U.; Puliyalil, H.; Labrincha, J.A.; Legat, A.; Sever Škapin, A. Hydrothermal synthesis of rare-earth modified titania: Influence on phase composition, optical properties, and photocatalytic activity. Materials 2019, 12, 713. [Google Scholar] [CrossRef] [Green Version]
- Thiehmed, Z.A.; Altahtamouni, T.M.; Talal, M. Morphology control of TiO2 nanorods using KBr salt for enhancing the photocatalytic activity of TiO2 and MoS2/TiO2 heterostructures. Nanomaterials 2022, 12, 2904. [Google Scholar] [CrossRef] [PubMed]
- Bjelajac, A.; Petrovic, R.; Socol, G.; Mihailescu, V.; Grumezescu, V.; Pavlovic, V. CdS quantum dots sensitized TiO2 nanotubes by matrix assisted pulsed laser evaporation method. Ceram. Intern. 2016, 42, 9011–9017. [Google Scholar] [CrossRef]
- Marien, C.; Cottineau, T.; Robert, D. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of paraquat. Appl. Catal. B 2016, 194, 1–6. [Google Scholar] [CrossRef]
- Borlaf, M.; Wu, H.-M.; Colomer, M.T.; Moreno, R.; Tseng, W.J. Synthesis and characterization of anatase-structured titania hollow spheres doped with erbium (III). J. Am. Ceram. Soc. 2012, 95, 3005–3011. [Google Scholar] [CrossRef]
- An, G.; Yang, C.; Jin, S.; Chen, G.; Zhao, X. Hollow TiO2: Sm3+ spheres with enhanced photoluminescence fabricated by a facile method using polystyrene as template. J. Mater. Sci. 2013, 48, 5483–5488. [Google Scholar] [CrossRef]
- Colomer, M.T.; Rodríguez, E.; Morán-Pedroso, M.; Vattier, F.; de Andrés, A. Impact of Tb4+ and morphology on the thermal evolution of Tb-doped TiO2 nanostructured hollow spheres and nanoparticles. J. Alloys Comp. 2021, 853, 156973. [Google Scholar] [CrossRef]
- Bao, R.; Li, R.; Chen, C.; Wu, H.; Xia, J.; Long, C.; Li, H. Biotemplated synthesis of 3D rare earth-doped TiO2 hollow spheres for photocatalytic application. J. Phys. Chem. Solids 2019, 126, 78–84. [Google Scholar] [CrossRef]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mat. 2008, 20, 3987–4019. [Google Scholar] [CrossRef]
- Wang, Y.; Tseng, W.J. A novel technique for synthesizing nanoshell hollow alumina particles. J. Am. Ceram. Soc. 2009, 92, S32–S37. [Google Scholar] [CrossRef]
- Tseng, W.J.; Chao, P.-S. Synthesis and photocatalysis of TiO2 hollow spheres by a facile template-implantation route. Ceram. Intern. 2013, 39, 3779–3787. [Google Scholar] [CrossRef]
- Tseng, T.-T.; Uan, J.Y.; Tseng, W.J. Synthesis, microstructure, and photocatalysis of In2O3 hollow particles. Ceram. Intern. 2011, 37, 1775–1780. [Google Scholar] [CrossRef]
- Caccioti, I.; Bianco, A.; Pezzotti, G.; Gusmano, G. Synthesis, thermal behaviour and luminiscence properties of rare earth-doped titania nanofibers. Mat. Chem. Phys. 2011, 126, 532–541. [Google Scholar]
- Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Domaradzki, J.; Prociow, E.L.; Mrawski, A.W.; Janus, M. Photocatalytic properties of nanocrystalline TiO2 thin films doped with Tb. Cent. Eur. J. Physics 2011, 9, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Borkowska, A. Proceedings of the 2007 International Students and Young Scientists Workshop Photonics and Microsystems; Sieradzka, K., Kaczmarek, D., Eds.; IEEE: Dresden, Germany, 2007; pp. 87–89. [Google Scholar]
- Scoca, D.L.S.; Cenim, F.; Bilmes, S.A.; Figueroa, C.A.; Zanatta, A.R.; Alvarez, F. Role of rare earth elements and entropy on the anatase-to-rutile phase transformation of TiO2 thin films deposited by ion beam sputtering. ACS Omega 2020, 5, 28027–28036. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Emen, R.; Amini, G. Cross-linked polystyrene/titanium tetrachloride as a tightly bound complex catalyzed the modified Mannich reaction for the synthesis of piperidin-4-ones. J. Organomet. Chem. 2019, 892, 24–33. [Google Scholar] [CrossRef]
- Pretch, E.; Clerc, T.; Seibl, J.; Simon, W. Tablas para la Elucidación Estructural de Compuestos Orgánicos por Métodos Espectroscópicos; Alhambra: Madrid, Spain, 1988; Volume 1. [Google Scholar]
- Li, M.; Li, W.; Liu, S.M. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef]
- Robertson, E.J.; Richmond, G.L. Chunks of charge: Effects at play in the assembly of macromolecules at fluid surfaces. Langmuir 2013, 35, 10980–10989. [Google Scholar] [CrossRef]
- Iconomopoulou, S.M.; Andreopoulou, A.K.; Soto, A.; Kallitsis, J.K.; Voyiatzis, G.A. Incorporation of low molecular weight biocides into polystyrene-divinyl benzene beads with controlled release characteristics. J. Control. Release 2005, 102, 223–233. [Google Scholar] [CrossRef]
- Busani, T.; Devine, R.A.B. Dielectric and infrared properties of TiO2 films containing anatase and rutile. Semicond. Sci. Technol. 2005, 20, 870–875. [Google Scholar] [CrossRef] [Green Version]
- Ayers, M.R.; Hunt, A.J. Titanium oxide aerogels prepared from titanium metal and hydrogen peroxide. Mater. Lett. 1998, 34, 290–293. [Google Scholar] [CrossRef]
- Vinosel, V.M.; Janifer, M.A.; Anand, S.; Pauline, S. Preparation and performance of Fe3O4/TiO2 nanocomposite with enhanced photo-Fenton activity for photocatalysis by facile hydrothermal method. Appl. Phys. A 2019, 125, 319. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.W.S. Physisorption of gases, with special reference to the evaluation of surface area and distribution pore size (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Song, H.; Chen, X. Enhancement of thermal stability of polydivynil benzene microspheres. Mater. Lett. 2008, 62, 179–182. [Google Scholar] [CrossRef]
- Fajardie, F.; Tempere, J.-F.; Manoli, J.-M.; Djega-Mariadassou, G.; Blanchard, G.J. Ceria lattice oxygen ion substitution by Cl− during the reduction of Rh(Cl)/CeO2 catalysts. Formation and stability of CeOCl. Chem. Soc. Faraday Trans. 1998, 94, 3727–3735. [Google Scholar] [CrossRef]
- Zhu, L.J.; Lu, Q.P.; Lv, L.F.Y.; Wang, Y.; Hu, Y.F.; Deng, Z.B.; Lou, Z.D.; Hou, Y.B.F.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Liu, Y.; Withers, R.L.; Frankcombe, T.J.; Norén, L.; Snashall, A.; Kitchin, M.; Sith, P.; Gong, B.; Chen, H.; et al. Electron-pinned defect-dipoles for high-performance colossal permittivity materials. Nature Mat. 2013, 12, 821–826. [Google Scholar] [CrossRef]
- Blanco, G.; Pintado, J.M.; Bernal, S.; Cauqui, M.A.; Corchado, M.P.; Galtayries, A.; Ghijsen, J.; Sporken, R.; Eickhoff, T.; Drube, W. Influence of the nature of the noble metal (Rh,Pt) on the low-temperature reducibility of a Ce/Tb mixed oxide with application as TWC component. Surf. Interface Anal. 2002, 34, 120–124. [Google Scholar] [CrossRef]
- Balaguer, M.; Yoo, C.; Bouwmeester, H.J.M.; Serra, J.M. Bulk transport and oxygen surface exchange of the mixed ionic–electronic conductor Ce1−xTbxO2−δ (x = 0.1, 0.2, 0.5). J. Mater. Chem. A 2013, 1, 10234–10242. [Google Scholar] [CrossRef]
- Nagpure, I.M.; Pitale, S.S.; Coetsee, E.; Ntwaeaborwa, O.M.; Terblans, J.J.; Swart, H.C. Low voltage electron induced cathodoluminescence degradation and surface characterization of Sr3(PO4)2:Tb phosphor. Appl. Surf. Sci. 2011, 257, 10147–10155. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Gu, S.; Wang, F.; Zhou, H. In-built Tb4+/Tb3+ redox centers in terbium-doped bismuth molybdate nanograss for enhanced photocatalytic activity. Catal. Sci. Technol. 2016, 6, 3510–3519. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V.; Cu and Zn. Appl. Surface Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Ketteler, G.; Yamamoto, S.; Bluhm, H.; Andersson, K.; Starr, D.E.; Ogletree, D.F.; Ogasawara, H.; Nilsson, A.; Salmeron, M. The nature of water nucleation sites on TiO2 (110) surfaces revealed by ambient pressure X-ray photoelectron spectroscopy. J. Phys. Chem. C 2007, 111, 8278–8282. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, P.; Liu, M.; Itty, P.A.; Liu, Z.; Rheinheimer, V.; Zhang, M.-H.; Monteiro, P.J.M.; Yu, L.E. Characterization of photocatalytic TiO2 powder under varied environments using near ambient pressure X-ray photoelectron spectroscopy. Sci. Rep. 2017, 7, 43298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouilleaue, J.; Devilliers, D.; Groult, H.; Marcus, P. Surface study of titanium-based ceramic electrode material by X-ray photoelectron spectroscopy. J. Mat. Sci. 1997, 32, 5645–5651. [Google Scholar] [CrossRef]
- Gennari, F.C.; Pasquevich, D.M. Enhancing effect of iron chlorides on the anatase-rutile transition in titanium dioxide. J. Am. Ceram. Soc. 1999, 82, 1919–1921. [Google Scholar] [CrossRef]
- Camps, I.; Borlaf, M.; Toudert, J.; de Andrés, A.; Colomer, M.T.; Moreno, R.; Serna, R. Evidencing early pyrochlore formation in rare-earth doped TiO2 nanocrystals: Structure sensing via VIS and NIR Er3+ light emission. J. Alloys Comp. 2018, 735, 2267–2274. [Google Scholar] [CrossRef]
- Borlaf, M.; Moreno, R.; Ortiz, A.L.; Colomer, M.T. Synthesis and photocatalytic activity of Eu3+-doped nanoparticulate TiO2 sols and thermal stability of the resulting xerogels. Mat. Chem. Phys. 2014, 144, 8–16. [Google Scholar] [CrossRef]
- Colomer, M.T.; Roa, C.; Ortiz, A.L.; Ballesteros, L.M.; Molina., P. Influence of Nd3+ on the structure, thermal evolution and photoluminescence properties of nanoparticulate TiO2 xerogels. J. Alloys Comp. 2020, 819, 152972. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii and crystal chemistry. J. Inorg. Nucl. Chem. 1970, 32, 1427–1441. [Google Scholar] [CrossRef]
- Novotny, J. Oxide Semiconductors for Solar Energy Conversion Titanium Dioxide, 1st ed.; Lee, S., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res. 2000, 15, 437–448. [Google Scholar] [CrossRef]





| SSA (m2/g) | |
|---|---|
| 0.00%_550 | 44 ± 3 |
| 0.12%_550 | 35 ± 4 |
| 1.70%_550 | 34 ± 4 |
| 3.00%_550 | 32 ± 4 |
| Tb (at. %) | Ti (at. %) | O (at. %) | O/Ti Ratio | Tb/Ti Ratio | |
|---|---|---|---|---|---|
| 0.00%_550 | - | 25.3 | 74.7 | 3.0 | - |
| 0.12%_550 | 0.1 | 27.4 | 72.5 | 2.6 | 0.004 |
| 1.70%_550 | 1.4 | 27.3 | 71.3 | 2.6 | 0.050 |
| 3.00%_550 | 2.7 | 27.9 | 69.4 | 2.5 | 0.100 |
| 1.70%_800 | 2.8 | 27.5 | 69.7 | 2.5 | 0.102 |
| 1.70%_1000 | 3.9 | 25.3 | 70.8 | 2.8 | 0.154 |
| Tb4+ (a) (at.%) | Tb3+ (a) (at.%) | Tb4+/Tb3+ Ratio | Ti-O (b) Lattice (at.%) | Ti-OH (b) Terminal (at.%) | |
|---|---|---|---|---|---|
| 0.00%_550 | - | - | - | 61.5 | 38.5 |
| 0.12%_550 | - | - | - | 83.0 | 17.0 |
| 1.70%_550 | 38.5 | 61.5 | 61.5 | 80.7 | 19.3 |
| 3.00%_550 | 41.2 | 58.8 | 58.8 | 83.5 | 16.5 |
| 1.70%_800 | 43.9 | 56.1 | 56.1 | 83.6 | 16.4 |
| 1.70%_1000 | 49.5 | 50.5 | 50.5 | 83.5 | 16.5 |
| a ± 0.004 (Å) | c ± 0.005 (Å) | V ± 0.02 (Å3) | Crystallite Size ± 0.2 (nm) | |
|---|---|---|---|---|
| 0.00%_550 | 3.786 | 9.520 | 136.44 | 9.9 |
| 0.12%_550 | 3.784 | 9.515 | 136.24 | 12.0 |
| 1.70%_550 | 3.786 | 9.517 | 136.41 | 12.5 |
| 3.00%_550 | 3.786 | 9.518 | 136.46 | 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomer, M.T.; Vattier, F. Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase. Int. J. Mol. Sci. 2022, 23, 13162. https://doi.org/10.3390/ijms232113162
Colomer MT, Vattier F. Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase. International Journal of Molecular Sciences. 2022; 23(21):13162. https://doi.org/10.3390/ijms232113162
Chicago/Turabian StyleColomer, María Teresa, and Florencia Vattier. 2022. "Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase" International Journal of Molecular Sciences 23, no. 21: 13162. https://doi.org/10.3390/ijms232113162
APA StyleColomer, M. T., & Vattier, F. (2022). Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase. International Journal of Molecular Sciences, 23(21), 13162. https://doi.org/10.3390/ijms232113162







